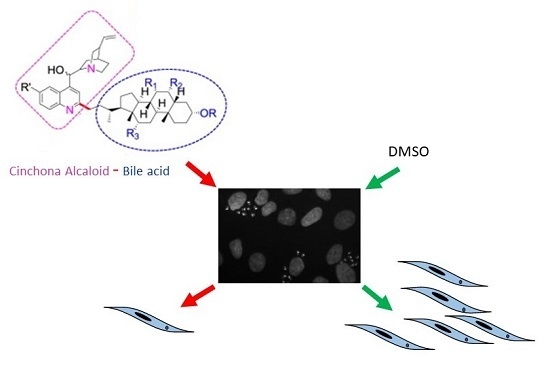
Hybrids of Cinchona Alkaloids and Bile Acids as Antiparasitic Agents Against Trypanosoma cruzi
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cells and Parasites
4.2. Parasite IC50 Estimation
4.3. Cells IC50 Estimation
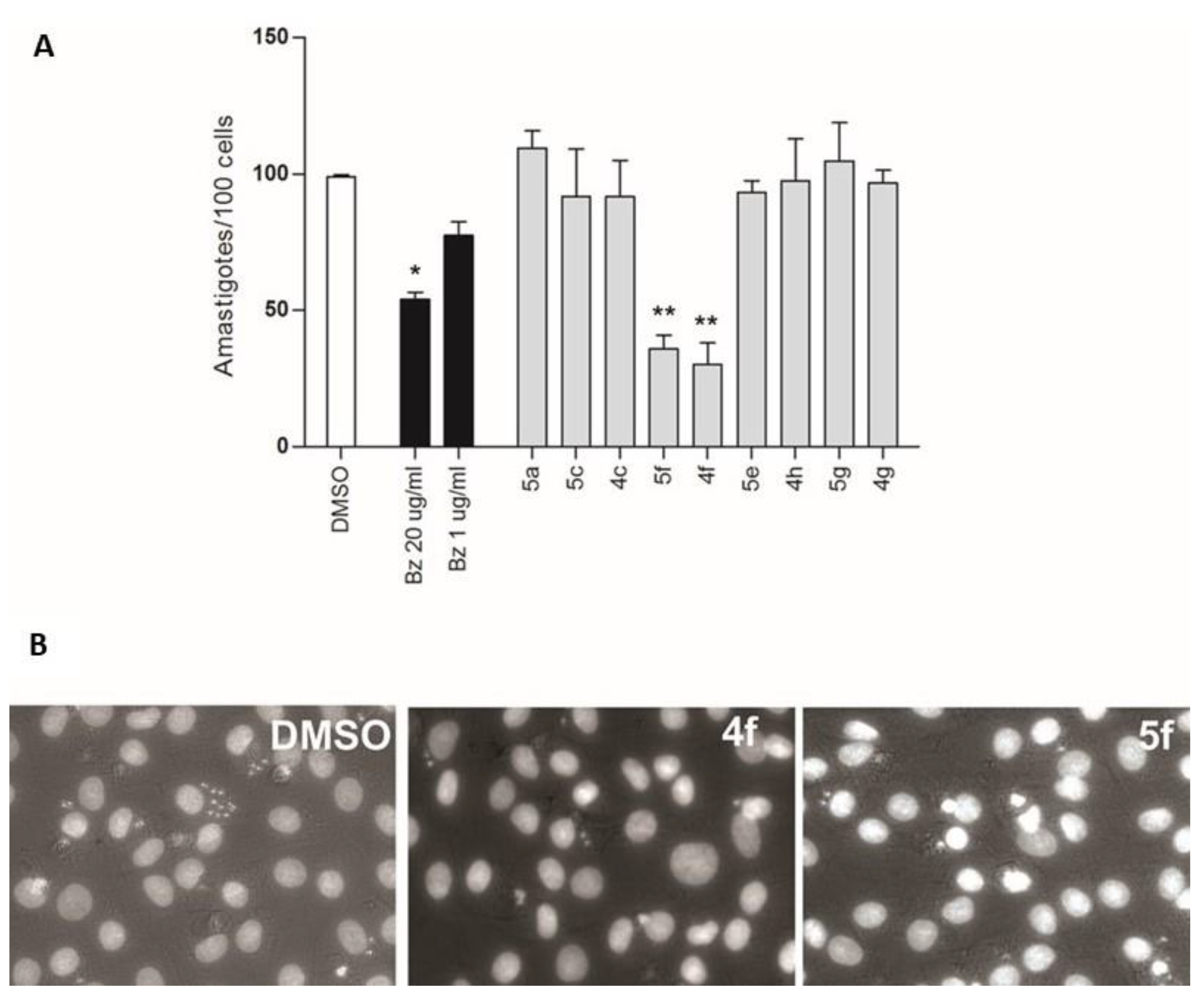
4.4. Amastigote Count
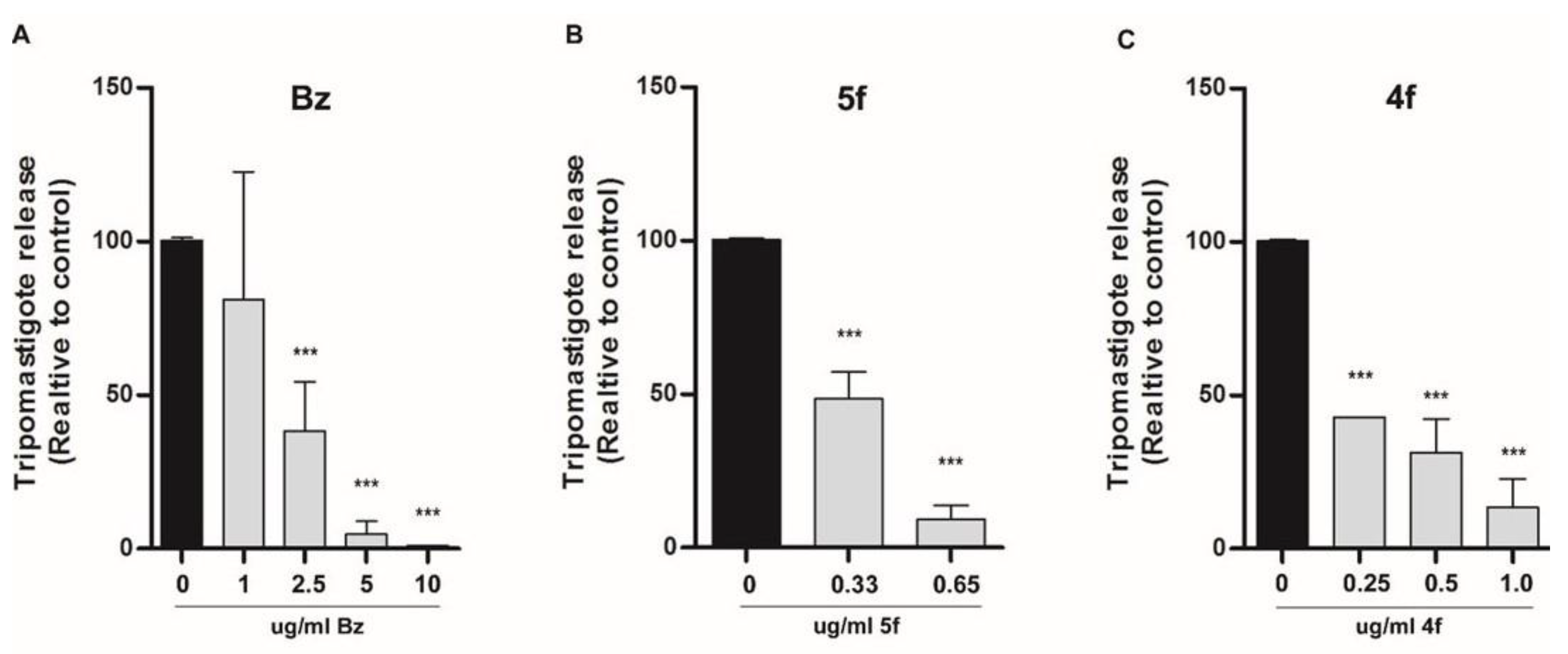
4.5. Trypomastigote Release Assay
4.6. Statistics
Author Contributions
Funding
Conflicts of Interest
References
- Lechuga, G.C.; Borges, J.C.; Calvet, C.M.; de Araujo, H.P.; Zuma, A.A.; do Nascimento, S.B.; Motta, M.C.M.; Bernardino, A.M.R.; Pereira, M.C.S.; Bourguignon, S.C. Interactions between 4-aminoquinoline and heme: Promising mechanism against Trypanosoma cruzi. Int. J. Parasitol. 2016, 6, 154–164. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kinnamon, K.E.; Poon, B.T.; Hanson, W.L.; Waits, V.B. Primaquine analogues that are potent anti-Trypanosoma cruzi agents in a mouse model. Ann. Trop. Med. Parasitol. 1996, 90, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Foley, M.; Tilley, L. Quinoline antimalarials: Mechanisms of action and resistance and prospects for new agents. Pharmacol. Ther. 1998, 79, 55–87. [Google Scholar] [CrossRef]
- Tiuman, T.S.; Santos, A.O.; Ueda-Nakamura, T.; Filho, B.P.; Nakamura, C.V. Recent advances in leishmaniasis treatment. Int. J. Infect. Dis. 2011, 15, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, K.; Ruszkowski, P.; Valentini, L.; Huczynski, A.; Steverding, D. Cytotoxic and trypanocidal activities of Cinchona alkaloid derivatives. Chem. Biol. Drug Des. 2018, 92, 1778–1787. [Google Scholar] [CrossRef] [PubMed]
