Characterization and Hemostatic Potential of Two Kaolins from Southern China
Abstract
1. Introduction
2. Results
2.1. Chemical Composition Analysis
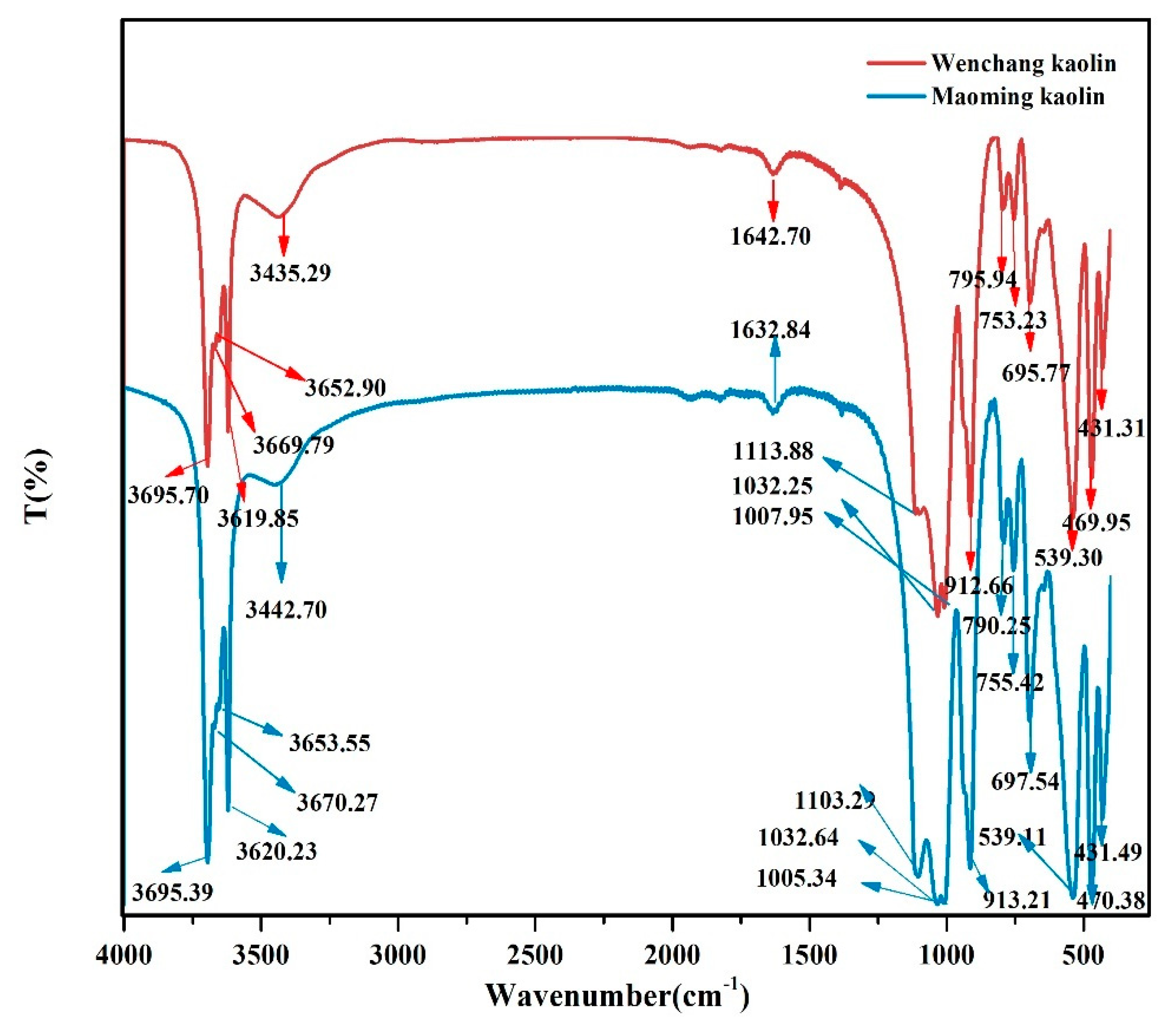
2.2. FTIR
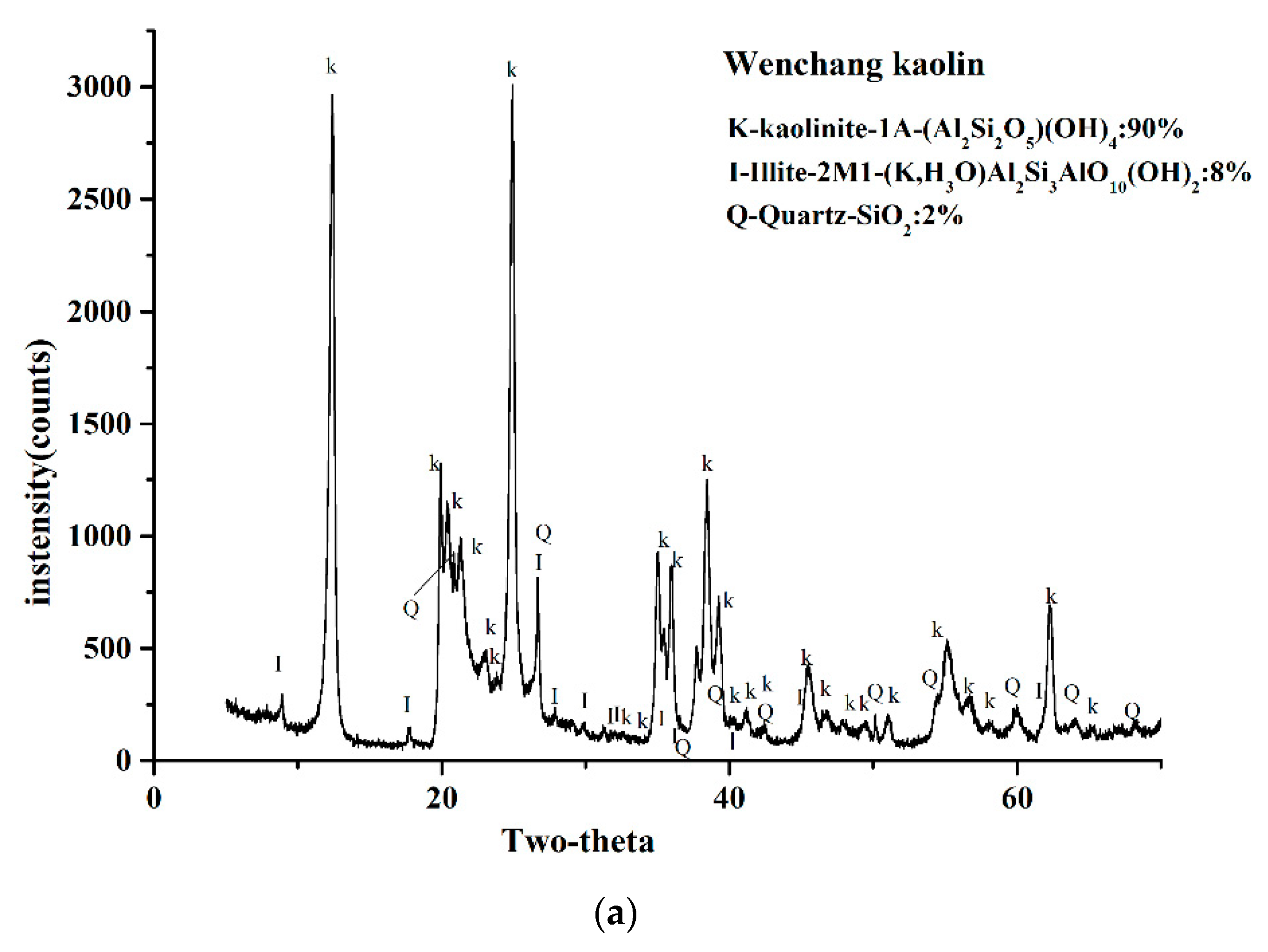
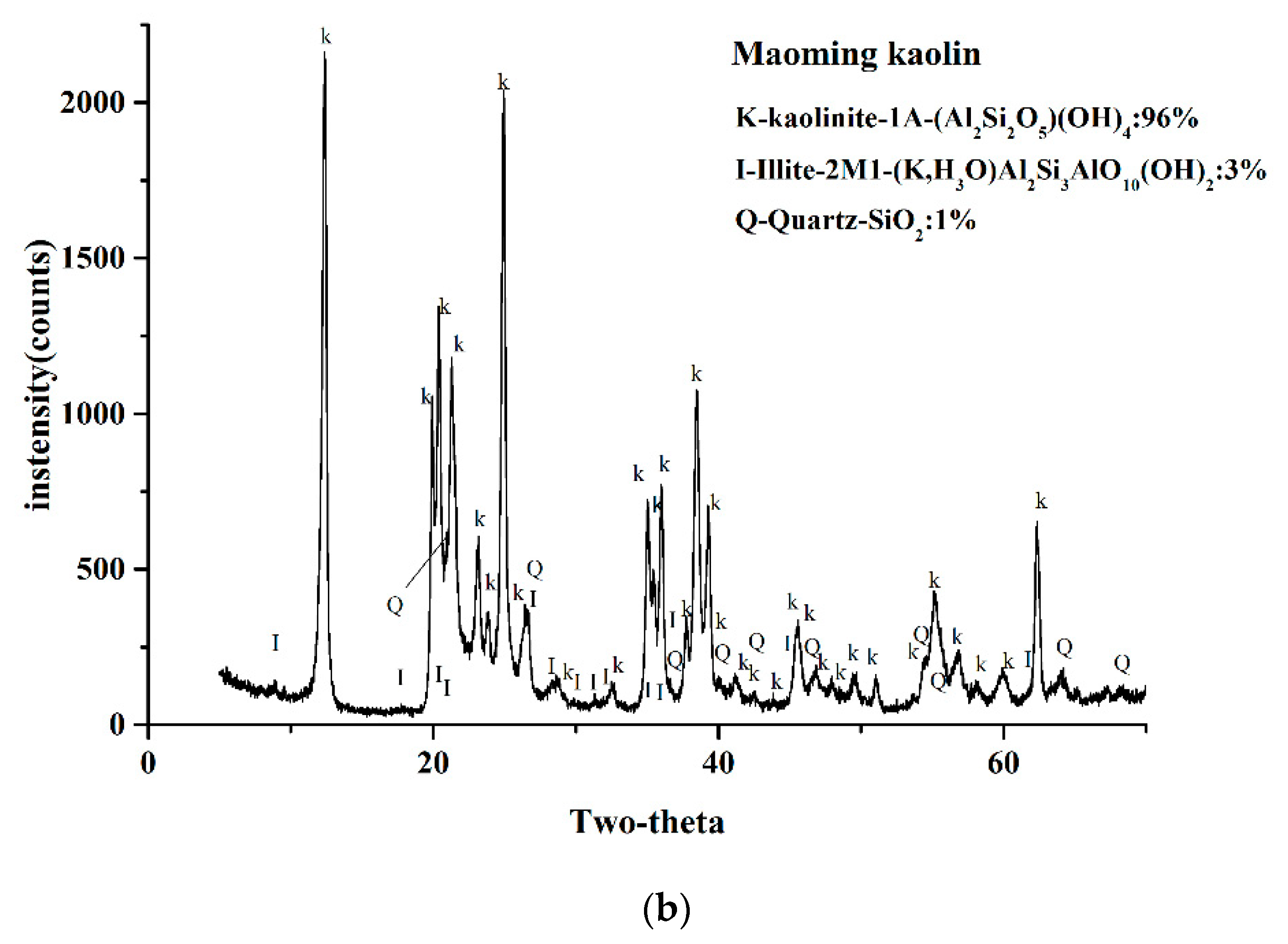
2.3. XRD Analysis
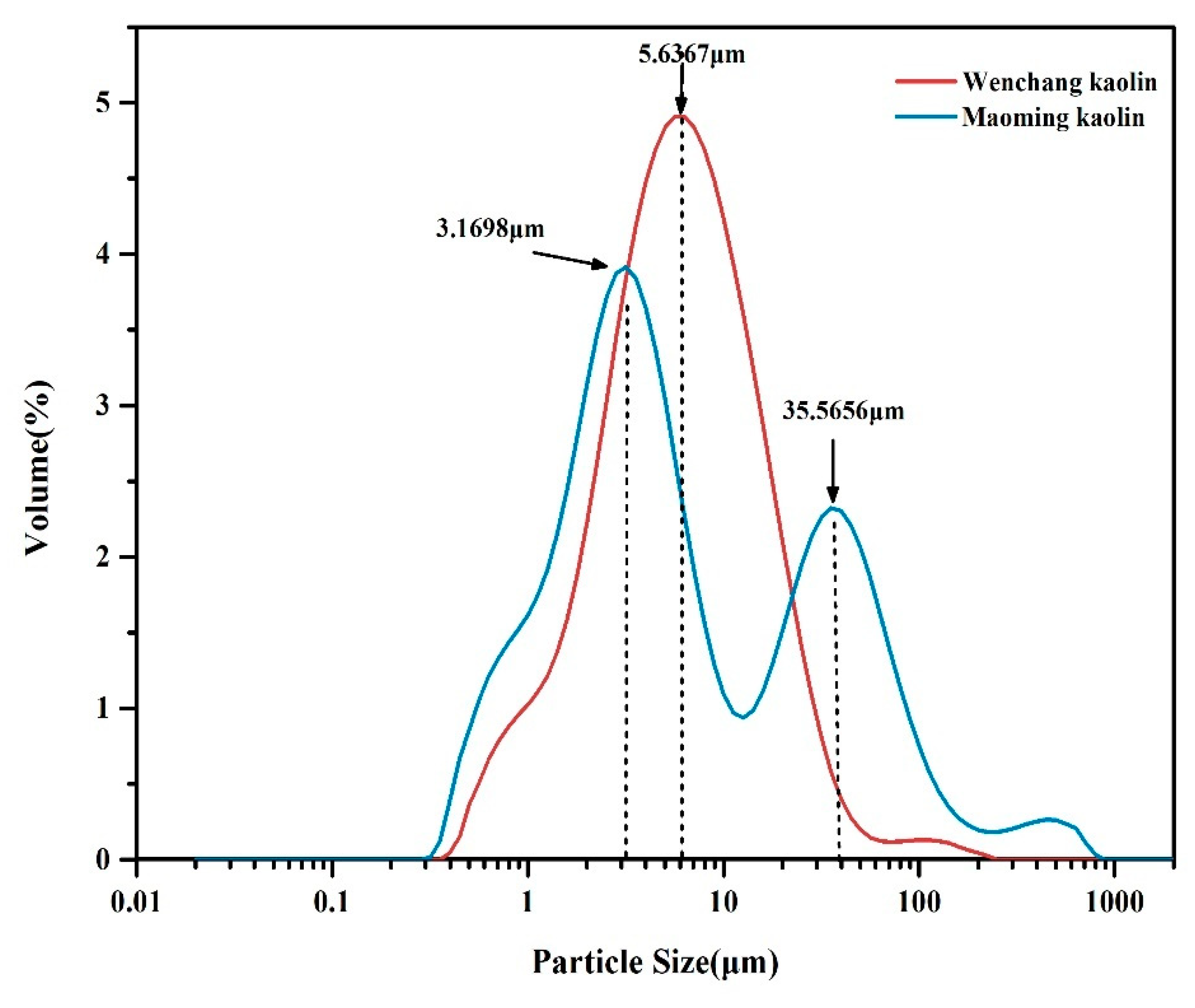
2.4. Particle Size Distributions
2.5. Surface Area Determination
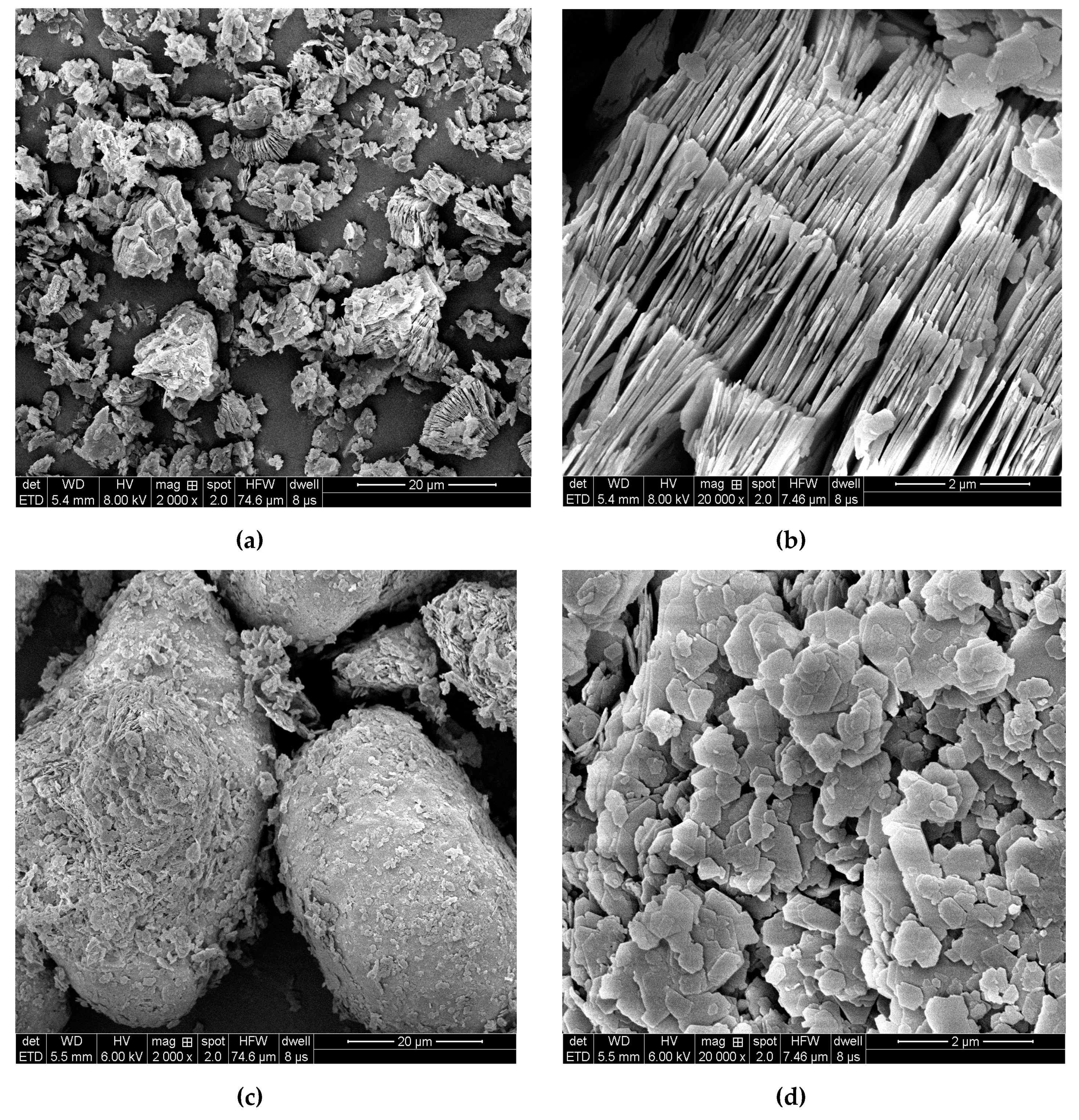
2.6. Kaolinite Micromorphology and Microtexture
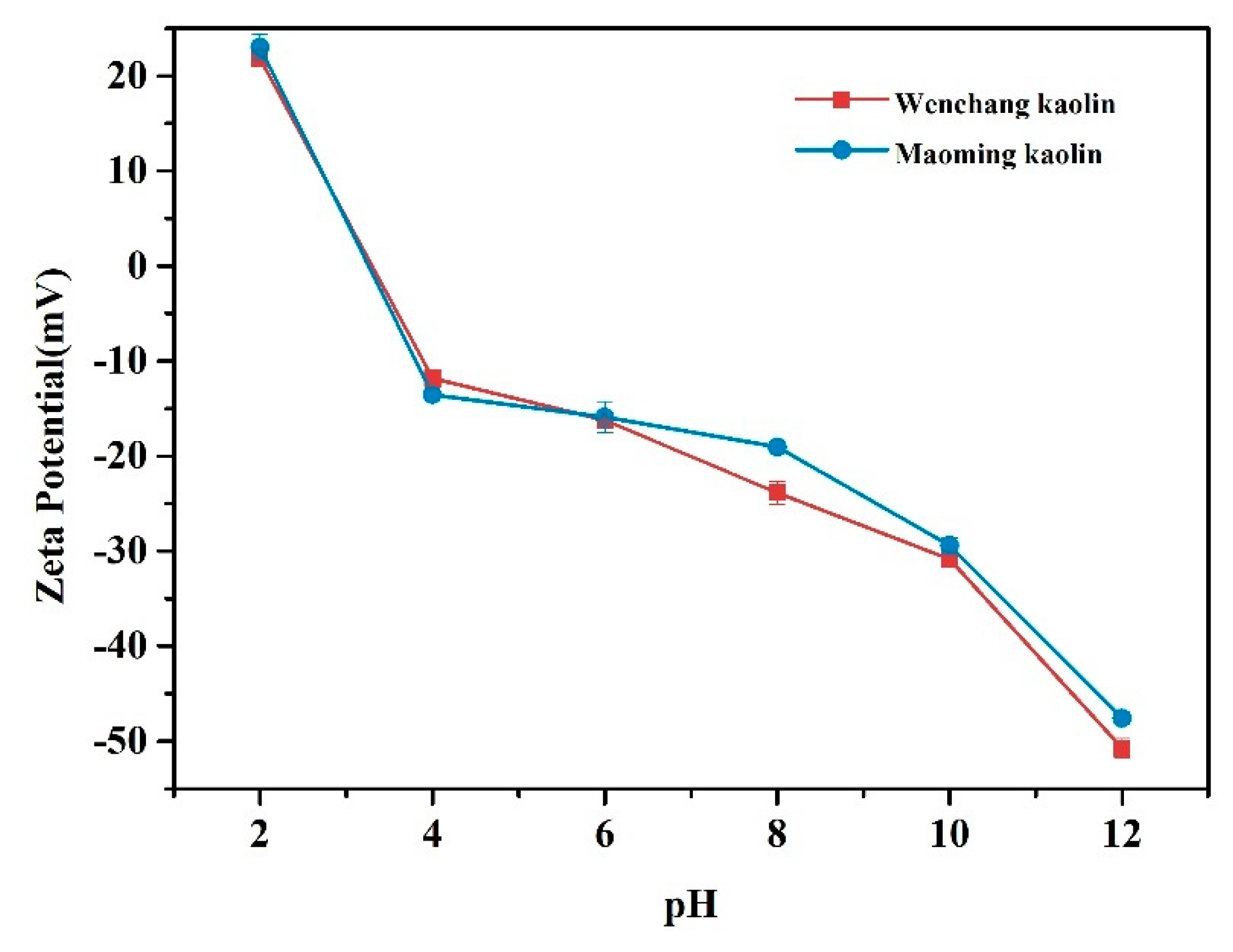
2.7. Zeta Potential Analysis
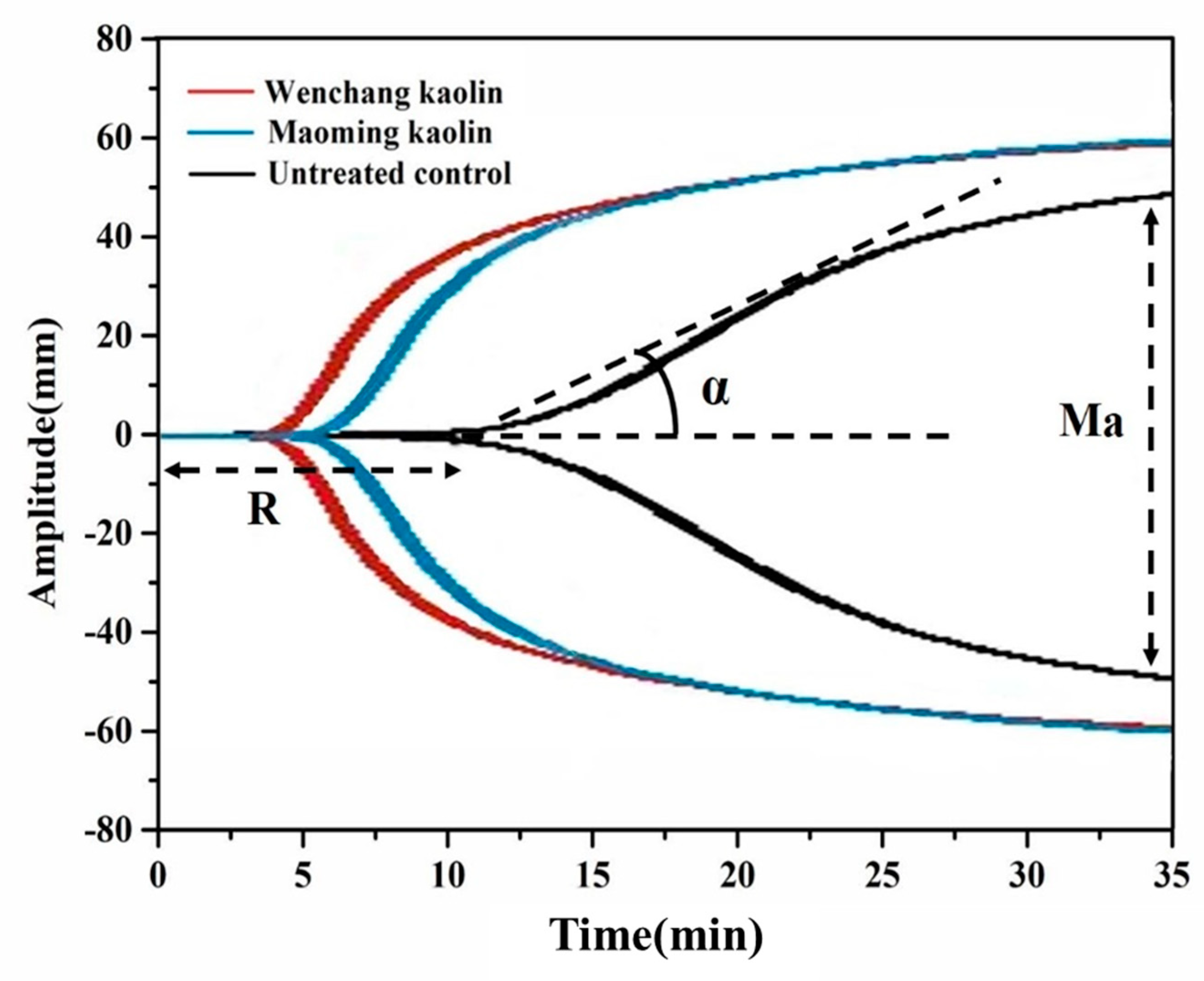
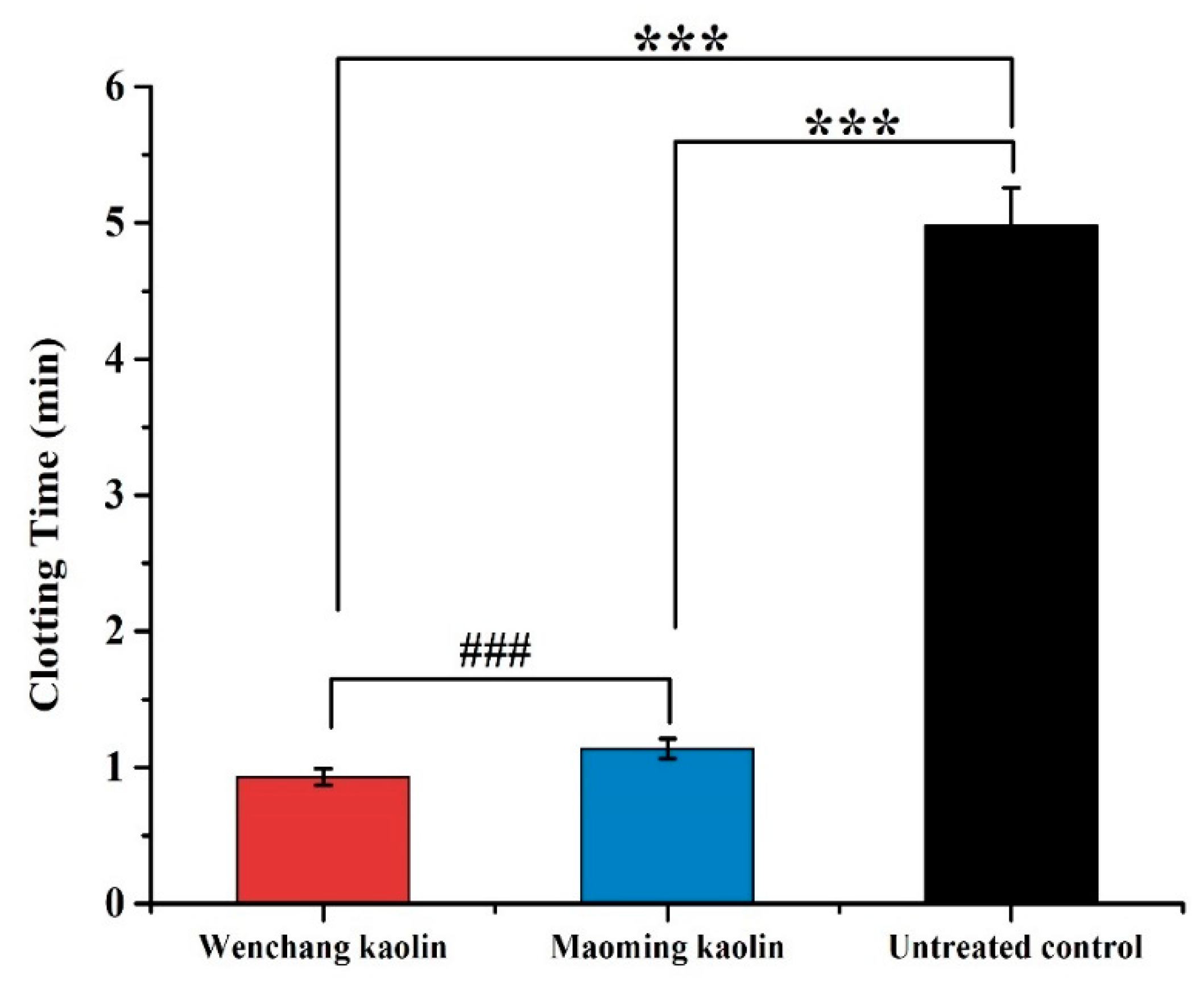
2.8. Thrombelastograph Measurements of Clotting Agents in Whole Blood
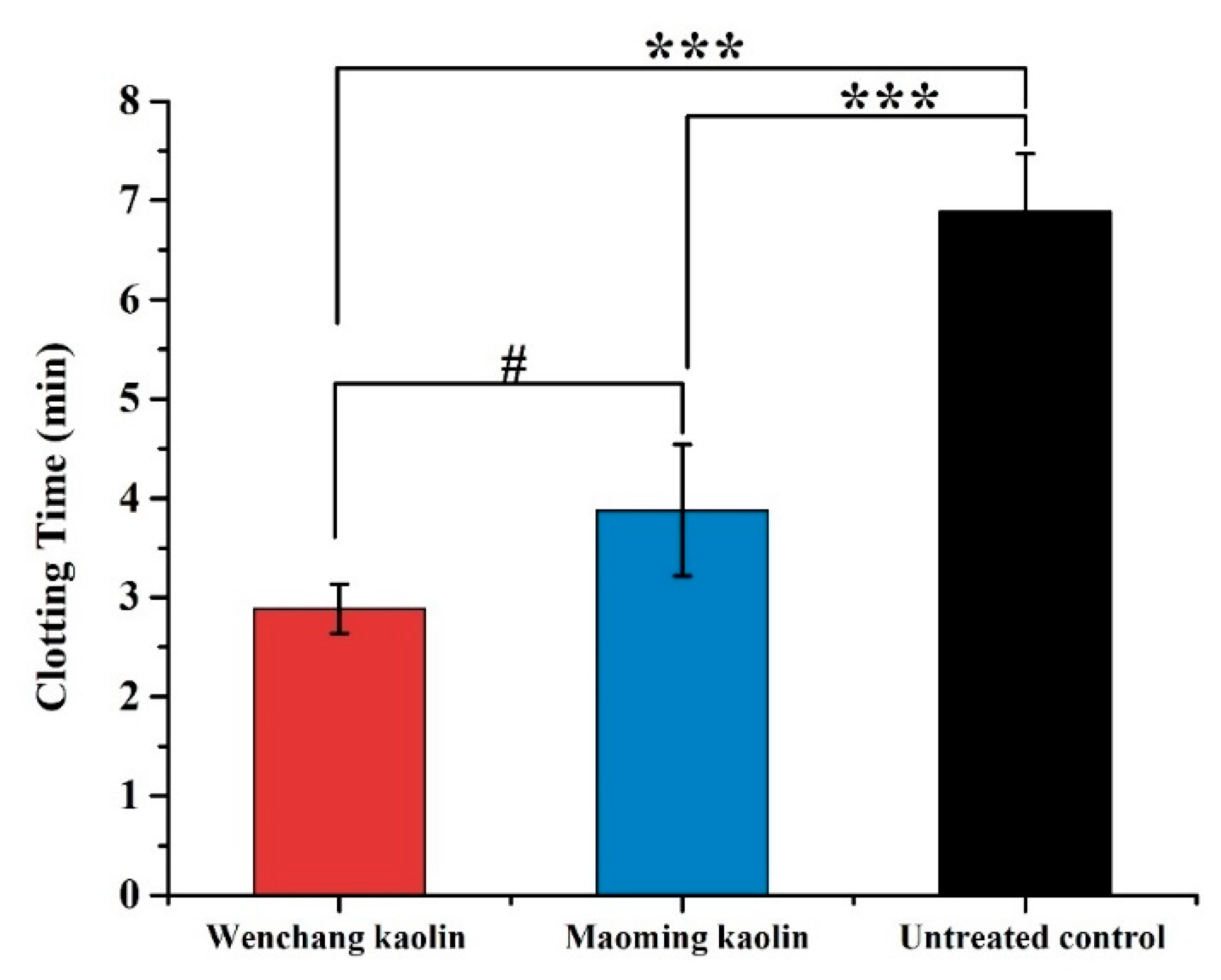
2.9. In Vitro WBCT
2.10. Plasma Recalcification Time Measurement
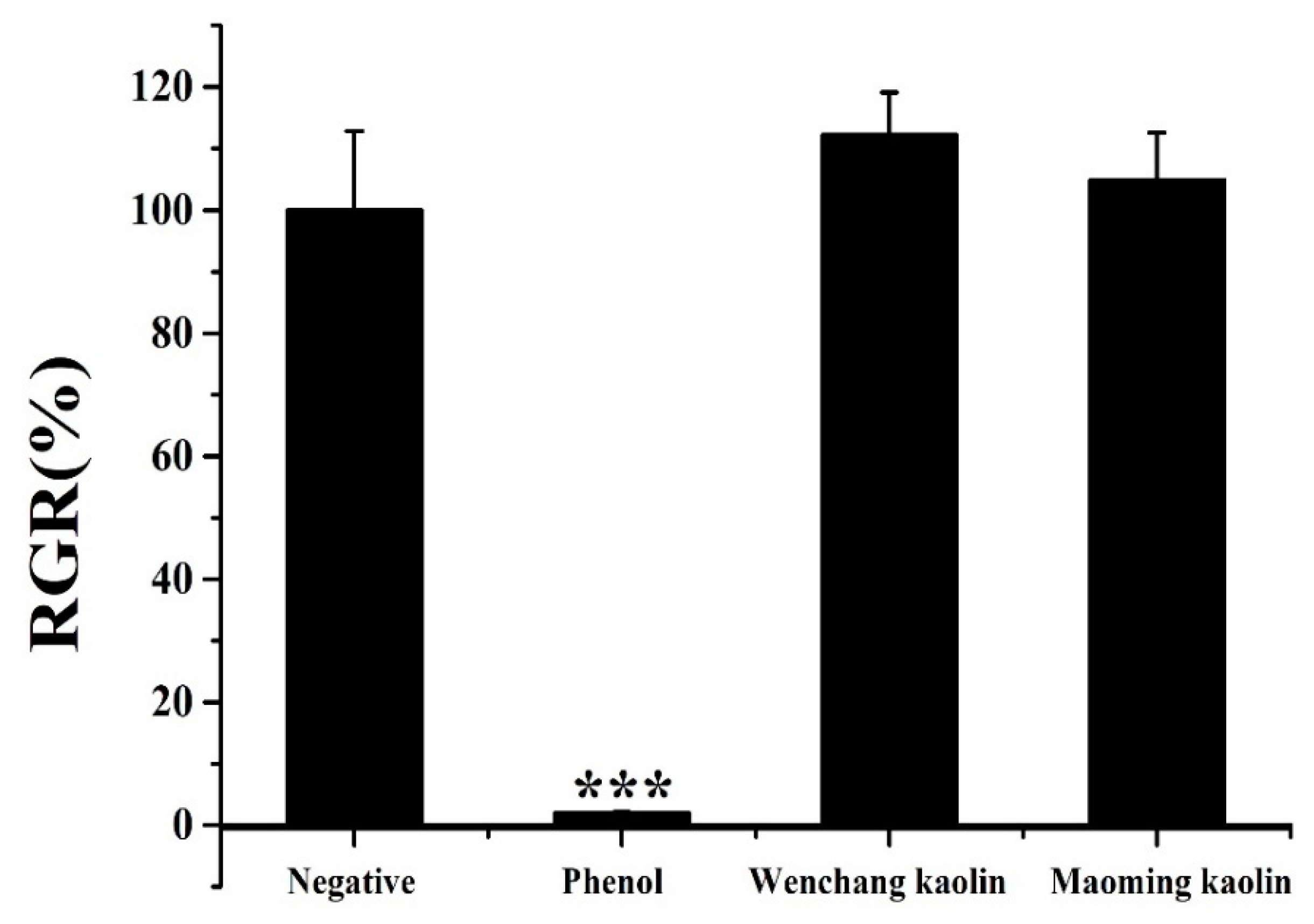
2.11. Cell Viability Assay
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Preparation and Analysis of the Clay Minerals
4.1.2. Experiment Materials
4.2. Chemical Composition Analysis
4.3. Fourier Transform Infrared (FTIR) Spectroscopy
4.4. X-ray diffraction (XRD) Measurements
4.5. Particle Size Distribution
4.6. Surface Area Determination
4.7. Scanning Electron Microscopy (SEM) Analysis
4.8. Zeta Potential Analysis
4.9. Thrombelastograph Measurements of Clotting Agents in Whole Blood
4.10. In Vitro Whole Blood Clotting Tests (WBCT)
4.11. Plasma Recalcification Time (PRT) Measurement
4.12. Cell Viability Assay
4.13. Data Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yaya, A.; Tiburu, E.K.; Vickers, M.E.; Efavi, J.K.; Onwona-Agyeman, B.; Knowles, K.M. Characterisation and identification of local kaolin clay from Ghana: A potential material for electroporcelain insulator fabrication. Appl. Clay Sci. 2017, 150, 125–130. [Google Scholar] [CrossRef]
- Segura, J.C.F.; Cruz, V.E.R.; Ascencio, E.M.L.; García, F.L. Characterization and electrochemical treatment of a kaolin. Appl. Clay Sci. 2017, 146, 264–269. [Google Scholar] [CrossRef]
