Melatonin Inhibits Apoptosis and Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism
Abstract
1. Introduction
2. Results
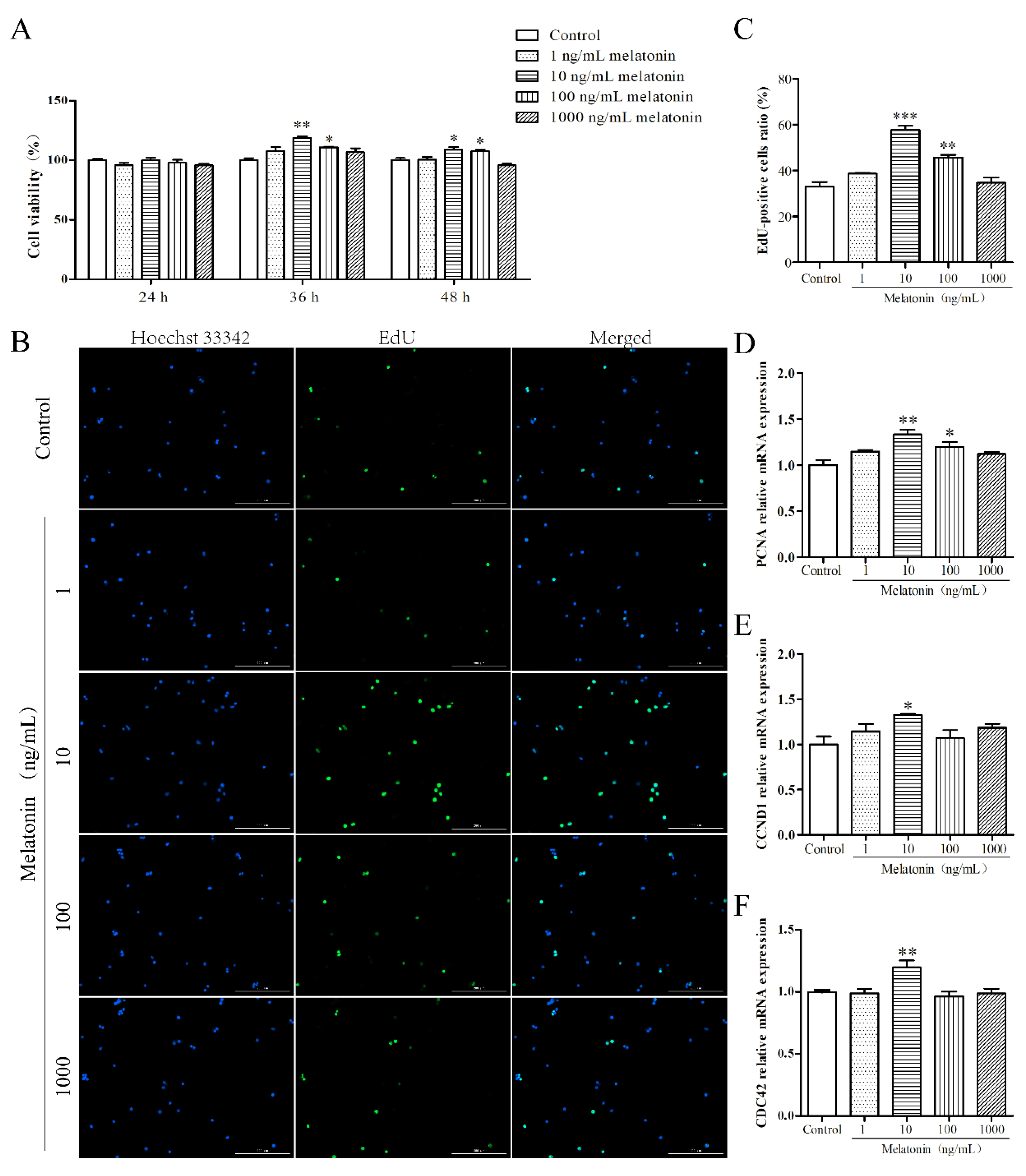
2.1. Melatonin Promoted Proliferation of Mouse Leydig Cells
2.2. Melatonin Inhibited Apoptosis of Mouse Leydig Cells
2.3. Melatonin Suppressed Oxidative Stress of Mouse Leydig Cells
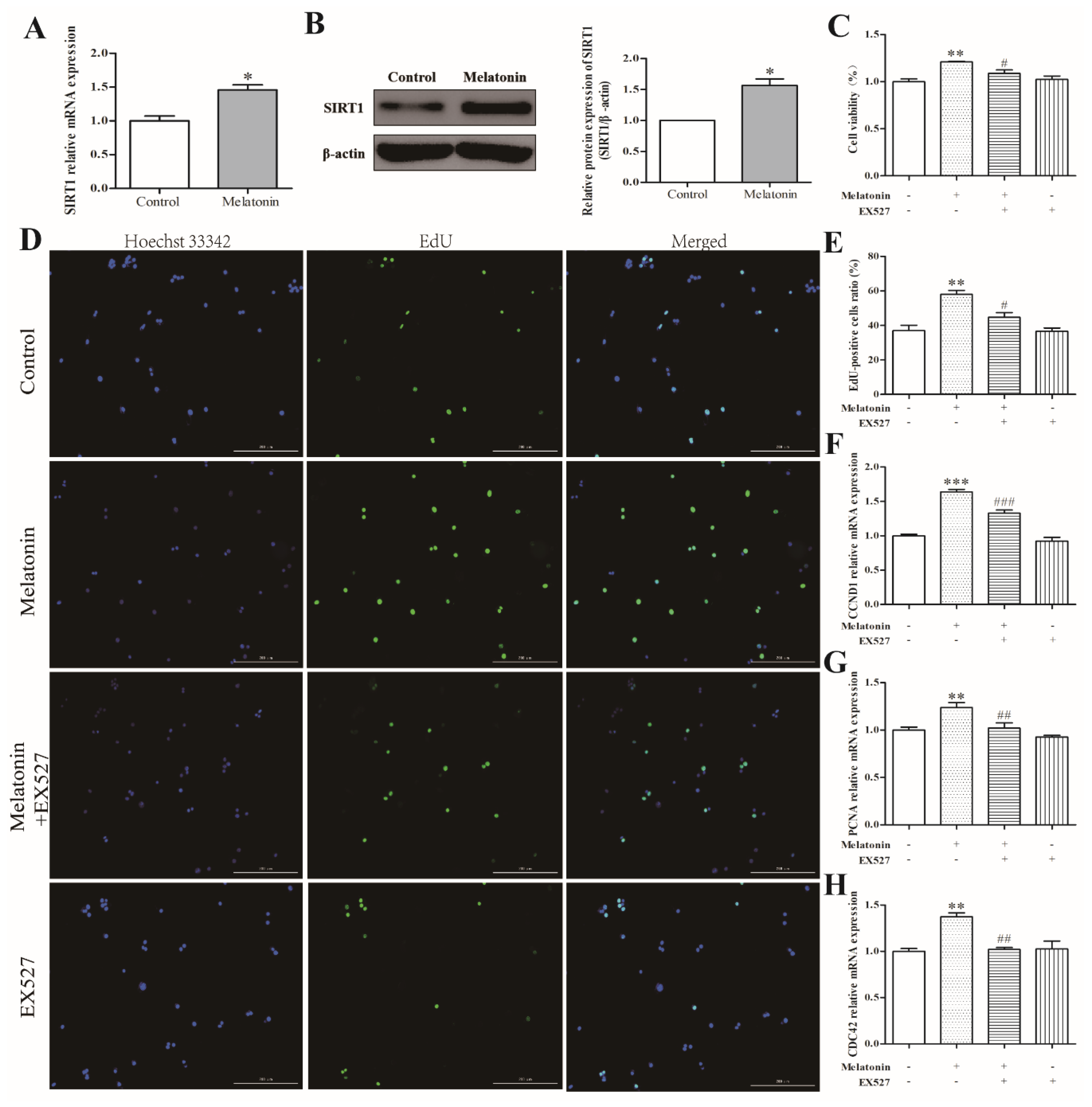
2.4. Melatonin Increased Cell Proliferation via a SIRT1-Dependent Mechanism in Mouse Leydig Cells
2.5. Melatonin Inhibited Apoptosis of Mouse Leydig Cells via a SIRT1-Dependent Mechanism
2.6. Melatonin Inhibited Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Cell Viability Assay
4.3. EdU Assay
4.4. Cell Apoptosis Analysis
4.5. ROS Analysis
4.6. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR) Analysis
4.7. Measurement of MDA, 8-OHdG, SOD and GSH-Px
4.8. Western Blot Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naher, Z.U.; Ali, M.; Biswas, S.K.; Mollah, F.H.; Fatima, P.; Hossain, M.M.; Arslan, M.I. Effect of oxidative stress in male infertility. Mymensingh Med. J. 2013, 22, 136–142. [Google Scholar] [PubMed]
- Hampl, R.; Drábková, P.; Kanďár, R.; Stěpán, J. Impact of oxidative stress on male infertility. Ceska Gynekol. 2012, 77, 241–245. [Google Scholar] [PubMed]
- Shukla, K.K.; Mahdi, A.A.; Rajender, S. Apoptosis, spermatogenesis and male infertility. Front. Biosci. (Elite Ed.) 2012, 4, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sharma, R.K.; Sikka, S.C.; Thomas, A.J.; Falcone, T.; Agarwal, A. Oxidative stress is associated with increased apoptosis leading to spermatozoa DNA damage in patients with male factor infertility. Fertil. Steril. 2003, 80, 531–535. [Google Scholar] [CrossRef]
- Othman, A.I.; Edrees, G.M.; El-Missiry, M.A.; Ali, D.A.; Aboel-Nour, M.; Dabdoub, B.R. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health 2016, 32, 1537–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ren, X.; Hu, X.; Zhou, L.; Zhang, C.; Zhang, M. Cadmium-induced apoptosis through reactive oxygen species-mediated mitochondrial oxidative stress and the JNK signaling pathway in TM3 cells, a model of mouse Leydig cells. Toxicol. Appl. Pharmacol. 2019, 368, 37–48. [Google Scholar] [CrossRef]
