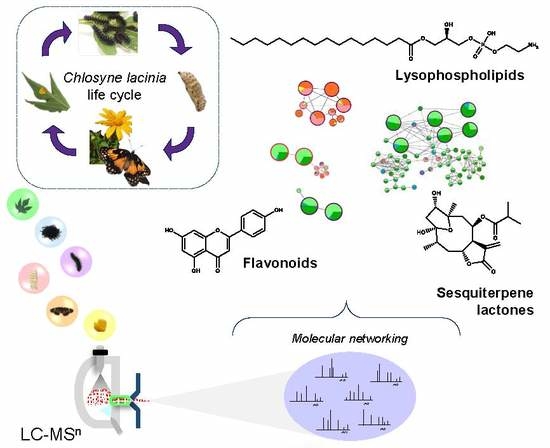
Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae)
Abstract
1. Introduction
2. Results and Discussion
2.1. C. lacinia Development
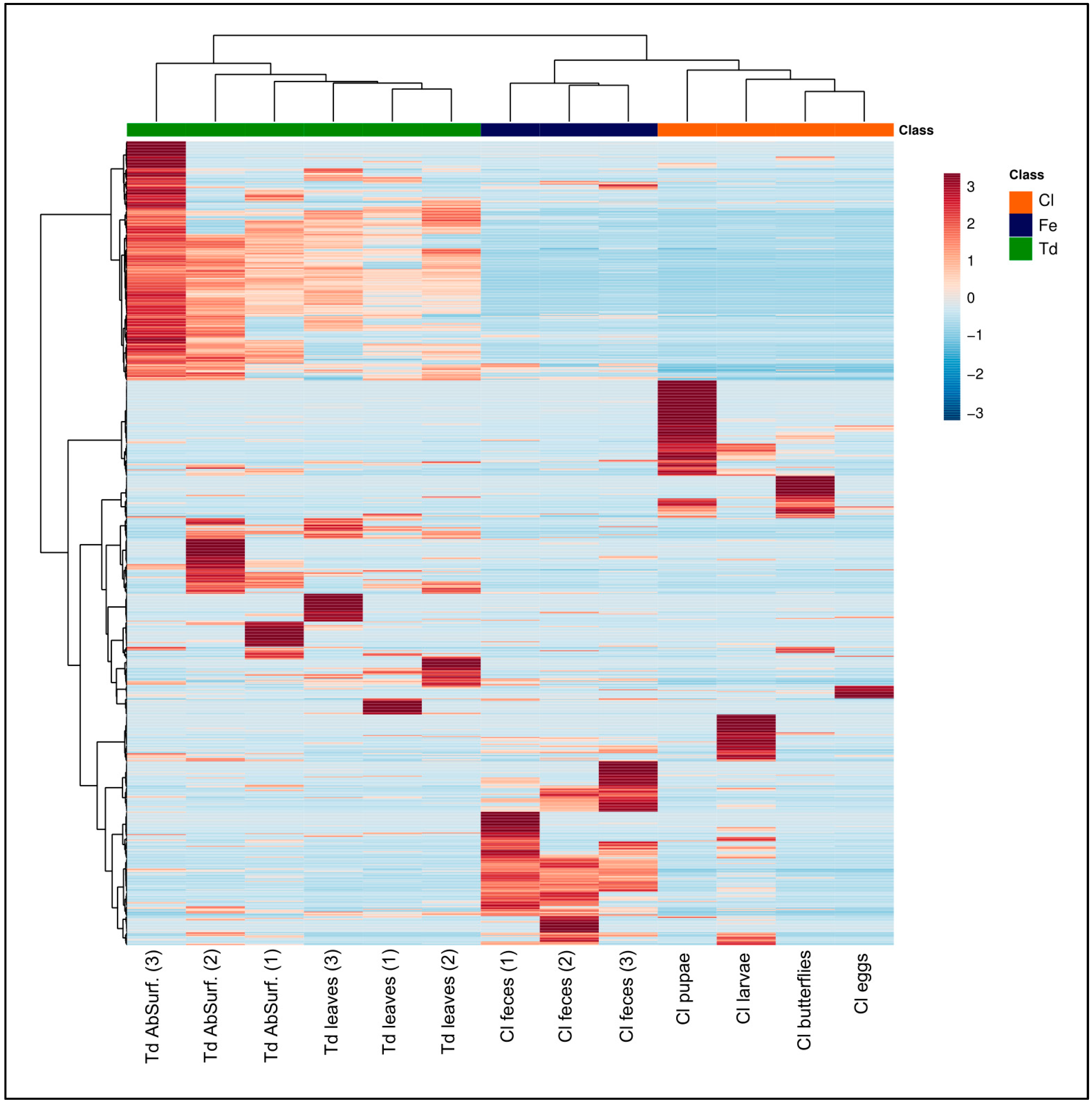
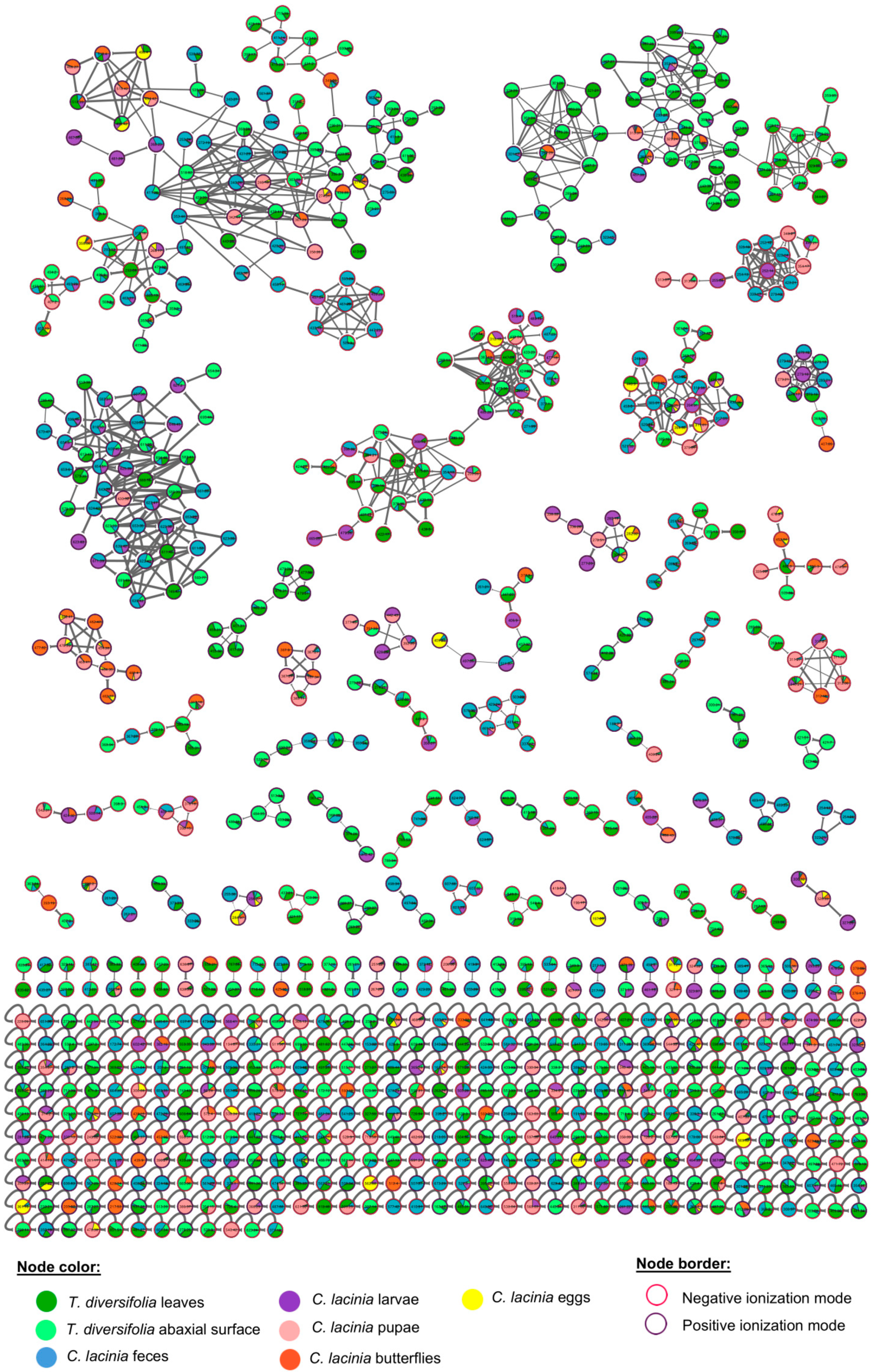
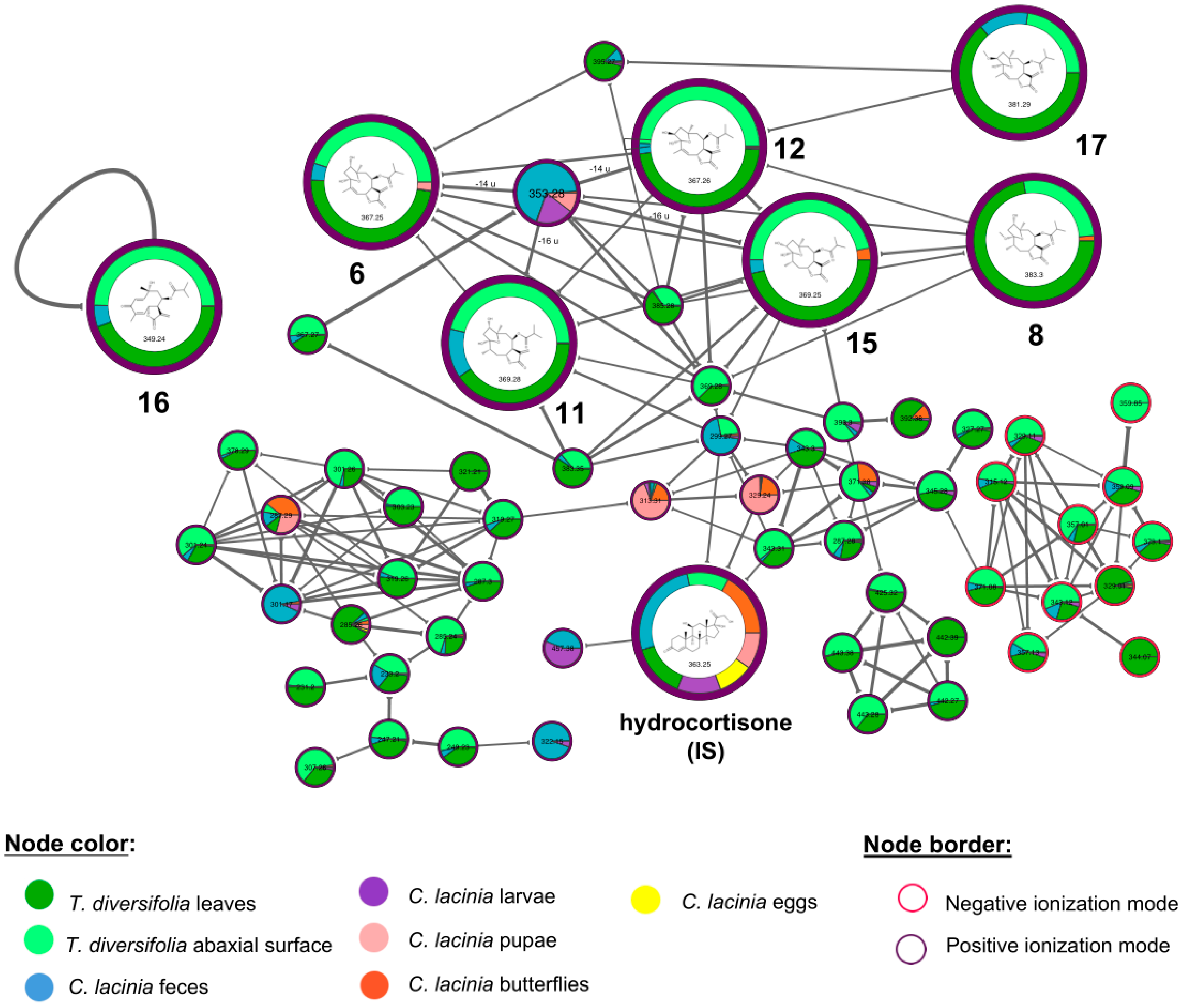
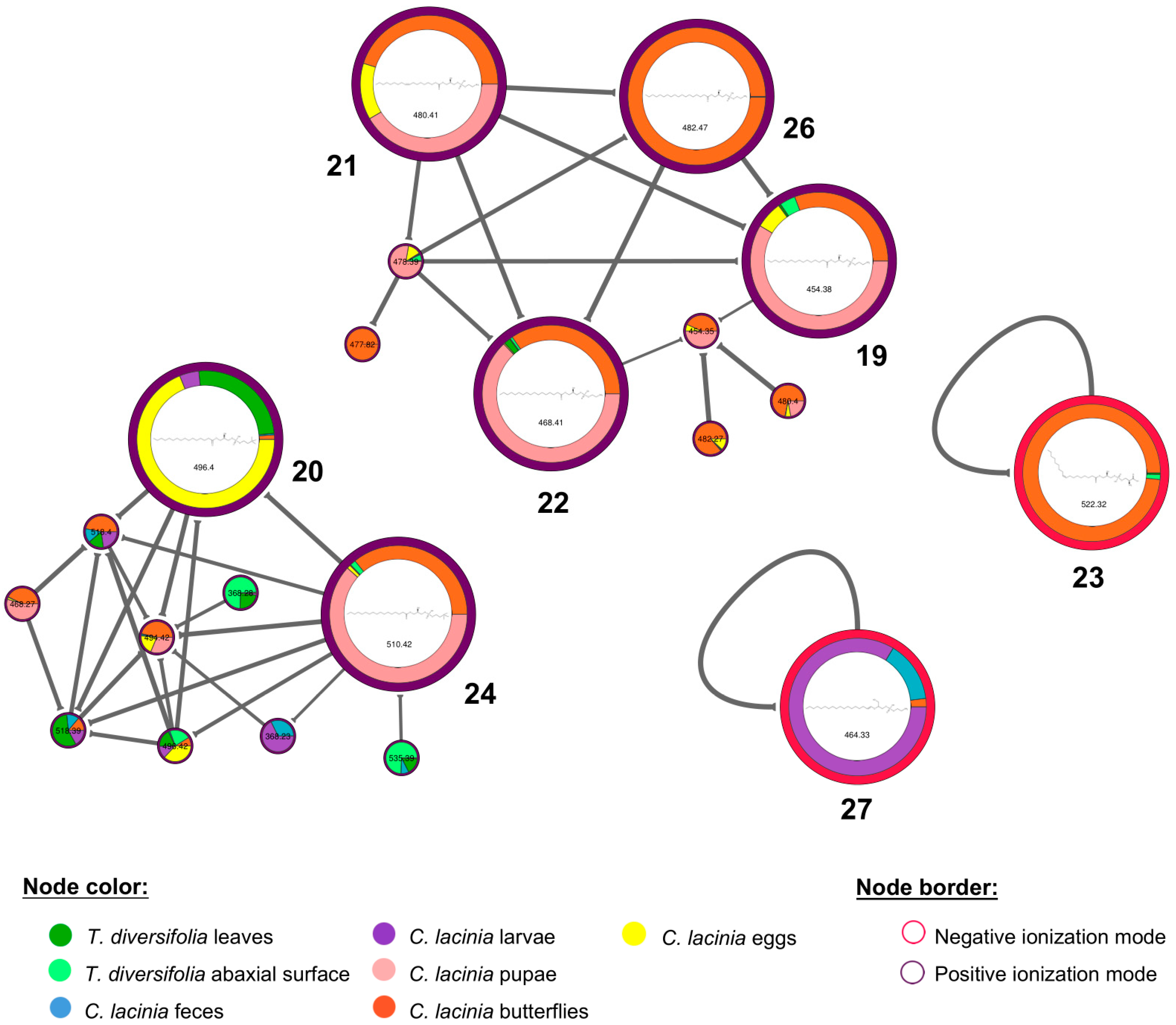
2.2. Natural Products Diversity
3. Material and Methods
3.1. Experimental Design
3.2. Sample Preparation
3.3. LC-MSn Analysis
3.4. Data Processing and Analysis
3.5. Chemical Analysis and Dereplication
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burkepile, D.E.; Parker, J.D. Recent advances in plant-herbivore interactions. F1000Research 2017, 6, 119. [Google Scholar] [CrossRef] [PubMed]
- Sedio, B.E. Recent breakthroughs in metabolomics promise to reveal the cryptic chemical traits that mediate plant community composition, character evolution and lineage diversification. New Phytol. 2017, 214, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Richards, L.A.; Dyer, L.A.; Forister, M.L.; Smilanich, A.M.; Dodson, C.D.; Leonard, M.D.; Jeffrey, C.S. Phytochemical diversity drives plant–insect community diversity. Proc. Natl. Acad. Sci. USA 2015, 112, 10973–10978. [Google Scholar] [CrossRef] [PubMed]
- Maag, D.; Erb, M.; Glauser, G. Metabolomics in plant-herbivore interactions: Challenges and applications. Entomol. Exp. Appl. 2015, 157, 18–29. [Google Scholar] [CrossRef]
- Sardans, J.; Peñuelas, J.; Rivas-Ubach, A. Ecological metabolomics: Overview of current developments and future challenges. Chemoecology 2011, 21, 191–225. [Google Scholar] [CrossRef]
- Kuhlisch, C.; Pohnert, G. Metabolomics in chemical ecology. Nat. Prod. Rep. 2015, 32, 937–955. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.E.; Neto, F.C.; Vera, M.C.; Taboada, C.; Pavarini, D.P.; Bauermeister, A.; Lopes, N.P. An integrative omics perspective for the analysis of chemical signals in ecological interactions. Chem. Soc. Rev. 2018, 47, 1574–1591. [Google Scholar] [CrossRef]
- Zhou, S.; Lou, Y.-R.; Tzin, V.; Jander, G. Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol. 2015, 169, 1488–1498. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Bauermeister, A.; Sakamoto, H.; Gouvea, D.; Lopes, J.; Lopes, N. Spatial and temporal variations in secondary metabolites content of the Brazilian arnica leaves (Lychnophora ericoides Mart., Asteraceae). J. Braz. Chem. Soc. 2017, 28, 2382–2390. [Google Scholar] [CrossRef]