- Awad, M.E.; Lopez-Galindo, A.; Setti, M.; El-Rahmany, M.M.; Iborra, C.V. Kaolinite in pharmaceutics and biomedicine. Int. J. Pharm. 2017, 533, 34–48. [Google Scholar] [CrossRef] [PubMed]
- Pruett, R.J. Kaolin deposits and their uses: Northern Brazil and Georgia, USA. Appl. Clay Sci. 2016, 131, 3–13. [Google Scholar] [CrossRef]
- Ward, K.R.; Tiba, M.H.; Holbert, W.H.; Blocher, C.R.; Draucker, G.T.; Proffitt, E.K.; Bowlin, G.L.; Ivatury, R.R.; Diegelmann, R.F. Comparison of a new hemostatic agent to current combat hemostatic agents in a Swine model of lethal extremity arterial hemorrhage. J. Trauma 2007, 63, 276–284. [Google Scholar] [CrossRef]
- Khoshmohabat, H.; Dalfardi, B.; Dehghanian, A.R.; Rasouli, H.R.; Mortazavi, S.M.J.; Paydar, S. The effect of CoolClot hemostatic agent on skin wound healing in rats. J. Surg. Res. 2016, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, B. Evaluation of topical hemostatic agents for combat wound treatment. US Army Med. Dep. J. 2011, 4–6, 25–37. [Google Scholar]
- Carraway, J.W.; Kent, D.; Young, K.; Cole, A.; Friedman, R.; Ward, K.R. Comparison of a new mineral based hemostatic agent to a commercially available granular zeolite agent for hemostasis in a swine model of lethal extremity arterial hemorrhage. Resuscitation 2008, 78, 230–235. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Gao, B.; Liu, X.W. Topical and effective hemostatic medicines in the battlefield. Int. J. Clin. Exp. Med. 2015, 8, 10–19. [Google Scholar]
- Englehart, M.S.; Cho, S.D.; Tieu, B.H.; Morris, M.S.; Underwood, S.J.; Karahan, A.; Schreiber, M.A. A novel highly porous silica and chitosan-based hemostatic dressing is superior to HemCon and gauze sponges. J. Trauma 2008, 65, 884–890. [Google Scholar] [CrossRef]
- Baker, S.E.; Sawvel, A.M.; Zheng, N.; Stucky, G.D. Controlling Bioprocesses with Inorganic Surfaces: Layered Clay Hemostatic Agents. Chem. Mater. 2007, 19, 4390–4392. [Google Scholar] [CrossRef]