- Weissová, K.; Škrabalová, J.; Skálová, K.; Červená, K.; Bendová, Z.; Miletínová, E.; Kopřivová, J.; Šonka, K.; Dudysová, D.; Bartoš, A.; et al. Circadian rhythms of melatonin and peripheral clock gene expression in idiopathic REM sleep behavior disorder. Sleep Med. 2018, 52, 1–6. [Google Scholar] [CrossRef]
- Tamura, H.; Kawamoto, M.; Sato, S.; Tamura, I.; Maekawa, R.; Taketani, T.; Aasada, H.; Takaki, E.; Nakai, A.; Reiter, R.J.; et al. Long-term melatonin treatment delays ovarian aging. J. Pineal Res. 2017, 62, e12381. [Google Scholar] [CrossRef]
- Li, R.; Luo, X.; Li, L.; Peng, Q.; Yang, Y.; Zhao, L.; Ma, M.; Hou, Z. The Protective Effects of Melatonin Against Oxidative Stress and Inflammation Induced by Acute Cadmium Exposure in Mice Testis. Biol. Trace Elem. Res. 2016, 170, 152–164. [Google Scholar] [CrossRef]
- Carloni, S.; Favrais, G.; Saliba, E.; Albertini, M.C.; Chalon, S.; Longini, M.; Gressens, P.; Buonocore, G.; Balduini, W. Melatonin modulates neonatal brain inflammation through endoplasmic reticulum stress, autophagy, and miR-34a/silent information regulator 1 pathway. J. Pineal Res. 2016, 61, 370–380. [Google Scholar] [CrossRef]
- Chen, Z.; Lei, L.; Wen, D.; Yang, L. Melatonin attenuates palmitic acid-induced mouse granulosa cells apoptosis via endoplasmic reticulum stress. J. Ovarian Res. 2019, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, X.; Bai, X.; Lin, Y.; Li, Z.; Fu, J.; Li, M.; Zhao, T.; Yang, H.; Xu, R.; et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR-223/NLRP3 axis. J. Pineal Res. 2018, 64, e12449. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, L.; Chen, K.; Li, C.; Wang, Y.; Wang, G. Melatonin alleviates β-zearalenol and HT-2 toxin-induced apoptosis and oxidative stress in bovine ovarian granulosa cells. Environ. Toxicol. Pharmacol. 2019, 68, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hibaoui, Y.; Roulet, E.; Ruegg, U.T. Melatonin prevents oxidative stress-mediated mitochondrial permeability transition and death in skeletal muscle cells. J. Pineal Res. 2009, 47, 238–252. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Cui, M.; Lin, H.; Zhao, L.; Wang, J.; Chen, S.; Shao, Z. Melatonin resists oxidative stress-induced apoptosis in nucleus pulposus cells. Life Sci. 2018, 199, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Zhang, P.; Guo, J.; Zhu, Z.; Li, X.; Xu, D.; Zeng, W. Melatonin protects mouse spermatogonial stem cells against hexavalent chromium-induced apoptosis and epigenetic histone modification. Toxicol. Appl. Pharmacol. 2018, 340, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ozen, O.A.; Kus, M.A.; Kus, I.; Alkoc, O.A.; Songur, A. Protective effects of melatonin against formaldehyde-induced oxidative damage and apoptosis in rat testes: An immunohistochemical and biochemical study. Syst. Biol. Reprod. Med. 2008, 54, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, J.; Li, T.; Zhang, Q.; Bu, S.; Wang, Q.; Lai, D. Melatonin ameliorates restraint stress-induced oxidative stress and apoptosis in testicular cells via NF-κB/iNOS and Nrf2/HO-1 signaling pathway. Sci. Rep. 2017, 7, 9599. [Google Scholar] [CrossRef]
- Bahrami, N.; Goudarzi, M.; Hosseinzadeh, A.; Sabbagh, S.; Reiter, R.J.; Mehrzadi, S. Evaluating the protective effects of melatonin on di(2-ethylhexyl) phthalate-induced testicular injury in adult mice. Biomed. Pharmacother. 2018, 108, 515–523. [Google Scholar] [CrossRef]
- Mohammadghasemi, F.; Jahromi, S.K. Melatonin ameliorates testicular damages induced by nicotine in mice. Iran. J. Basic Med. Sci. 2018, 21, 639–644. [Google Scholar]
- Persengiev, S.; Kehajova, J. Inhibitory action of melatonin and structurally related compounds on testosterone production by mouse Leydig cells in vitro. Cell. Biochem. Funct. 1991, 9, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Zhang, J.; Zan, L.; Guo, W.; Wang, J.; Chen, L.; Cao, Y.; Shen, O.; Tong, J. Inhibitory effect of melatonin on testosterone synthesis is mediated via GATA-4/SF-1 transcription factors. Reprod. Biomed. Online 2015, 31, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.Y.; Xu, X.W.; Deng, Y.Z.; Ma, Z.X.; Li, X.R.; Zhao, L.; Qiu, L.J.; Liu, H.Y.; Chen, H.P. Resveratrol attenuates myocardial hypoxia/reoxygenation-induced cell apoptosis through DJ-1-mediated SIRT1-p53 pathway. Biochem. Biophys. Res. Commun. 