- Gobbo-Neto, L.; Guaratini, T.; Pessoa, C.; De Moraes, M.O.; Costa-Lotufo, L.V.; Vieira, R.F.; Colepicolo, P.; Lopes, N.P. Differential metabolic and biological profiles of Lychnophora ericoides mart. (Asteraceae) from different localities in the Brazilian “campos rupestres”. J. Braz. Chem. Soc. 2010, 21, 750–759. [Google Scholar] [CrossRef]
- Barah, P.; Bones, A.M. Multidimensional approaches for studying plant defence against insects: From ecology to omics and synthetic biology. J. Exp. Bot. 2015, 66, 479–493. [Google Scholar] [CrossRef]
- Dyer, L.A.; Philbin, C.S.; Ochsenrider, K.M.; Richards, L.A.; Massad, T.J.; Smilanich, A.M.; Forister, M.L.; Parchman, T.L.; Galland, L.M.; Hurtado, P.J.; et al. Modern approaches to study plant–insect interactions in chemical ecology. Nat. Rev. Chem. 2018, 2, 50–64. [Google Scholar] [CrossRef]
- Baruah, N.C.; Sharma, R.P.; Madhusudanan, K.P.; Thyagarajan, G.; Herz, W.; Murari, R. Sesquiterpene lactones of Tithonia diversifolia. Stereochemistry of the tagitinins and related compounds. J. Org. Chem. 1979, 44, 1831–1835. [Google Scholar] [CrossRef]
- Zhao, G.-J.; Xi, Z.-X.; Chen, W.-S.; Li, X.; Sun, L.; Sun, L.-N. Chemical constituents from Tithonia diversifolia and their chemotaxonomic significance. Biochem. Syst. Ecol. 2012, 44, 250–254. [Google Scholar] [CrossRef]
- Sampaio, B.L.; Edrada-Ebel, R.; Da Costa, F.B. Effect of the environment on the secondary metabolic profile of Tithonia diversifolia: A model for environmental metabolomics of plants. Sci. Rep. 2016, 6, 29265. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Paula, D.A.; Oliveira, R.B.; Rocha, B.A.; Da Costa, F.B.; Chagas-Paula, D.A. Ethnobotany, chemistry, and biological activities of the genus Tithonia (Asteraceae). Chem. Biodivers. 2012, 9, 210–235. [Google Scholar] [CrossRef] [PubMed]
- Ajao, A.A.; Moteetee, A.N. Tithonia diversifolia (Hemsl) A. Gray. (Asteraceae: Heliantheae), an invasive plant of significant ethnopharmacological importance: A review. S. Afr. J. Bot. 2017, 113, 396–403. [Google Scholar] [CrossRef]
- Drummond, B.A., III; Bush, G.L.; Emmel, T.C. The biology and laboratory culture of Chlosyne lacinia Geyer (Nymphalidae). J. Lepid. Soc. 1970, 24, 135–142. [Google Scholar]
- Justus, C.M.; Pasini, A.; De Oliveira, É.D. Biologia e preferência da lagarta do girassol, Chlosyne lacinia saundersii (Lepidoptera: Nymphalidae) na planta daninha losna branca, Parthenium hysterophorus (Asteraceae). Neotrop. Entomol. 2003, 32, 163–166. [Google Scholar] [CrossRef]
- Neck, R.W. Foodplant ecology of the butterfly Chlosyne lacinia (Geyer) (Nymphalidae). I. Larval foodplants. J. Lepid. Soc. 1973, 27, 22–33. [Google Scholar]
