Sequence Identification of Bioactive Peptides from Amaranth Seed Proteins (Amaranthus hypochondriacus spp.)
Abstract
:1. Introduction
2. Results
2.1. Free Amine Groups Analysis during Enzymatic Hydrolysis
2.2. Hydrolysates Bioactivity Analysis
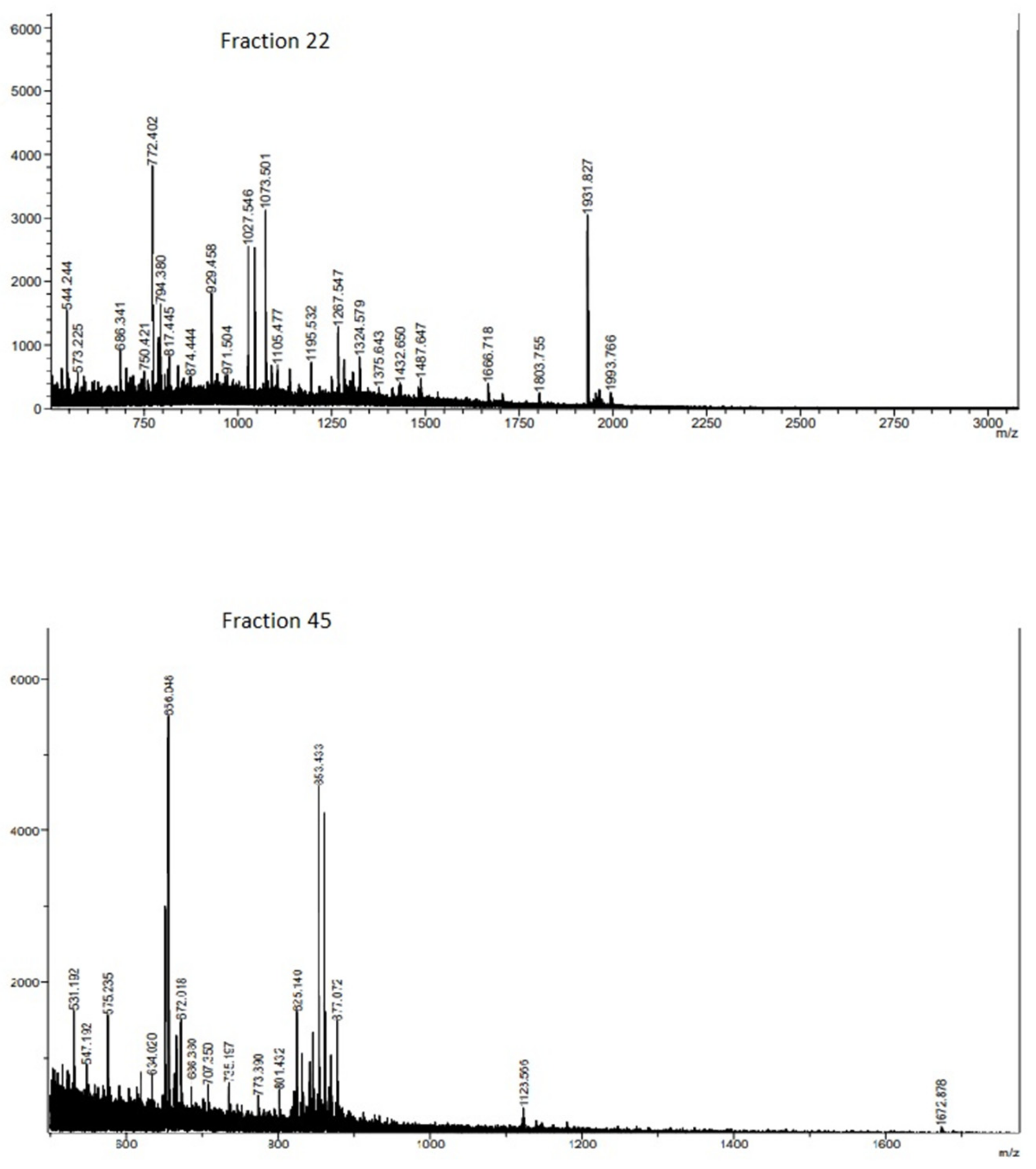
2.3. RP-HPLC Separation and Fraction Bioactivity
3. Discussion
4. Materials and Methods
4.1. Sample and Treatments
4.2. Enzymatic Hydrolysis
4.3. Free Amine Groups Analysis by TNBS Test
4.4. Antihypertensive Activity
4.5. Antithrombotic Activity
4.6. Antioxidant Activity
4.6.1. ABTS test
4.6.2. DPPH Test
4.6.3. FRAP Test
4.7. Identification of Bioactive Peptides by RP-HPLC
4.7.1. Sample Preparation
4.7.2. RP-HPLC Separation
4.7.3. MALDI-TOF Spectrometry
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A

References
- Jew, S.; AbuMweis, S.S.; Jones, P.J.H. Evolution of the human diet: Linking our ancestral diet to modern functional foods as a means of chronic disease prevention. J. Med. Food 2009, 12, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Mazorra-Manzano, M.A.; Ramírez-Suarez, J.C.; Yada, R.Y. Plant proteases for bioactive peptides release: A review. Crit Rev. Food Sci. Nutr. 2018, 58, 2147–2163. [Google Scholar] [CrossRef] [PubMed]
- Wattanasirithama, L.; Theerakulkaita, C.; Wickramasekara, S.; Maierb, C.S.; Stevensc, J.F. Isolation and identification of antioxidant peptides from enzymatically hydrolyzed rice bran protein. Food Chem. 2016, 192, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Najafian, L.; Babji, A.S. Production of bioactive peptides using enzymatic hydrolysis and identification antioxidative peptides from patin (Pangasius sutchi) sarcoplasmic protein hydrolysate. J. Funct. Foods 2014, 9, 280–289. [Google Scholar] [CrossRef]
- Baltia, R.; Bougatef, A.; Silaa, A.; Guillochon, D.; Dhulster, P.; Nedjar-Arroume, N. Nine novel angiotensin I-converting enzyme (ACE) inhibitory peptides from cuttlefish (Sepia officinalis) muscle protein hydrolysates and antihypertensive effect of the potent active peptide in spontaneously hypertensive rats. Food Chem. 2015, 170, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V.; Dassios, T. Health-promoting properties of bioactive peptides derived from milk proteins in infant food: A review. Dairy Sci. Technol. 2014, 94, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Tovar, L.R. Biotechnology for an Ancient Crop- Amaranth. In Amaranth Biology, Chemistry and Technology, 1st ed.; Paredes-López, O., Ed.; CRC Press: Boca Raton, FL, USA, 2018; Volume 1, pp. 14–36. [Google Scholar]
- Mendonça, S.; Saldiva, P.H.; Cruz, R.J.; Areas, A.G. Amaranth protein presents cholesterol-lowering. Food Chem. 2009, 116, 738–742. [Google Scholar] [CrossRef]