- Fakhfakh, M.A.; Fournet, A.; Prina, E.; Mouscadet, J.F.; Franck, X.; Hocquemiller, R.; Figadere, B. Synthesis and biological evaluation of substituted quinolines: Potential treatment of protozoal and retroviral co-infections. Bioorganic Med. Chem. 2003, 11, 5013–5023. [Google Scholar] [CrossRef]
- Kacprzak, K.M. Chemistry and Biology of Cinchona Alkaloids. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 605–641. [Google Scholar]
- Pranay, G.; Puspal, D. Spectrum of biological properties of Cinchona alkaloids: A brief review. J. Pharmacogn. Phytochem. 2017, 6, 162–166. [Google Scholar]
- Dawson, P.A. Role of the intestinal bile acid transporters in bile acid and drug disposition. Handb. Exp. Pharmacol. 2011, 201, 169–203. [Google Scholar]
- Leverrier, A.; Bero, J.; Frederich, M.; Quetin-Leclercq, J.; Palermo, J. Antiparasitic hybrids of Cinchona alkaloids and bile acids. Eur. J. Med. Chem. 2013, 66, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Musikant, D.; Ferri, G.; Durante, I.M.; Buscaglia, C.A.; Altschuler, D.L.; Edreira, M.M. Host Epac1 is required for cAMP-mediated invasion by Trypanosoma cruzi. Mol. Biochem. Parasitol. 2017, 211, 67–70. [Google Scholar] [CrossRef] [PubMed]
- Zingales, B.; Andrade, S.G.; Briones, M.R.; Campbell, D.A.; Chiari, E.; Fernandes, O.; Guhl, F.; Lages-Silva, E.; Macedo, A.M.; Machado, C.R.; et al. A new consensus for Trypanosoma cruzi intraspecific nomenclature: Second revision meeting recommends TcI to TcVI. Memorias do Instituto Oswaldo Cruz 2009, 104, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.; D’Avila, D.A.; Silva, M.N.; Galvao, L.M.; Macedo, A.M.; Chiari, E.; Gontijo, E.D.; Zingales, B. Trypanosoma cruzi benznidazole susceptibility in vitro does not predict the therapeutic outcome of human Chagas disease. Memorias do Instituto Oswaldo Cruz 2010, 105, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, D.; Barnabe, C.; Sereno, D.; Tibayrenc, M. Lack of correlation between in vitro susceptibility to Benznidazole and phylogenetic diversity of Trypanosoma cruzi, the agent of Chagas disease. Exp. Parasitol. 