- Lopes-Da-Silva, M.; Casagrande, M.M. Color polymorphism and allele frequency in a Brazilian population of the sunflower caterpillar Chlosyne lacinia saundersi (Doubleday) (Lepidoptera: Nymphalidae). Neotrop. Entomol. 2003, 32, 159–161. [Google Scholar] [CrossRef]
- Neck, R.W. Larval morph variation in Chlosyne lacinia (Nymphalidae). J. Lepid. Soc. 1976, 30, 91–94. [Google Scholar]
- Ambrósio, S.R.; Oki, Y.; Heleno, V.C.G.; Chaves, J.S.; Nascimento, P.G.B.D.; Lichston, J.E.; Constantino, M.G.; Varanda, E.M.; Da Costa, F.B. Constituents of glandular trichomes of Tithonia diversifolia: Relationships to herbivory and antifeedant activity. Phytochemistry 2008, 69, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Aksenov, A.A.; Da Silva, R.; Knight, R.; Lopes, N.P.; Dorrestein, P.C. Global chemical analysis of biology by mass spectrometry. Nat. Rev. Chem. 2017, 1, 54. [Google Scholar] [CrossRef]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beger, R.; Beale, M.H.; Daykin, C.A.; Fan, T.W.M.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis: Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Fiehn, O. Strategies for dereplication of natural compounds using high-resolution tandem mass spectrometry. Phytochem. Lett. 2017, 21, 313–319. [Google Scholar] [CrossRef]
- Crotti, A.E.M.; Lopes, J.L.C.; Lopes, N.P. Triple quadrupole tandem mass spectrometry of sesquiterpene lactones: A study of goyazensolide and its congeners. J. Mass Spectrom. 2005, 40, 1030–1034. [Google Scholar] [CrossRef] [PubMed]
- Herz, W. Biogenetic aspects of sesquiterpene lactone chemistry. Isr. J. Chem. 1977, 16, 32–44. [Google Scholar] [CrossRef]
- Dewick, P.M. The mevalonate and methylerythritol phosphate pathways: Terpenoids and steroids. In Medicinal Natural Products; Wiley: Hoboken, NJ, USA, 2009; pp. 187–310. [Google Scholar]
- Seaman, F.C. Sesquiterpene lactones as taxonomic characters in the Asteraceae. Bot. Rev. 1982, 48, 121–594. [Google Scholar] [CrossRef]
- Emerenciano, V.D.P.; Ferreira, Z.S.; Kaplan, M.A.C.; Gottlieb, O.R. A chemosystematic analysis of tribes of Asteraceae involving sesquiterpene lactones and flavonoids. Phytochemistry 1987, 26, 3103–3115. [Google Scholar] [CrossRef]
- Zdero, C.; Bohlmann, F. Systematics and evolution within the Compositae, seen with the eyes of a chemist. Plant Syst. Evol. 1990, 171, 1–14. [Google Scholar] [CrossRef]