- Li, Y.; Liao, X.; Zhang, X.; Ma, G.; Zuo, S.; Xiao, L.; Stucky, G.D.; Wang, Z.; Chen, X.; Shang, X. In situgenerated thrombin in the protein corona of zeolites: Relevance of the functional proteins to its biological impact. Nano Res. 2014, 7, 1457–1465. [Google Scholar] [CrossRef]
- Li, G.; Quan, K.; Liang, Y.; Li, T.; Yuan, Q.; Tao, L.; Xie, Q.; Wang, X. Graphene-Montmorillonite Composite Sponge for Safe and Effective Hemostasis. ACS Appl. Mater. Interfaces 2016, 8, 35071–35080. [Google Scholar] [CrossRef] [PubMed]
- Pourshahrestani, S.; Zeimaran, E.; Djordjevic, I.; Kadri, N.A.; Towler, M.R. Inorganic hemostats: The state-of-the-art and recent advances. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Trabattoni, D.; Gatto, P.; Bartorelli, A.L. A new kaolin-based hemostatic bandage use after coronary diagnostic and interventional procedures - International Journal of Cardiology. Int. J. Cardiol. 2012, 156, 53–54. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.J.; Geoffrey, D.; Gerlach, T.; Grayson, J.K.; Pichakron, K.O.; Dustin, Z. A pilot study of the use of kaolin-impregnated gauze (Combat Gauze) for packing high-grade hepatic injuries in a hypothermic coagulopathic swine model. J. Surg. Res. 2013, 183, 704–709. [Google Scholar] [CrossRef]
- Trabattoni, D.; Montorsi, P.; Fabbiocchi, F.; Lualdi, A.; Gatto, P.; Bartorelli, A.L. A new kaolin-based haemostatic bandage compared with manual compression for bleeding control after percutaneous coronary procedures. Eur. Radiol. 2011, 21, 1687–1691. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Zhang, Y.; Huang, P.; Chang, S.; Hu, Y.; Yang, Q.; Yang, H. Emerging Nanoclay Composite for Effective Hemostasis. Adv. Funct. Mater. 2018, 28, 1704452. [Google Scholar] [CrossRef]
- Chávez, M.E. Topic usage of kaolin-impregnated gauze as a hemostatic in tonsillectomy. J. Surg. Res. 2014, 192, 678–685. [Google Scholar] [CrossRef]
- Gegel, B.; Burgert, J.; Gasko, J.; Campbell, C.; Martens, M.; Keck, J.; Reynolds, H.; Loughren, M.; Johnson, D. The effects of QuikClot Combat Gauze and movement on hemorrhage control in a porcine model. Mil. Med. 2012, 177, 1543–1547. [Google Scholar] [CrossRef]