2004, 108, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.J.; Bahia, M.T.; Carneiro, C.M.; Martins-Filho, O.A.; Tibayrenc, M.; Barnabe, C.; Tafuri, W.L.; de Lana, M. Chemotherapy with benznidazole and itraconazole for mice infected with different Trypanosoma cruzi clonal genotypes. Antimicrob. Agents Chemother. 2003, 47, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Murta, S.M.; Gazzinelli, R.T.; Brener, Z.; Romanha, A.J. Molecular characterization of susceptible and naturally resistant strains of Trypanosoma cruzi to benznidazole and nifurtimox. Mol. Biochem. Parasitol. 1998, 93, 203–214. [Google Scholar] [CrossRef]
- Filardi, L.S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef]
- Zingales, B.; Miles, M.A.; Moraes, C.B.; Luquetti, A.; Guhl, F.; Schijman, A.G.; Ribeiro, I. Drug discovery for Chagas disease should consider Trypanosoma cruzi strain diversity. Memorias do Instituto Oswaldo Cruz 2014, 109, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Quebrada Palacio, L.P.; Gonzalez, M.N.; Hernandez-Vasquez, Y.; Perrone, A.E.; Parodi-Talice, A.; Bua, J.; Postan, M. Phenotypic diversity and drug susceptibility of Trypanosoma cruzi TcV clinical isolates. PLoS ONE 2018, 13, e0203462. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Berzal, C.; Aran, V.J.; Escario, J.A.; Gomez-Barrio, A. Experimental models in Chagas disease: A review of the methodologies applied for screening compounds against Trypanosoma cruzi. Parasitol. Res. 2018, 117, 3367–3380. [Google Scholar] [CrossRef] [PubMed]