- Da Costa, F.; Terfloth, L.; Gasteiger, J. Sesquiterpene lactone-based classification of three Asteraceae tribes: A study based on self-organizing neural networks applied to chemosystematics. Phytochemistry 2005, 66, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Gallon, M.E.; Monge, M.; Casoti, R.; Da Costa, F.B.; Semir, J.; Gobbo-Neto, L. Metabolomic analysis applied to chemosystematics and evolution of megadiverse Brazilian Vernonieae (Asteraceae). Phytochemistry 2018, 150, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Gonzalez, G.F.; Dos Santos, F.A.; Da Costa, F.B. Sesquiterpene lactones: More than protective plant compounds with High Toxicity. Crit. Rev. Plant Sci. 2016, 35, 1–20. [Google Scholar] [CrossRef]
- Martucci, M.E.P.; Gobbo-Neto, L. Differential secondary metabolite accumulation and performance of Chlosyne lacinia fed with Tithonia diversifolia or Vernonia polyanthes. Biochem. Syst. Ecol. 2016, 68, 156–162. [Google Scholar] [CrossRef]
- Ahern, J.R.; Whitney, K.D. Sesquiterpene lactone stereochemistry influences herbivore resistance and plant fitness in the field. Ann. Bot. 2014, 113, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.H.R.; Gil Da Costa, R.M.; Lopes, C.; Bastos, M.M.S.M. Sesquiterpene lactones: Adverse health effects and toxicity mechanisms. Crit. Rev. Toxicol. 2013, 43, 559–579. [Google Scholar] [CrossRef]
- Isman, M.B. Toxicity and tolerance of sesquiterpene lactones in the migratory grasshopper, Melanoplus sanguinipes (Acrididae). Pestic. Biochem. Physiol. 1985, 24, 348–354. [Google Scholar] [CrossRef]
- Dewick, P.M. The shikimate pathway: Aromatic amino acids and phenylpropanoids. In Medicinal Natural Products; Wiley: Hoboken, NJ, USA, 2009; pp. 137–186. [Google Scholar]
- Bone, K.; Mills, S. Principles of herbal pharmacology. In Principles and Practice of Phytotherapy: Modern Herbal Medicine; Churchill Livingstone: New York, NY, USA, 2013; p. 1056. ISBN 9780443069925. [Google Scholar]
- Silva, D.B.; Turatti, I.C.C.; Gouveia, D.R.; Ernst, M.; Teixeira, S.P.; Lopes, N.P. Mass spectrometry of flavonoid vicenin-2, based sunlight barriers in Lychnophora species. Sci. Rep. 2014, 4, 4309. [Google Scholar] [CrossRef]
- Simmonds, M.S. Importance of flavonoids in insect-plant interactions: Feeding and oviposition. Phytochemistry 2001, 56, 245–252. [Google Scholar] [CrossRef]
- Patel, K.; Patel, D.K. Medicinal importance, pharmacological activities, and analytical aspects of hispidulin: A concise report. J. Tradit. Complement. Med. 2017, 7, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.S. Flavonoid–insect interactions: Recent advances in our knowledge. Phytochemistry 2003, 64, 21–30. [Google Scholar] [CrossRef]