- Wilson, I.R. Kaolin and halloysite deposits of China. Clay Miner. 2004, 39, 1–15. [Google Scholar] [CrossRef]
- Yuan, J.; Murray, H.H. Mineralogical and Physical Properties of the Maoming Kaolin from Guandong Province, South China. Clay Miner. Soc. Spec. Pub. 1990, 1, 249–259. [Google Scholar]
- Zhu, X.; He, J.; Su, S.; Zhang, X.; Wang, F. Concept model of the formation process of humic acid–kaolin complexes deduced by trichloroethylene sorption experiments and various characterizations. Chemosphere 2016, 151, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Sun, M.; Liu, X.; Chen, Z. Functional kaolin supported nanoscale zero-valent iron as a Fenton-like catalyst for the degradation of Direct Black G. Chemosphere 2017, 184, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zheng, H.; Sun, Y.; Zhang, S.; Liang, J.; Liu, Y.; An, Y. Evaluation of a novel dextran-based flocculant on treatment of dye wastewater: Effect of kaolin particles. Sci. Total Environ. 2018, 640, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Ma, X.; He, J.; Feng, J.; Liu, S.; Yao, Y.; Hou, L.; Liu, X. Facile selective and diverse fabrication of superhydrophobic, superoleophobic-superhydrophilic and superamphiphobic materials from kaolin. ACS Appl. Mater. Interfaces 2016, 9, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Konduri, M.K.R.; Fatehi, P. Dispersion of kaolin particles with carboxymethylated xylan. Appl. Clay Sci. 2017, 137, 183–191. [Google Scholar] [CrossRef]
- Liu, Q.; Shuai, Z.; Cheng, H.; Ding, W.; Li, X.; Hou, X.; Frost, R.L. Thermal behavior of kaolinite–urea intercalation complex and molecular dynamics simulation for urea molecule orientation. J. Therm. Anal. Calorim. 2014, 117, 189–196. [Google Scholar] [CrossRef]
- Bich, C.; Ambroise, J.; Péra, J. Influence of degree of dehydroxylation on the pozzolanic activity of metakaolin. Appl. Clay Sci. 2009, 44, 194–200. [Google Scholar] [CrossRef]
