Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity
Abstract
1. Introduction
2. Results
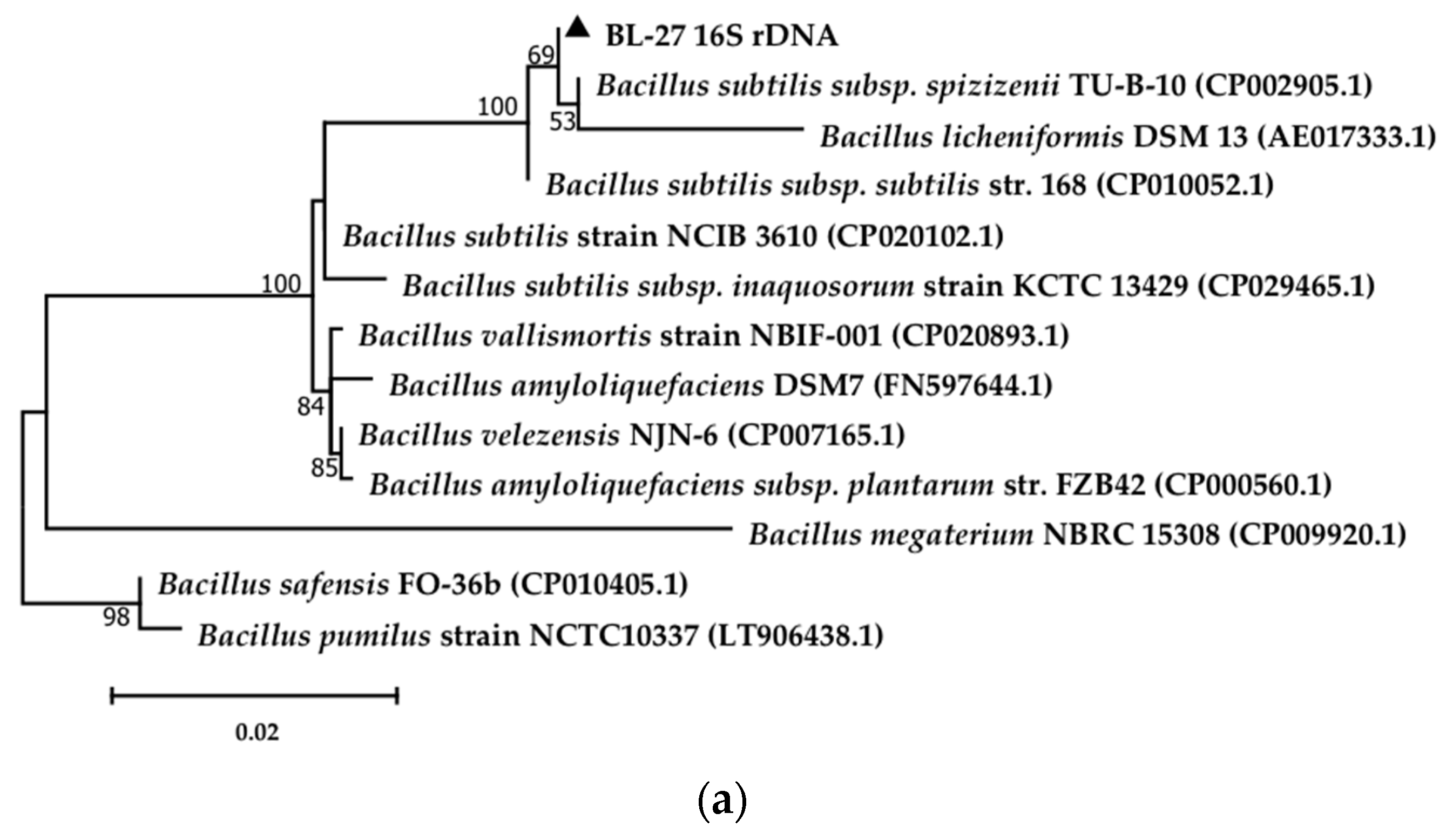
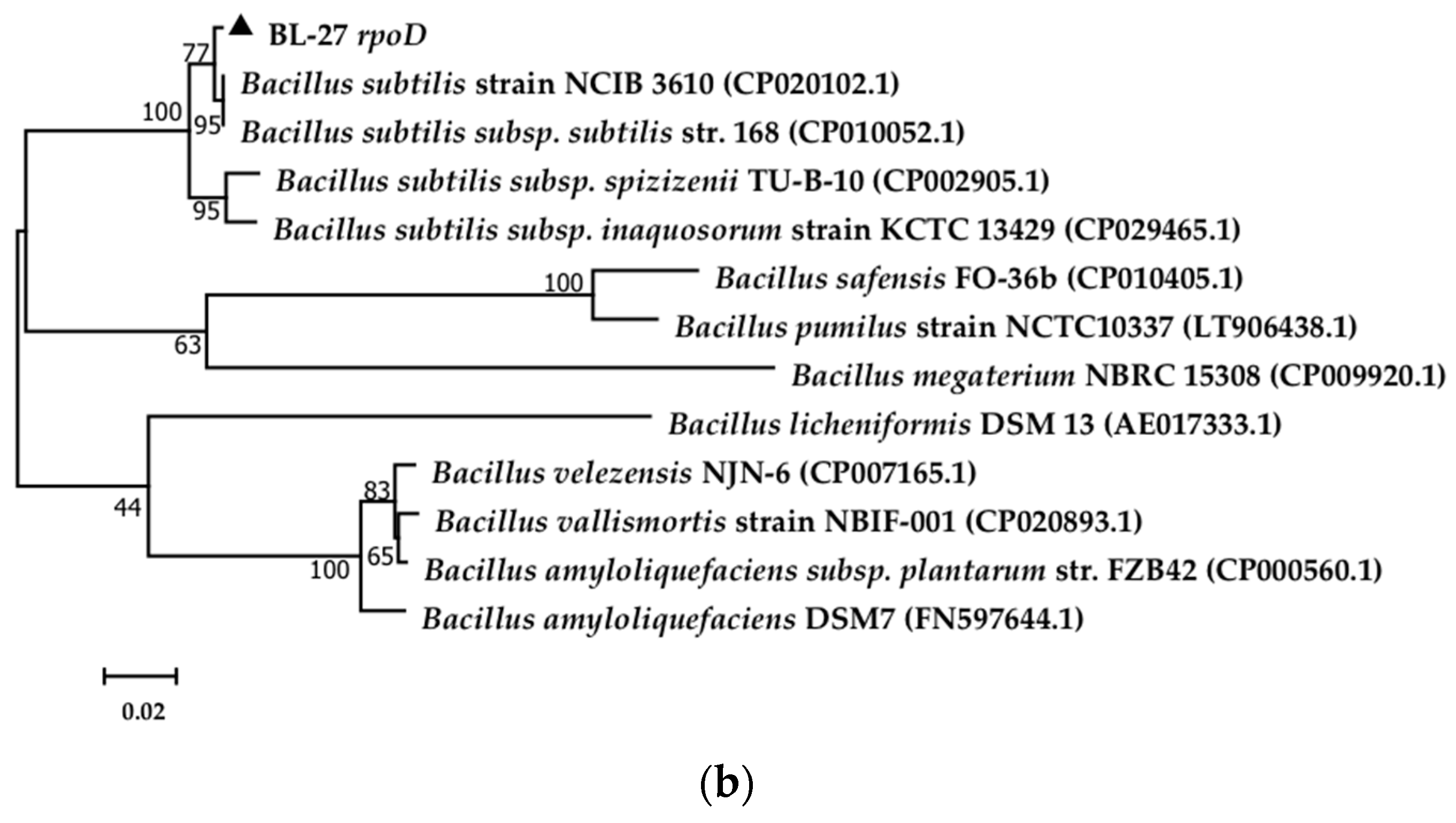
2.1. Isolation and Identification of