- Tovar-Pérez, E.G.; Guerrero-Legarreta, B.C.; Ferrés-González, A.; Soriano-Santos, J. Angiotensin I-converting enzyme-inhibitory peptide fractions from albumin 1 and globulin as obtained of amaranth grain. Food Chem. 2009, 116, 437–444. [Google Scholar] [CrossRef]
- Orsini-Delgado, M.C.; Torini, V.A.; Añón, M.C. Antioxidant activity of amaranth protein or their hydrolysates under simulated gastrointestinal digestion. Food Sci. Technol. 2011, 44, 1752–1760. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Scilingo, A.; Añón, M.A. Potential antithrombotic activity detected in amaranth proteins and its hydrolysates. Food Sci. Technol. 2015, 60, 171–177. [Google Scholar] [CrossRef]
- Tiengo, A.; Faria, M.; Neitto, E.M. Characterization and ACE-Inhibitory activity of Amaranth proteins. J. Food Sci. 2009, 74, H121–H126. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, S.; Lunow, D.; Kaiser, S.; Henle, T. Identification and quantification of ACE-inhibiting peptides in enzymatic hydrolysates of plant proteins. Food Chem. 2017, 224, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Otte, J.; De Gobba, C.; Osman, A.; Hamad, E. Angiotensin I-converting enzyme inhibitory activity and antioxidant capacity of bioactive peptides derived from enzymatic hydrolysis of buffalo milk proteins. Int. Dairy J. 2017, 66, 91–98. [Google Scholar] [CrossRef]
- Orsini-Delgado, A.; Galleano, M.; Añón, M.C.; Tironi, V.A. Amaranth peptides from simulated gastrointestinal digestión: Antioxidant Activity against Reactive species. Plants Foods Human Nutr. 2015, 70, 27–34. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wang, L.; Guo, X.; Wang, G.; Wang, X.; Yao, H. Isolation and identification of antioxidative peptides from rice endosperm protein enzymatic hydrolysates by consecutive chromatography and MALDI-TOF/TOF MS/MS. Food Chem. 2010, 119, 226–234. [Google Scholar] [CrossRef]
- Zhang, B.S. In vitro antithrombotic activities of peanut protein hydrolysates. Food Chem. 2016, 202, 1–8. [Google Scholar] [CrossRef]
- Zhuang, H.; Tang, N.; Yuan, Y. Purification and identification of antioxidant peptides from corn gluten meal. J. Funct. Foods 2013, 5, 1810–1821. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, L.; Sun, X.; Zhang, J.; Wang, J.; Li, Y. Study on Hydrolysis Conditions of Flavourzyme in Soybean Polypeptide Alcalase Hydrolysate and Soybean Polypeptide Refining Process. Adv. J. Food Sci. Technol. 2014, 6, 1027–1032. [Google Scholar] [CrossRef]
- Foh, M.B.; Amadou, I.; Foh, B.M.; Kamara, M.T.; Xia, W. Functionality and antioxidant properties of Tilipa (Oreochromis niloticus) as influences by degree of hydrolysis. Int. J. Mol. Sci. 2010, 11, 1851–1869. [Google Scholar] [CrossRef]