- Ece, O.I.; Nakagawa, Z.E.; Schroeder, P. Alteration of volcanic rocks and genesis of kaolin deposits in the sile region, northern Istanbul, Turkey. I: Clay mineralogy. Clay Miner. 2003, 51, 675–688. [Google Scholar]
- Irfan Khan, M.; Khan, H.U.; Azizli, K.; Sufian, S.; Man, Z.; Siyal, A.A.; Muhammad, N.; Faiz ur Rehman, M. The pyrolysis kinetics of the conversion of Malaysian kaolin to metakaolin. Appl. Clay Sci. 2017, 146, 152–161. [Google Scholar] [CrossRef]
- Cheng, H.; Xu, P.; Ding, W.; Frost, R.L. Thermal decomposition behavior and de-intercalation kinetics of kaolinite/quaternary ammonium salt complexes. J. Therm. Anal. Calorim. 2016, 126, 1–13. [Google Scholar] [CrossRef]
- Souri, A.; Golestani-Fard, F.; Naghizadeh, R.; Veiseh, S. An investigation on pozzolanic activity of Iranian kaolins obtained by thermal treatment. Appl. Clay Sci. 2015, 103, 34–39. [Google Scholar] [CrossRef]
- Lee, S.; Kim, Y.J.; Moon, H.S. Energy-Filtering Transmission Electron Microscopy (EF-TEM) Study of a Modulated Structure in Metakaolinite, Represented by a 14 Å Modulation. J. Am. Ceram. Soc. 2010, 86, 174–176. [Google Scholar] [CrossRef]
- Mehrosadat, A.; Alireza, T.; Mohammad Ali, O.; Motahareh, M.; Peyman, R.; Mohammad, A. The effect of a new impregnated gauze containing bentonite and halloysite minerals on blood coagulation and wound healing. Blood Coagul. Fibrinolysis 2014, 25, 856–859. [Google Scholar]
- Ostomel, T.A.; Qihui, S.; Stoimenov, P.K.; Stucky, G.D. Metal oxide surface charge mediated hemostasis. Langmuir ACS J. Surf. Colloids 2007, 23, 11233–11238. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, J.; Park, C.M. Magnesium therapy improves thromboelastographic findings before liver transplantation: A preliminary study. Can. J. Anaesth. 2005, 52, 156–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anne, M.-P.; Aurélie, L.; Alba, M.; Liliane, L.; Didier, L.; Ludwik, L. Organ repair, hemostasis, and in vivo bonding of medical devices by aqueous solutions of nanoparticles. Angew. Chem. 2014, 53, 6369–6373. [Google Scholar]
- Murray, H.H. Traditional and new applications for kaolin, smectite, and palygorskite: A general overview. Appl. Clay Sci. 2000, 17, 207–221. [Google Scholar] [CrossRef]