- Preiß, S.; Degenhardt, J.; Gershenzon, J. Plant-animal dialogues. In Ecological Biochemistry: Enviromental and Interspecies Interactions; Wiley-VCH: Weinheim, Germany, 2014; pp. 312–330. ISBN 9783527322909. [Google Scholar]
- Downer, R.G.H.; Matthews, J.R. Patterns of lipid distribution and utilization in insects. Am. Zool. 1976, 16, 733–745. [Google Scholar] [CrossRef]
- Fast, P.G. A comparative study of the phospholipids and fatty acids of some insects. Lipids 1966, 1, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Visser, B.; Willett, D.S.; Harvey, J.A.; Alborn, H.T. Concurrence in the ability for lipid synthesis between life stages in insects. R. Soc. Open Sci. 2017, 4, 160815. [Google Scholar] [CrossRef] [PubMed]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Well, M.A. Fat metabolism in insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.I. Lipid Metabolism and Function in Insects. Adv. Insect Physiol. 1967, 4, 69–211. [Google Scholar]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Riessberger-Gallé, U.; Hernández-López, J.; Rechberger, G.; Crailsheim, K.; Schuehly, W. Lysophosphatidylcholine acts in the constitutive immune defence against American foulbrood in adult honeybees. Sci. Rep. 2016, 6, 30699. [Google Scholar] [CrossRef]
- Pintea, A.M. Other natural pigments. In Food Colorants: Chemical and Functional Properties; Socaciu, C., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2008; pp. 101–124. [Google Scholar]
Sample Availability: Samples are not available from the authors. |

| Stage | 1st Instar | 2nd Instar | 3rd Instar | 4th Instar | 5th Instar | Pupation | Adult Stage | Total Development |
|---|---|---|---|---|---|---|---|---|
| Mean 1 | 5.25 | 3.70 | 3.25 | 3.45 | 3.55 | 7.65 | 11.35 | 38.20 |
| SD 2 | 1.12 | 0.65 | 0.44 | 0.83 | 0.69 | 0.67 | 1.04 | 0.85 |
| Rt | Usual Name (InChI) | Compound Class | Samples |
|---|---|---|---|
| 1.2 | pantothenic acid (1) (1/C9H17NO5/c1-9(2,5-11)7(14)8(15)10-4-3-6(12)13/h7,11,14H,3-5H2,1-2H3,(H,10,15)(H,12,13)/t7-/s2) | vitamin | T. diversifolia leaves and abaxial surface; C. lacinia feces, larvae, pupae, butterflies and eggs |
| 1.4 | tryptophan (2) (1/C11H12N2O2/c12-9(11(14)15)5-7-6-13-10-4-2-1-3-8(7)10/h1-4,6,9,13H,5,12H2,(H,14,15)/t9-/s2) | amino acid | T. diversifolia leaves and abaxial surface; C. lacinia larvae, pupae and butterflies |