- Leverrier, A.; Bero, J.; Cabrera, J.; Frederich, M.; Quetin-Leclercq, J.; Palermo, J.A. Structure-activity relationship of hybrids of Cinchona alkaloids and bile acids with in vitro antiplasmodial and antitrypanosomal activities. Eur. J. Med. Chem. 2015, 100, 10–17. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4f and 5f are available from the authors. |
| Hybrid (Alkaloyd + Bile Acid) | Peracetylated | Non-Acetylated |
|---|---|---|
| Quinine + Litocholic | 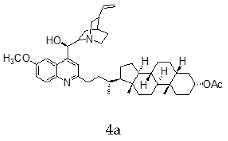 |  |
| Quinine + Chenodeoxycholic | 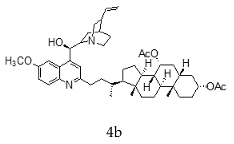 | 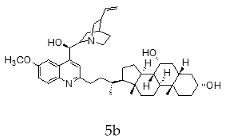 |
| Quinidine + Litocholic | 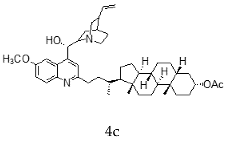 |  |
| Quinidine + Chenodeoxycholic | 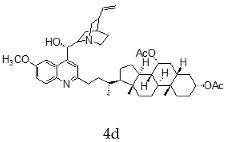 | 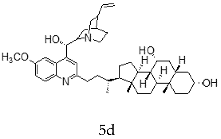 |
| Cinchonine + Litocholic | 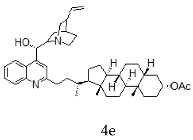 | 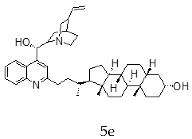 |
| Cinchonine + Chenodeoxycholic | 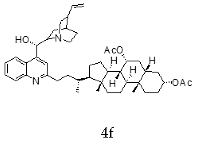 | 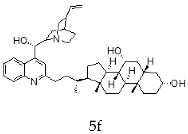 |
| Cinchonidine + Litocholic | 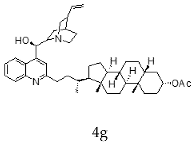 |  |
| Cinchonidine + Chenodeoxycholic | 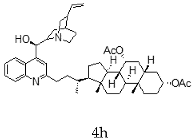 | 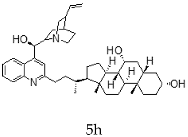 |
| T. cruzi CL Brener | NRK Cells | Selectivity | |
|---|---|---|---|
| Hybrid | IC50 (μg/mL) | IC50 (μg/mL) | NRK/T. cruzi |
| 5b | 0.90 ± 0.10 | 5.10 ± 0.76 | 5.67 |
| 4b | 0.80 ± 0.13 | 3.91 ± 0.29 | 4.89 |
| 5a | 3.71 ± 0.13 | 15.30 ± 4.04 | 4.12 |
| 4a | 0.64 ± 0.15 | 1.41 ± 0.13 | 2.20 |
| 5d | 0.40 ± 0.05 | 1.59 ± 0.54 | 3.98 |
| 4d | 0.72 ± 0.07 | 3.30 ± 0.08 | 4.58 |
| 5c | 0.78 ± 0.11 | >11 | >10 |
| 4c | 1.08 ± 0.23 | 16.53 ± 0.18 | 15.30 |
| 5f | 0.34 ± 0.03 | 4.02 ± 0.55 | 12.18 |