- Li, J.; Cao, W.; Lv, X.-X.; Jiang, L.; Li, Y.-J.; Li, W.-Z.; Chen, S.-Z.; Li, X.-Y. Zeolite-based hemostat QuikClot releases calcium into blood and promotes blood coagulation in vitro. Acta Pharmacol. Sin. 2013, 34, 367–372. [Google Scholar] [CrossRef]
- Selvam, T.; Schwieger, W.; Dathe, W. Histamine-binding capacities of different natural zeolites: A comparative study. Environ. Geochem. Health 2018, 40, 2657–2665. [Google Scholar] [CrossRef]
- Vogler, E.A.; Siedlecki, C.A. Contact activation of blood-plasma coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Sperling, C.; Fischer, M.; Maitz, M.F.; Werner, C. Blood coagulation on biomaterials requires the combination of distinct activation processes. Biomaterials 2009, 30, 4447–4456. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Tang, Z.; Pan, M.; Wang, Z.; Yang, H.; Liu, H. Chitosan/kaolin composite porous microspheres with high hemostatic efficacy. Carbohydr. Polym. 2017, 177, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Udangawa, R.N.; Mikael, P.E.; Mancinelli, C.; Chapman, C.; Willard, C.F.; Simmons, T.J.; Linhardt, R.J. Novel Cellulose-Halloysite Hemostatic Nanocomposite Fibers with a Dramatic Reduction in Human Plasma Coagulation Time. ACS Appl. Mater. Interfaces 2019, 11, 15447–15456. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B.; Blazejak, S. Application of Sodium Selenite in the Prevention and Treatment of Cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Kieliszek, M.; Lipinski, B. Pathophysiological significance of protein hydrophobic interactions: An emerging hypothesis. Med. Hypotheses 2018, 110, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, B.; Pretorius, E. Novel pathway of iron-induced blood coagulation: Implications for diabetes mellitus and its complications. Pol. Arch. Med. Wewnętrznej 2012, 122, 115–122. [Google Scholar] [CrossRef]