- Cumby, N.; Zhong, Y.; Naczk, M.; Shahidi, F. Antioxidant activity and water-holding capacity of canola protein hydrolysates. Food Chem. 2008, 109, 144–148. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzón, A.G.; Martínez-Augustin, O.; Botto, C.C.; Drago, S.R. Antithrombotic activity of Brewer’s spent grain peptides and their effects on blood coagulation pathways. Plant. Foods Hum. Nutr. 2018, 73, 241–246. [Google Scholar] [CrossRef]
- Kasiwut, J.; Sirinupong, N.; Wirote, Y. The anticoagulant and angiotensin I-Converting Enzyme (ACE) Inhibitory peptides from tuna cooking juice produced by alcalase. Curr. Nutr. Food Sci. 2018, 14, 225–234. [Google Scholar] [CrossRef]
- Sabbione, A.C.; Nardo, E.A.; Añón, M.C.; Scilingo, A. Amaranth peptides with antithrombotic activity released by sumulated gastrointestinal digestion. J. Funct. Foods 2016, 20, 204–214. [Google Scholar] [CrossRef]
- Cheng, S.; Tu, M.; Liu, H.; Zhao, G.; Du, M. Food-derived antithrombotic peptides: Preparation, identification and interactions with thrombin. Crit. Rev. Food Sci. Nutr. 2019, 2019. [Google Scholar] [CrossRef] [PubMed]
- Laudano, A.P.; Doolittle, R.F. Synthetic peptides derivatives that bind to fibrinogen and prevent the polymerization of fibrin monomers. PNAS USA 1978, 75, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Fiat, A.M.; Levy-Toledano, S.; Caen, J.P.; Jolles, P. Biologically active peptides of casein and lactoferrin implicated in platelet function. J. Dairy Res. 1989, 56, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Ambigaipalan, P.; Al-Khalifa, A.S.; Shahidi, F. Antioxidant and angiotensin I converting enzyme (ACE) inhibitory activities of date seed protein hydrolysate prepared using Alcalase, Flavourzyme and Thermolysin. J. Funct. Foods 2015, 18, 1125–1137. [Google Scholar] [CrossRef]
- Jung, W.K.; Mendis, E.; Je, J.; Park, P.J.; Son, B.; Kim, H.C.; Choi, Y.K.; Kim, S.K. Angiotensin I-converting enzyme inhibitory peptide from yellowfin sole (Limanda aspera) frame protein and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2006, 94, 26–32. [Google Scholar] [CrossRef]
- Wijesekara, I.; Kim, S.K. Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: Prospects in the pharmaceutical industry. Mar. Drugs 2010, 8, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- Hanafi, M.A.; Hashim, S.N.; Yea, C.S.; Ebrahimpour, A.; Zarei, M.; Muhammad, K.; Abdul-Hamid, A.; Saari, N. High angiotensin-I converting enzyme (ACE) inhibitory activity of Alcalase-digested green soybean (Glycine max) hydrolysates. Food Res. Int. 2018, 106, 589–597. [Google Scholar] [CrossRef] [PubMed]