| 4f | 0.51 ± 0.06 | 6.69 ± 1.51 | 13.04 |
| 5e | 3.96 ± 2.69 | >30 | ND |
| 4e | 1.16 ± 0.15 | 7.50 ± 0.29 | 6.46 |
| 5h | 0.30 ± 0.00 | 0.67 ± 0.05 | 2.23 |
| 4h | 0.70 ± 0.18 | 6.50 ± 0.28 | 9.28 |
| 5g | 2.56 ± 0.58 | 27.11 ± 4.54 | 10.58 |
| 4g | 1.30 ± 0.01 | 12.30 ± 0.81 | 9.46 |
| Bz | 2.5 ± 0.01 | ND | ND |
| T. cruzi RA (DTU VI) | T. cruzi Y (DTU II) | |
|---|---|---|
| Hybrid | IC50 (μg/mL) | IC50 (μg/mL) |
| 5c | 0.79 ± 0.19 | ≥2.00 |
| 4c | ND | ≥1.00 |
| 5f | 0.31 ± 0.10 | 0.65 ± 0.07 |
| 4f | 0.25 ± 0.10 | 0.50 ± 0.03 |
| 4h | 1.50 ± 0.50 | ≥2.00 |
| 5g | 3.03 ± 0.24 | 3.25 ± 0.67 |
| 4g | 1.10 ± 0.21 | 3.05 ± 0.71 |
| Bz | 2.5 ± 0.32 | ≥15.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musikant, D.; Leverrier, A.; Bernal, D.; Ferri, G.; Palermo, J.A.; Edreira, M.M. Hybrids of Cinchona Alkaloids and Bile Acids as Antiparasitic Agents Against Trypanosoma cruzi. Molecules 2019, 24, 3168. https://doi.org/10.3390/molecules24173168
Musikant D, Leverrier A, Bernal D, Ferri G, Palermo JA, Edreira MM. Hybrids of Cinchona Alkaloids and Bile Acids as Antiparasitic Agents Against Trypanosoma cruzi. Molecules. 2019; 24(17):3168. https://doi.org/10.3390/molecules24173168
Chicago/Turabian StyleMusikant, Daniel, Aurélie Leverrier, Diana Bernal, Gabriel Ferri, Jorge A. Palermo, and Martin M. Edreira. 2019. "Hybrids of Cinchona Alkaloids and Bile Acids as Antiparasitic Agents Against Trypanosoma cruzi" Molecules 24, no. 17: 3168. https://doi.org/10.3390/molecules24173168
APA StyleMusikant, D., Leverrier, A., Bernal, D., Ferri, G., Palermo, J. A., & Edreira, M. M. (2019). Hybrids of Cinchona Alkaloids and Bile Acids as Antiparasitic Agents Against Trypanosoma cruzi. Molecules, 24(17), 3168. https://doi.org/10.3390/molecules24173168