- Wang, X.; Hu, L.; Li, C.; Gan, L.; He, M.; He, X.; Tian, W.; Li, M.; Xu, L.; Li, Y. Improvement in physical and biological properties of chitosan/soy protein films by surface grafted heparin. Int. J. Biol. Macromol. 2016, 83, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Senoussi, H.; Osmani, H.; Courtois, C.; Bourahli, M.e.H. Mineralogical and chemical characterization of DD3 kaolin from the east of Algeria. Boletín De La Soc. Española De Cerámica Y Vidr. 2016, 55, 121–126. [Google Scholar] [CrossRef]
- Zhu, X.J.; Jiang-Tao, H.E.; Si-Hui, S.U. Forming Mechanism of Humic Acid-Kaolin Complexes and the Adsorption of Trichloroethylene. Environ. Sci. 2015, 36, 227–236. [Google Scholar]
- Kheirabadi, B.S.; Scherer, M.R.; Estep, J.S.; Dubick, M.A.; Holcomb, J.B. Determination of efficacy of new hemostatic dressings in a model of extremity arterial hemorrhage in swine. J. Trauma Acute Care Surg. 2009, 67, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Feng, R.; Jiao, Y.; Zhou, C. Effect of halloysite nanotubes on the structure and function of important multiple blood components. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 75, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, A.; Dowling, M.B.; Gustin, J.P.; Scalea, T.M.; Raghavan, S.R.; Pasley, J.D.; Narayan, M. Hydrophobically modified chitosan gauze: A novel topical hemostat. J. Surg. Res. 2017, 207, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Gan, H.; Meng, Z.; Gu, R.; Wu, Z.; Zhang, L.; Zhu, X.; Sun, W.; Li, J.; Zheng, Y. Effects of genipin cross-linking of chitosan hydrogels on cellular adhesion and viability. Colloids Surf. B Biointerfaces 2014, 117, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Xu, C.; Li, G.; Liu, T.; Liang, J.F.; Wang, X. Graphene-kaolin composite sponge for rapid and riskless hemostasis. Colloids Surf. B Biointerfaces 2018, 169, 168–175. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Oxides | Wenchang Kaolin | Maoming Kaolin |
|---|---|---|
| SiO2 | 46.950 | 46.960 |
| Al2O3 | 35.960 | 36.410 |
| K2O | 1.570 | 0.664 |
| Fe2O3 | 1.070 | 0.784 |
| TiO2 | 0.327 | 0.407 |
| Na2O | 0.236 | 0.134 |