- González-González, C.R.; Tuohy, K.M.; Jauregi, P. Production of angiotensin-I-converting enzyme (ACE) inhibitory activity in milk fermented with probiotic strains: Effects of calcium, pH and peptides on the ACE-inhibitory activity. Int. Dairy J. 2011, 21, 615–622. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Dhaheri, A.S.; Mahadin, S.A.; Kizhakkayil, J.; Abushelaibi, A. In vitro investigation of anticancer, antihypertensive, antidiabetic and antioxidant activities of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. J. Dairy Sci. 2018, 101, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vecchi, B.; Añón, M.C. ACE inhibitory tetrapeptides from Amaranthus hypochondriacus 11S globulin. Phytochemistry 2009, 70, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Miralles, B.; Amigo, L.; Recio, I. Critical Review and Perspective on Food-Derived Antihypertensive Peptides. J. Agric. Food Chem. 2018, 66, 9384–9390. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.B.; He, T.P.; Li, H.B.; Tang, H.W.; Xia, W.Q. The structure-activity relationship of the antioxidant peptides from natural proteins. Molecules 2016, 12, 72. [Google Scholar] [CrossRef]
- Virtanen, T.; Pihlanto, A.; Akkanen, S.; Korhonen, H. Development of antioxidant activity in milk whey during fermentation with lactic acid bacteria. J. Appl. Microbiol. 2007, 102, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Je, J.Y.; Lee, K.H.; Lee, M.H.; Ahn, C.B. Antioxidant and antihypertensive protein hydrolysates produces from tuna liver by enzymatic hydrolysis. Food Res. Int. 2009, 42, 1266–1272. [Google Scholar] [CrossRef]
- Chirinos, R.; Ochoa, K.; Aguilar-Galvez, A.; Carpentier, S.; Pedreschi, R.; Campos, D. Obtaining of peptides with in vitro antioxidant and angiotensin I converting enzyme inhibitory activities from cañihua protein (Chenopodium pallidicaule Aellen). J. Cereal. Sci. 2018, 83, 139–146. [Google Scholar] [CrossRef]
- Choi, Y.; Lim, T.; He, Y.; Hwang, T. Chemical characteristics and antioxidant properties of wheat gluten hydrolysates produced by single and sequential enzymatic hydrolyses using commercial proteases and their application in beverage systems. J. Food Meas. Charact. 2019, 13, 745–754. [Google Scholar] [CrossRef]
- Moronta, J.; Smaldini, P.L.; Docena, G.H.; Añón, M.C. Peptides of amaranth were targeted as containing sequences with potential anti-inflammatory properties. J. Funct. Foods 2018, 21, 463–473. [Google Scholar] [CrossRef]
- Ijaritomi, O.S.; Malomo, S.A.; Alashi, A.M.; Nwachukwu, I.D.; Fagbemi, T.N.; Osundahunsi, O.F.; Aluko, R.E. Antioxidant and antihypertensive activities of wonderful cola (Buchholzia coriacea) seed protein and enzymatic protein hydrolysates. J. Food Bioact. 2018, 3, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Montoya-Rodríguez, A.; Gómez-Fávela, M.A.; Reyes-Moreno, C.; Milán-Carrillo, J.; González de Mejía, E. Identification of Bioactive Peptides Sequences from Amaranth (Amaranthus hypochodriacus) Seeds Proteins and Their Potential Role in the Prevention of Chronic Diseases. Compr. Rev. Food Sci. Food Saf. 2015, 14, 139–159. [Google Scholar] [CrossRef]
- Balgir, P.P.; Sharma, M. Biopharmaceutical potential of ACE-Inhibitory peptides. J. Proteomics Bioinform. 2017, 10, 171–177. [Google Scholar] [CrossRef]