2019, 514, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Pierucci, F.; Bruno, G.; Di Cesare Mannelli, L.; Ghelardini, C.; Brandi, M.L.; Iantomasi, T.; Meacci, E.; Vincenzini, M.T. Blueberry juice protects osteocytes and bone precursor cells against oxidative stress partly through SIRT1. FEBS Open Bio 2019, 9, 1082–1096. [Google Scholar] [PubMed]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; An, R.; Yang, Y.; Yang, X.; Liu, H.; Yue, L.; Li, X.; Lin, Y.; Reiter, R.J.; Qu, Y. Melatonin alleviates brain injury in mice subjected to cecal ligation and puncture via attenuating inflammation, apoptosis, and oxidative stress: The role of SIRT1 signaling. J. Pineal Res. 2015, 59, 230–239. [Google Scholar] [CrossRef]
- Yu, L.; Sun, Y.; Cheng, L.; Jin, Z.; Yang, Y.; Zhai, M.; Pei, H.; Wang, X.; Zhang, H.; Meng, Q.; et al. Melatonin receptor-mediated protection against myocardial ischemia/reperfusion injury: Role of SIRT1. J. Pineal Res. 2014, 57, 228–238. [Google Scholar] [CrossRef]
- Yu, L.; Liang, H.; Dong, X.; Zhao, G.; Jin, Z.; Zhai, M.; Yang, Y.; Chen, W.; Liu, J.; Yi, W.; et al. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia-reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J. Pineal Res. 2015, 59, 376–390. [Google Scholar] [CrossRef]
- Zhang, W.X.; He, B.M.; Wu, Y.; Qiao, J.F.; Peng, Z.Y. Melatonin protects against sepsis-induced cardiac dysfunction by regulating apoptosis and autophagy via activation of SIRT1 in mice. Life Sci. 2019, 217, 8–15. [Google Scholar] [CrossRef]
- Wang, S.J.; Liu, W.J.; Wu, C.J.; Ma, F.H.; Ahmad, S.; Liu, B.R.; Han, L.; Jiang, X.P.; Zhang, S.J.; Yang, L.G. Melatonin suppresses apoptosis and stimulates progesterone production by bovine granulosa cells via its receptors (MT1 and MT2). Theriogenology 2012, 78, 1517–1526. [Google Scholar] [CrossRef]
- Chen, Z.; Chua, C.C.; Gao, J.; Chua, K.W.; Ho, Y.S.; Hamdy, R.C.; Chua, B.H. Prevention of ischemia/reperfusion-induced cardiac apoptosis and injury by melatonin is independent of glutathione peroxdiase 1. J. Pineal Res. 2009, 46, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wang, Y.; Chen, J.; Zhang, J.; Yu, P.; Zhang, R.; Li, S.; Tao, B.; Qiu, Y.; Xu, M.; et al. Activation of melatonin receptor 2 but not melatonin receptor 1 mediates melatonin-conferred cardioprotection against myocardial ischemia/reperfusion injury. J. Pineal Res. 2019, 67, e12571. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Zhang, J.; Ji, Q.; Yu, K.; Wang, P.; Song, M.; Cao, Z.; Zhang, X.; Li, Y. Melatonin alleviates aluminium chloride-induced immunotoxicity by inhibiting oxidative stress and apoptosis associated with the activation of Nrf2 signaling pathway. Ecotoxicol. Environ. Saf. 2019, 173, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Y.; Li, W.; Ao, H.; Zhang, Y.; Zhou, R.; Li, K. Effects of melatonin on the synthesis of estradiol and gene expression in pig granulosa cells. J. Pineal Res. 2019, 66, e12546. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Jia, S.; Ma, Q.; Zhong, M.; Gao, P.; Yu, Z.; Zhang, Y. Suppressive effects of subchronic aluminum overload on the splenic immune function may be related to oxidative stress in mice. Biol. Trace Elem. Res. 2014, 157, 249–255. [Google Scholar] [CrossRef]
- Sun, J.; Wang, H.; Liu, B.; Shi, W.; Shi, J.; Zhang, Z.; Xing, J. Rutin attenuates H2O2-induced oxidation damage and apoptosis in Leydig cells by activating PI3K/Akt signal pathways. Biomed. Pharmacother. 2017, 88, 500–506. [Google Scholar] [CrossRef]
- Mukherjee, A.; Haldar, C.; Vishwas, D.K. Melatonin prevents dexamethasone-induced testicular oxidative stress and germ cell apoptosis in golden hamster, Mesocricetus auratus. Andrologia 2015, 47, 920–931. [Google Scholar] [CrossRef]
- Tanabe, M.; Tamura, H.; Taketani, T.; Okada, M.; Lee, L.; Tamura, I.; Maekawa, R.; Asada, H.; Yamagata, Y.; Sugino, N. Melatonin protects the integrity of granulosa cells by reducing oxidative stress in nuclei, mitochondria, and plasma membranes in mice. J. Reprod. Dev. 2015, 61, 35–41. [Google Scholar] [CrossRef]
- Sun, Y.; Zong, L.; Gao, Z.; Zhu, S.; Tong, J.; Cao, Y. Mitochondrial DNA damage and oxidative damage in HL-60 cells exposed to 900MHz radiofrequency fields. Mutat. Res. 2017, 797, 7–14. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, S.; Dong, Y.; Fan, C.; Zhao, L.; Yang, X.; Li, J.; Di, S.; Yue, L.; Liang, G.; et al. Melatonin prevents cell death and mitochondrial dysfunction via a SIRT1-dependent mechanism during ischemic-stroke in mice. J. Pineal Res. 2015, 58, 61–70. [Google Scholar] [CrossRef]