| Cl | 0.219 | 0.016 |
| CaO | 0.089 | 0.053 |
| CeO2 | 0.045 | 0.014 |
| P2O5 | 0.044 | 0.318 |
| La2O3 | 0.038 | 0.022 |
| SO3 | 0.036 | 0.176 |
| MgO | 0.033 | 0.034 |
| Nd2O3 | 0.021 | 0.004 |
| ZrO2 | 0.015 | 0.006 |
| ThO2 | 0.014 | 0.001 |
| Rb2O | 0.013 | 0.004 |
| Ga2O3 | 0.009 | 0.008 |
| Y2O3 | 0.008 | 0.002 |
| Pr6O11 | 0.004 | 0.003 |
| Nb2O5 | 0.004 | 0.001 |
| ZnO | 0.004 | 0.003 |
| MnO | 0.004 | 0.003 |
| PbO | 0.003 | 0.003 |
| IrO2 | 0.002 | 0.002 |
| GeO2 | 0.002 | 0.001 |
| V2O5 | 0.001 | 0.007 |
| NiO | 0.001 | 0.002 |
| Sc2O3 | 0.001 | 0.004 |
| MoO3 | - | 0.011 |
| Cr2O3 | - | 0.003 |
| a LOI | 13.28 | 13.95 |
| Materials | BET Surface Area (m2/g) | T-Plot External Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) | Blood Clotting Time (min) |
|---|---|---|---|---|---|
| Wenchang kaolin | 15.18 | 18.65 | 0.07 | 19.04 | 2.88 ± 0.25 |
| Maoming kaolin | 17.28 | 18.00 | 0.11 | 24.87 | 3.88 ± 0.66 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, C.; Hu, H.; Meng, Z.; Zhu, X.; Gu, R.; Wu, Z.; Wang, H.; Wang, D.; Gan, H.; Wang, J.; et al. Characterization and Hemostatic Potential of Two Kaolins from Southern China. Molecules 2019, 24, 3160. https://doi.org/10.3390/molecules24173160
Gan C, Hu H, Meng Z, Zhu X, Gu R, Wu Z, Wang H, Wang D, Gan H, Wang J, et al. Characterization and Hemostatic Potential of Two Kaolins from Southern China. Molecules. 2019; 24(17):3160. https://doi.org/10.3390/molecules24173160
Chicago/Turabian StyleGan, Changjiao, Hongjie Hu, Zhiyun Meng, Xiaoxia Zhu, Ruolan Gu, Zhuona Wu, Hongliang Wang, Donggen Wang, Hui Gan, Jinglin Wang, and et al. 2019. "Characterization and Hemostatic Potential of Two Kaolins from Southern China" Molecules 24, no. 17: 3160. https://doi.org/10.3390/molecules24173160
APA StyleGan, C., Hu, H., Meng, Z., Zhu, X., Gu, R., Wu, Z., Wang, H., Wang, D., Gan, H., Wang, J., & Dou, G. (2019). Characterization and Hemostatic Potential of Two Kaolins from Southern China. Molecules, 24(17), 3160. https://doi.org/10.3390/molecules24173160