| 3.2 | riboflavin (3) (1/C17H22N4O6/c1-7-3-9-10(4-8(7)2)21(5-11(23)14(25)12(24)6-22)15-13(18-9)16(26)20-17(27)19-15/h3-4,11-12,14-15,22-25H,5-6H2,1-2H3,(H2,19,20,26,27)/t11-,12+,14-,15?/s2) | vitamin | T. diversifolia leaves and abaxial surface; C. lacinia butterflies |
| 4.8 | phenylalanine-acetyl (4) (1/C11H13NO3/c1-8(13)12-10(11(14)15)7-9-5-3-2-4-6-9/h2-6,10H,7H2,1H3,(H,12,13)(H,14,15)/t10-/s2) | amino acid | T. diversifolia leaves; C. lacinia larvae, pupae and butterflies |
| 7.2 | isorhamnetin 3-O-hexoside (5) (1/C22H22O12/c1-31-12-4-8(2-3-10(12)25)20-21(17(28)15-11(26)5-9(24)6-13(15)32-20)34-22-19(30)18(29)16(27)14(7-23)33-22/h2-6,14,16,18-19,22-27,29-30H,7H2,1H3/t14-,16+,18+,19-,22+/s2) | flavonoid | T. diversifolia leaves and abaxial surface; C. lacinia feces and larvae |
| 7.6 | orizabin (6) (1/C19H26O7/c1-9(2)16(21)25-13-7-18(5)14(20)8-19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h6,9,12-15,20,23H,4,7-8H2,1-3,5H3/b10-6-/t12-,13-,14+,15+,18-,19-/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces and pupae |
| 7.7 | hispidulin 4′-O-hexoside (7) (1/C22H22O11/c1-30-21-12(25)7-14-16(18(21)27)11(24)6-13(32-14)9-2-4-10(5-3-9)31-22-20(29)19(28)17(26)15(8-23)33-22/h2-7,15,17,19-20,22-23,25-29H,8H2,1H3/t15-,17-,19+,20-,22-/s2) | flavonoid | C. lacinia feces and larvae |
| 8.7 | 1-hydroxy-3-O-methyltirotundin (8) (1/C20H30O7/c1-10(2)17(22)26-14-8-19(5)15(21)9-20(24-6,27-19)11(3)7-13-16(14)12(4)18(23)25-13/h10-11,13-16,21H,4,7-9H2,1-3,5-6H3/t11-,13+,14+,15-,16-,19+,20+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia butterflies |
| 9.4 | luteolin (9) (1S/C15H10O6/c16-8-4-11(19)15-12(20)6-13(21-14(15)5-8)7-1-2-9(17)10(18)3-7/h1-6,16-19H) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces |
| 9.6 | nepetin (10) (1S/C16H12O7/c1-22-16-11(20)6-13-14(15(16)21)10(19)5-12(23-13)7-2-3-8(17)9(18)4-7/h2-6,17-18,20-21H,1H3) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces |
| 9.9 | tagitinin A (11) (1/C19H28O7/c1-9(2)16(21)25-13-7-18(5)14(20)8-19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h9-10,12-15,20,23H,4,6-8H2,1-3,5H3/t10-,12+,13+,14-,15-,18+,19+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 10.3 | tagitinin B (12) (1/C19H26O7/c1-9(2)16(21)25-13-7-18(5)8-14(20)19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h6,9,12-15,20,23H,4,7-8H2,1-3,5H3/b10-6-/t12-,13-,14+,15+,18-,19+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 10.9 | apigenin (13) (1S/C15H10O5/c16-9-3-1-8(2-4-9)13-7-12(19)15-11(18)5-10(17)6-14(15)20-13/h1-7,16-18H) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces |
| 11.2 | hispidulin (14) (1S/C16H12O6/c1-21-16-11(19)7-13-14(15(16)20)10(18)6-12(22-13)8-2-4-9(17)5-3-8/h2-7,17,19-20H,1H3) | flavonoid | T. diversifolia leaves and abaxial surface, C. lacinia feces and larvae |
| 11.4 | 2-hydroxytirotundin (15) (1/C19H28O7/c1-9(2)16(21)25-13-7-18(5)8-14(20)19(23,26-18)10(3)6-12-15(13)11(4)17(22)24-12/h9-10,12-15,20,23H,4,6-8H2,1-3,5H3/t10-,12+,13+,14+,15-,18+,19-/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces and butterflies |
| 11.7 | tagitinin C (16) (1/C19H24O6/c1-10(2)17(21)25-15-9-19(5,23)7-6-13(20)11(3)8-14-16(15)12(4)18(22)24-14/h6-8,10,14-16,23H,4,9H2,1-3,5H3/b7-6+,11-8-/t14-,15+,16-,19-/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 14.8 | 2-O-methyltagitinin B (17) (1/C20H28O7/c1-10(2)17(21)26-14-8-19(5)9-15(24-6)20(23,27-19)11(3)7-13-16(14)12(4)18(22)25-13/h7,10,13-16,23H,4,8-9H2,1-3,5-6H3/b11-7-/t13-,14-,15+,16+,19-,20+/s2) | STL | T. diversifolia leaves and abaxial surface; C. lacinia feces |
| 18.2 | 12,13-DiHOME (18) (1/C18H34O4/c1-2-3-10-13-16(19)17(20)14-11-8-6-4-5-7-9-12-15-18(21)22/h8,11,16-17,19-20H,2-7,9-10,12-15H2,1H3,(H,21,22)/b11-8-) | fatty acid derivative | T. diversifolia leaves and abaxial surface; C. lacinia feces, larvae, pupae, butterflies and eggs |
| 22.1 | 1-hexadecanoyl-glycero-3-phosphoethanolamine (19) (1/C21H44NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-21(24)27-18-20(23)19-29-30(25,26)28-17-16-22/h20,23H,2-19,22H2,1H3,(H,25,26)/t20-/s2) | lysoPL | T. diversifolia leaves and abaxial surface; C. lacinia pupae, butterflies and eggs |
| 22.5 | 1-palmitoyl-glycerol-3-phosphorylcholine (20) (1/C24H50NO7P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-24(27)30-21-23(26)22-32-33(28,29)31-20-19-25(2,3)4/h23,26H,5-22H2,1-4H3/p+1/t23-/s2) | lysoPL | T. diversifolia leaves; C. lacinia larvae, butterflies and eggs |
| 22.9 | 1-(9-octadecenoyl)-glycero-3-phosphoethanolamine (21) (1/C23H46NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-23(26)29-20-22(25)21-31-32(27,28)30-19-18-24/h9-10,22,25H,2-8,11-21,24H2,1H3,(H,27,28)/b10-9-/t22-/s2) | lysoPL | C. lacinia pupae, butterflies, eggs |
| 23.6 | 1-heptadecanoyl-glycero-3-phosphoethanolamine (22) (1/C22H46NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-22(25)28-19-21(24)20-30-31(26,27)29-18-17-23/h21,24H,2-20,23H2,1H3,(H,26,27)/t21-/s2) | lysoPL | T. diversifolia leaves; C. lacinia pupae and butterflies |
| 23.8 | 1-(9-octadecenoyl)-glycero-3-phosphoserine (23) (1/C24H46NO9P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-23(27)32-18-21(26)19-33-35(30,31)34-20-22(25)24(28)29/h9-10,21-22,26H,2-8,11-20,25H2,1H3,(H,28,29)(H,30,31)/b10-9-/t21-,22+/s2) | lysoPL | T. diversifolia leaves and abaxial surface; C. lacinia butterflies |