- Gómez-Ruiz, J.A.; Ramos, M.; Recio, I. Identification of a novel angiotensin-converting enzyme-inhibitory peptides ovine milk proteins by CE-MS and chromatographic techniques. Electrophoresis 2007, 28, 4202–4211. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, S.; Xu, D.; Xie, D.; Guo, H. Inhibitor and substrate binding by angiotensin-converting enzyme: Quantum Mechanical/Molecular Mechanical Molecular Dynamics Studies. J. Chem. Inf. Model. 2011, 51, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- De Gobba, C.; Tompa, G.; Otte, J. Bioctive peptides from caseins released by cold active proteolytic enzymes from Arsukibacterium ikkense. Food Chem. 2014, 15, 205–215. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory peptides: Quantitative Structure-Activity Reletionship Study of di and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef]
- Ueno, T.; Tanaka, M.; Matsiu, T.; Matsumoto, K. Determination of antihypertensive small peptides, Val-Tyr and Ile-Val-Tyr, by fluorometric high-performance liquid chromatography combined with a double heart-cut column-switching technique. Anal. Sci. 2005, 21, 997–1000. [Google Scholar] [CrossRef]
- Umayaparvathi, S.; Meenakshi, S.; Vimalraj, V.; Arumugam, M.; Sivagami, G.; Balasubramanian, T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomed. Prev. Nutr. 2014, 4, 343–353. [Google Scholar] [CrossRef]
- Haung, W.Y.; Majumder, K.; Wu, J. Oxygen radical absorbance capacity of peptuides from egg white protein ovotransferrin and their interaction with phytochemicals. Food Chem. 2010, 123, 635–641. [Google Scholar] [CrossRef]
- Nimalaratne, C.; Lopes-Lutz, D.; Scheiber, A.; Wu, J. Free aromatic amino acids in egg yolk show antioxidant properties. Food Chem. 2011, 129, 151–161. [Google Scholar] [CrossRef]
- Liu, C.; Ren, D.; Li, J.; Fang, L.; Wang, J.; Liu, J.; Min, W. Cytoprotective effect and purification of novel antioxidant peptides from hazelnut (C. heterophylla Fish) protein hydrolysates. J. Funct. Foods 2018, 42, 203–215. [Google Scholar] [CrossRef]
- Wang, X.Q.; Yu, H.H.; Xing, R.; Li, P.C. Characterization, preparation, and purification of marine bioactive peptides. Bio Med. Res. Int. 2017, 2017, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Escalante, E.; González-Olivares, L.G.; Cruz-Guerrero, A.E.; Galán-Vidal, C.A.; Páez-Hernández, M.E.; Álvarez-Romero, G.A. Size exclusion chromatography (SEC-HPLC) as an alternative to study thrombin inhibition. J. Chromatogr. B 2018, 1074, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Martínez, N.E.; Añón, C.M. Composition and structural characterization of amaranth protein isolates: An electrophoretic and calorimetric study. J. Agric. Food Chem. 1996, 44, 2523–2530. [Google Scholar] [CrossRef]