- He, B.; Zhang, W.; Qiao, J.; Peng, Z.; Chai, X. Melatonin protects against COPD by attenuating apoptosis and endoplasmic reticulum stress via upregulating SIRT1 expression in rats. Can. J. Physiol. Pharmacol. 2019, 97, 386–391. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |




| Genes | Primer Sequence (5′–3′) | Genebank No. | Size (bp) |
|---|---|---|---|
| BCL-2 | F: ACGGTGGTGGAGGAACTCTTCAG | XM_021173243.1 | 168 |
| R: GGTGTGCAGATGCCGGTTCAG | |||
| BAX | F: CGTGAGCGGCTGCTTGTCTG | XM_021195914.1 | 128 |
| R: ATGGTGAGCGAGGCGGTGAG | |||
| PCNA | F: TGAAGAAGGTGCTGGAGGCTCTC | NM_011045.2 | 115 |
| R: AGCTGTACCAAGGAGACGTGAGAC | |||
| CCND1 | F: TGGATGCTGGAGGTCTGTGAGG | XM_011241977.1 | 112 |
| R: GCAGGCGGCTCTTCTTCAAGG | |||
| CDC42 | F: GGCTGTCAAGTATGTGGAGTGCTC | XM_021159845.1 | 111 |
| R: CTGCGGCTCTTCTTCGGTTCTG | |||
| SIRT1 | F: CGTCTTGTCCTCTAGTTCCTGTG | NM_001159589.2 | 134 |
| R: GCCTCTCCGTATCATCTTCCAAG | |||
| β-actin | F: GTGCTATGTTGCTCTAGACTTCG | NM_007393.5 | 174 |
| R: ATGCCACAGGATTCCATACC |
| Antibodies | Cat NO. | Source | Dilution |
|---|---|---|---|
| BCL-2 | A11025 | ABclonal, Wuhan, China | 1:1000 |
| BAX | A12009 | ABclonal, Wuhan, China | 1:1000 |
| SIRT1 | AB110304 | Abcam, Cambridge, UK | 1:1000 |
| β-actin | 60008-1-lg | ProteinTech, Chicago, IL, USA | 1:5000 |
| Goat Anti-Rabbit IgG | SA00001-2 | ProteinTech, Chicago, IL, USA | 1:5000 |
| Goat Anti-mouse IgG | SA00001-1 | ProteinTech, Chicago, IL, USA | 1:5000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, G.; Zhao, J.; Liu, H.; Wang, J.; Lu, W. Melatonin Inhibits Apoptosis and Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism. Molecules 2019, 24, 3084. https://doi.org/10.3390/molecules24173084
Xu G, Zhao J, Liu H, Wang J, Lu W. Melatonin Inhibits Apoptosis and Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism. Molecules. 2019; 24(17):3084. https://doi.org/10.3390/molecules24173084
Chicago/Turabian StyleXu, Gaoqing, Jing Zhao, Hongyu Liu, Jun Wang, and Wenfa Lu. 2019. "Melatonin Inhibits Apoptosis and Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism" Molecules 24, no. 17: 3084. https://doi.org/10.3390/molecules24173084
APA StyleXu, G., Zhao, J., Liu, H., Wang, J., & Lu, W. (2019). Melatonin Inhibits Apoptosis and Oxidative Stress of Mouse Leydig Cells via a SIRT1-Dependent Mechanism. Molecules, 24(17), 3084. https://doi.org/10.3390/molecules24173084