| 24.2 | 1-heptadecanoyl-glycero-3-phosphocholine (24) (1/C25H52NO7P/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18-19-25(28)31-22-24(27)23-33-34(29,30)32-21-20-26(2,3)4/h24,27H,5-23H2,1-4H3/p+1/t24-/s2) | lysoPL | T. diversifolia leaves and abaxial surface; C. lacinia pupae, butterflies and eggs |
| 24.4 | linolenoyl-tyrosine (25) (1/C27H39NO4/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-26(30)28-25(27(31)32)22-23-18-20-24(29)21-19-23/h3-4,6-7,9-10,18-21,25,29H,2,5,8,11-17,22H2,1H3,(H,28,30)(H,31,32)/b4-3-,7-6-,10-9-/t25-/s2) | fatty acid derivative | C. lacinia feces, larvae and pupae |
| 25.3 | 1-stearoyl-2-hydroxy-glycero-3-phosphoethanolamine (26) (1/C23H48NO7P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-23(26)29-20-22(25)21-31-32(27,28)30-19-18-24/h22,25H,2-21,24H2,1H3,(H,27,28)/t22-/s2) | lysoPL | C. lacinia butterflies |
| 26.3 | phosphatidylethanolamine lyso alkenyl 18:0 (27) (1/C23H48NO6P/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-19-28-23(21-25)22-30-31(26,27)29-20-18-24/h17,19,23,25H,2-16,18,20-22,24H2,1H3,(H,26,27)/b19-17+) | lysoPL | C. lacinia feces, larvae and butterflies |
| 29.3 | oleamide (28) (1S/C18H35NO/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h9-10H,2-8,11-17H2,1H3,(H2,19,20)/b10-9-) | fatty acid derivative | T. diversifolia leaves and abaxial surface; C. lacinia butterflies and eggs |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallon, M.E.; Silva-Junior, E.A.; Amaral, J.G.; Lopes, N.P.; Gobbo-Neto, L. Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae). Molecules 2019, 24, 3118. https://doi.org/10.3390/molecules24173118
Gallon ME, Silva-Junior EA, Amaral JG, Lopes NP, Gobbo-Neto L. Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae). Molecules. 2019; 24(17):3118. https://doi.org/10.3390/molecules24173118
Chicago/Turabian StyleGallon, Marília Elias, Eduardo Afonso Silva-Junior, Juliano Geraldo Amaral, Norberto Peporine Lopes, and Leonardo Gobbo-Neto. 2019. "Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae)" Molecules 24, no. 17: 3118. https://doi.org/10.3390/molecules24173118
APA StyleGallon, M. E., Silva-Junior, E. A., Amaral, J. G., Lopes, N. P., & Gobbo-Neto, L. (2019). Natural Products Diversity in Plant-Insect Interaction between Tithonia diversifolia (Asteraceae) and Chlosyne lacinia (Nymphalidae). Molecules, 24(17), 3118. https://doi.org/10.3390/molecules24173118