- Tironi, V.A.; Añón, M.C. Amaranth proteins as a source of antioxidant peptides: Effect of proteolysis. Food Res. Int. 2010, 43, 315–322. [Google Scholar] [CrossRef]
- Sashidhar, R.B.; Capoor, A.K.; Ramana, D. Quantification of amino group using amino acids as reference standards by trinitrobenzene sulfonic acid: A simple spectrophotometric method for the estimation of hapten to carrier protein ratio. J. Inmunol. Methods 1995, 167, 121–127. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.B.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin converting enzyme. Carboxylalkanoyl and mercaptoalkanoyl aminoacids. Biochemistry 1977, 16, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.S.; Zhang, W.; Xu, S.Y. Antioxidant and antithrombotic activity of raoeseed peptides. J. Am. Oil Chem. Soc. 2008, 85, 521–527. [Google Scholar] [CrossRef]
- Kuskoski, E.; Asuero, A.; Troncoso, A.; Mancini-Filho, J.; Fett, R. Aplicación de diversos métodos químicos para determinar actividad antioxidante en pulpa de fruto. Ciênc Tecnol. Aliment. 2005, 25, 726–732. [Google Scholar] [CrossRef]
- Delgado-Andrade, C.; Rufian-Henares, J.A.; Morales, F.J. Assessing the antioxidant activity of melanoidins from coffee brews by different antioxidant methods. J. Agric. Food Chem. 2005, 53, 7832–7836. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.; Strain, J. The Ferric Reducing Ability of Plasma (FRAP) as a measure if “Antioxidant power”. The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

| Hydrolysis | ACE Inhibition (%) | Thrombin Inhibition (%) | Antioxidant Activity | ||
|---|---|---|---|---|---|
| DPPH (µmol Trolox E/100 g) | ABTS (mg Trolox E/100 g) | FRAP (µmol Fe2 E/100 g) | |||
| Amaranth Protein | 10.58 ± 1.19 d | 11.90 ± 10.10 c | 76.66 ± 1.60 d | 115.65 ± 10.30 d | 63.37 ± 5.72 c |
| H1 | 49.49 ± 1.47 b | 92.85 ± 3.36 a | 340.17 ± 10.95 b | 425.86 ± 0.66 a | 241.70 ± 9.38 b |
| H2 | 39.77 ± 2.15 c | 80.95 ± 13.46 b | 274.03 ± 10.84 c | 398.36 ± 3.62 c | 226.29 ± 11.20 b |
| H3 | 58.53 ± 2.58 a | 92.85 ± 3.36 a | 388.94 ± 2.73 a | 404.90 ± 1.52 b | 592.54 ± 29.29 a |
| Fraction | ACE (IC50) | Thrombin (IC50) | ABTS (SC50) | Peptide Concentration (mg/L) |
|---|---|---|---|---|
| 2 | 0.332 cd | 38.46 i | 4.204 e | 0.2125 |
| 3 | 0.442 e | 4.36 h | NI | 0.8375 |
| 9 | NI | 0.426 f | NI | 0.9062 |
| 18 | 0.614 f | 2.65 g | 2.538 d | 0.7125 |
| 19 | 0.173 b | 0.183 b | NI | 0.3750 |
| 22 | 0.158 ab | 0.167 ab | 1.375 b | 0.4687 |
| 23 | NI | 0.349 e | 2.809 d | 0.5625 |
| 27 | 0.808 c | 0.402 f | 1.616 c | 0.3125 |
| 28 | 0.346 d | 0.135 a | 1.728 c | 0.0937 |
| 32 | 0.192 b | 0.298 d | 6.931 g | 0.4687 |
| 34 | 0.317 cd | 0.247 c | 2.593 d | 0.4937 |
| 39 | NI | 0.247 c | 5.561 f | 0.4375 |
| 40 | 0.298 c | 0.26 cd | 4.547 e | 0.8375 |
| 45 | 0.134 a | 0.155 a | 0.992 a | 0.8125 |
| Fraction | Mass m/z | Calc MH+ | Sequence | Protein |
|---|---|---|---|---|
| Fraction 22 | ||||
| 34 | 1375.6435 | 1375.6341 | ITASANEPDENKS | Agglutinin |
| 3 | 573.2252 | 573.3516 | LVRW | Agglutinin |
| 16 | 874.4448 | 874.4813 | NIDMLRL | Granule bound starch synthase I |
| 12 | 794.3805 | 794.4203 | RPVFEF | Granule bound starch synthase I |
| 5 | 686.3414 | 686.4081 | DPKLTL | Granule bound starch synthase I |
| 3 | 573.2251 | 573.3617 | IKEAL | Granule bound starch synthase I |
| 13 | 812.3607 | 812.4265 | NVEVHKS | Cystatin |
| Fraction 45 | ||||
| 27 | 853.4330 | 853.4329 | HVQLGHY | Agglutinin |
| 14 | 707.3505 | 707.3212 | SQIDTGS | Agglutinin |
| 14 | 707.3502 | 707.3185 | NWACTL | Agglutinin |
| 4 | 547.1921 | 547.2997 | VRWS | Agglutinin |
| 29 | 861.3847 | 861.4299 | CIHNIVY | Granule bound starch synthase I |
| 26 | 845.4098 | 845.4254 | EGTESIPL | Granule bound starch synthase I |
| 24 | 841.4242 | 841.3841 | PRYDQY | Granule bound starch synthase I |
| 19 | 823.4281 | 823.3696 | MSNIDML | Granule bound starch synthase I |
| 13 | 686.3805 | 686.4080 | DPKLTL | Granule bound starch synthase I |
| 6 | 619.2805 | 619.3566 | IPSRF | Granule bound starch synthase I |
| 3 | 531.1927 | 531.3042 | ARVW | Granule bound starch synthase I |
| 2 | 505.1907 | 505.2447 | CQAAL | Granule bound starch synthase I |
| 1 | 503.1730 | 503.2715 | EELL | Granule bound starch synthase I |
| 1 | 503.1731 | 503.2823 | LGVAGS | Granule bound starch synthase I |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayala-Niño, A.; Rodríguez-Serrano, G.M.; González-Olivares, L.G.; Contreras-López, E.; Regal-López, P.; Cepeda-Saez, A. Sequence Identification of Bioactive Peptides from Amaranth Seed Proteins (Amaranthus hypochondriacus spp.). Molecules 2019, 24, 3033. https://doi.org/10.3390/molecules24173033
Ayala-Niño A, Rodríguez-Serrano GM, González-Olivares LG, Contreras-López E, Regal-López P, Cepeda-Saez A. Sequence Identification of Bioactive Peptides from Amaranth Seed Proteins (Amaranthus hypochondriacus spp.). Molecules. 2019; 24(17):3033. https://doi.org/10.3390/molecules24173033
Chicago/Turabian StyleAyala-Niño, Alexis, Gabriela Mariana Rodríguez-Serrano, Luis Guillermo González-Olivares, Elizabeth Contreras-López, Patricia Regal-López, and Alberto Cepeda-Saez. 2019. "Sequence Identification of Bioactive Peptides from Amaranth Seed Proteins (Amaranthus hypochondriacus spp.)" Molecules 24, no. 17: 3033. https://doi.org/10.3390/molecules24173033
APA StyleAyala-Niño, A., Rodríguez-Serrano, G. M., González-Olivares, L. G., Contreras-López, E., Regal-López, P., & Cepeda-Saez, A. (2019). Sequence Identification of Bioactive Peptides from Amaranth Seed Proteins (Amaranthus hypochondriacus spp.). Molecules, 24(17), 3033. https://doi.org/10.3390/molecules24173033