the Bacterial Strain
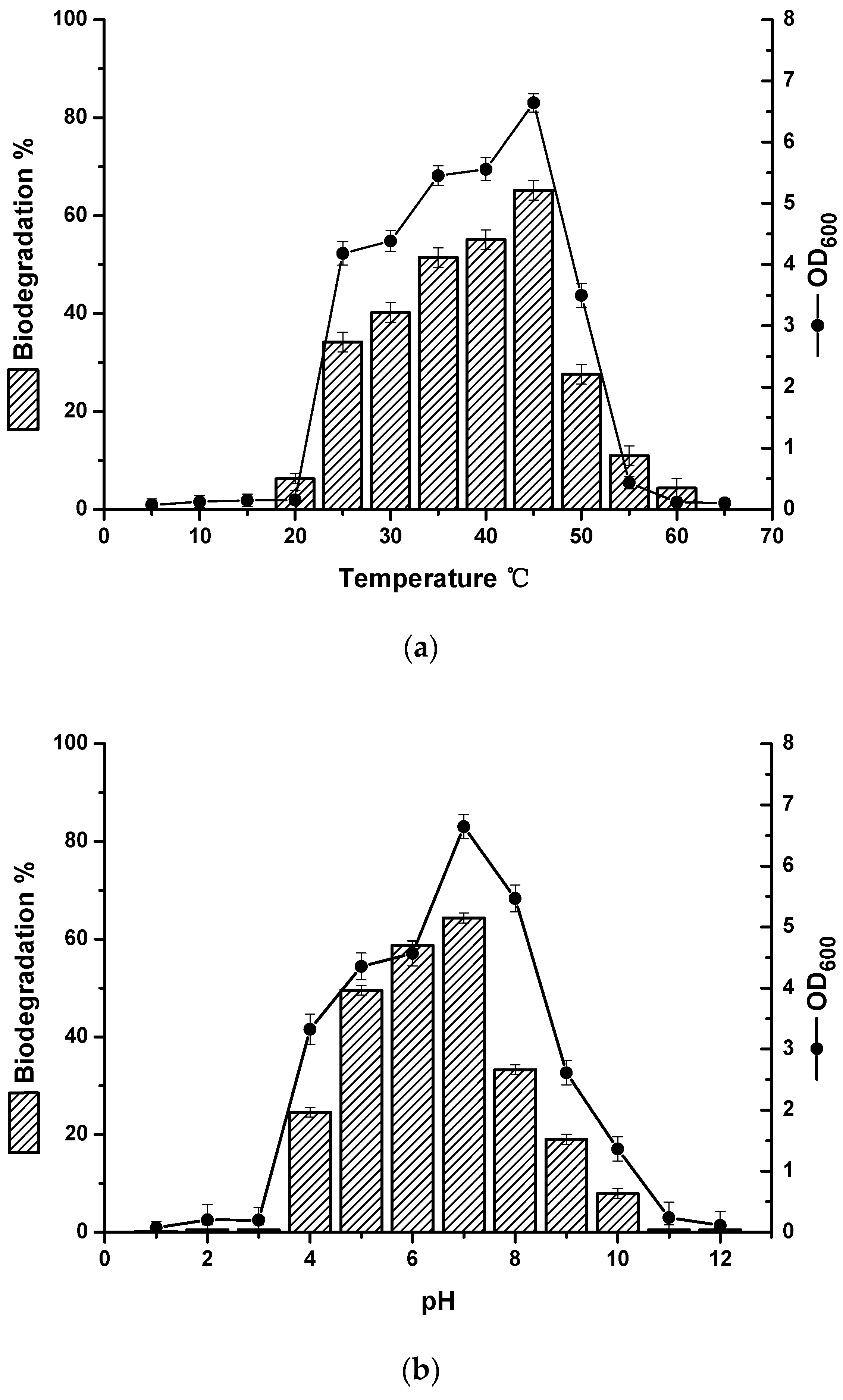
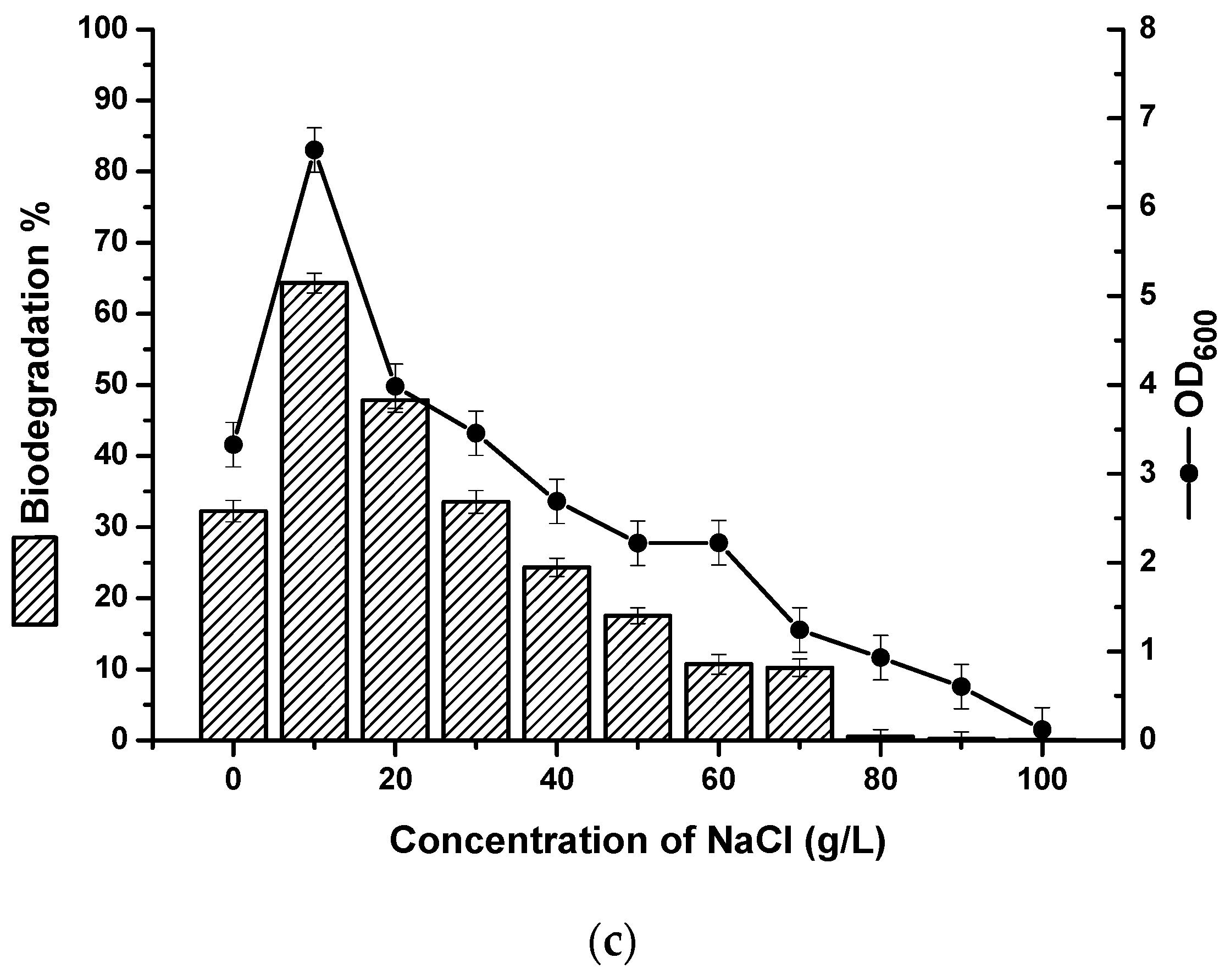
2.2. The Effects of Carbon Source, Temperature, pH and Salinity on Strain Growth and Biodegradation of Crude Oil
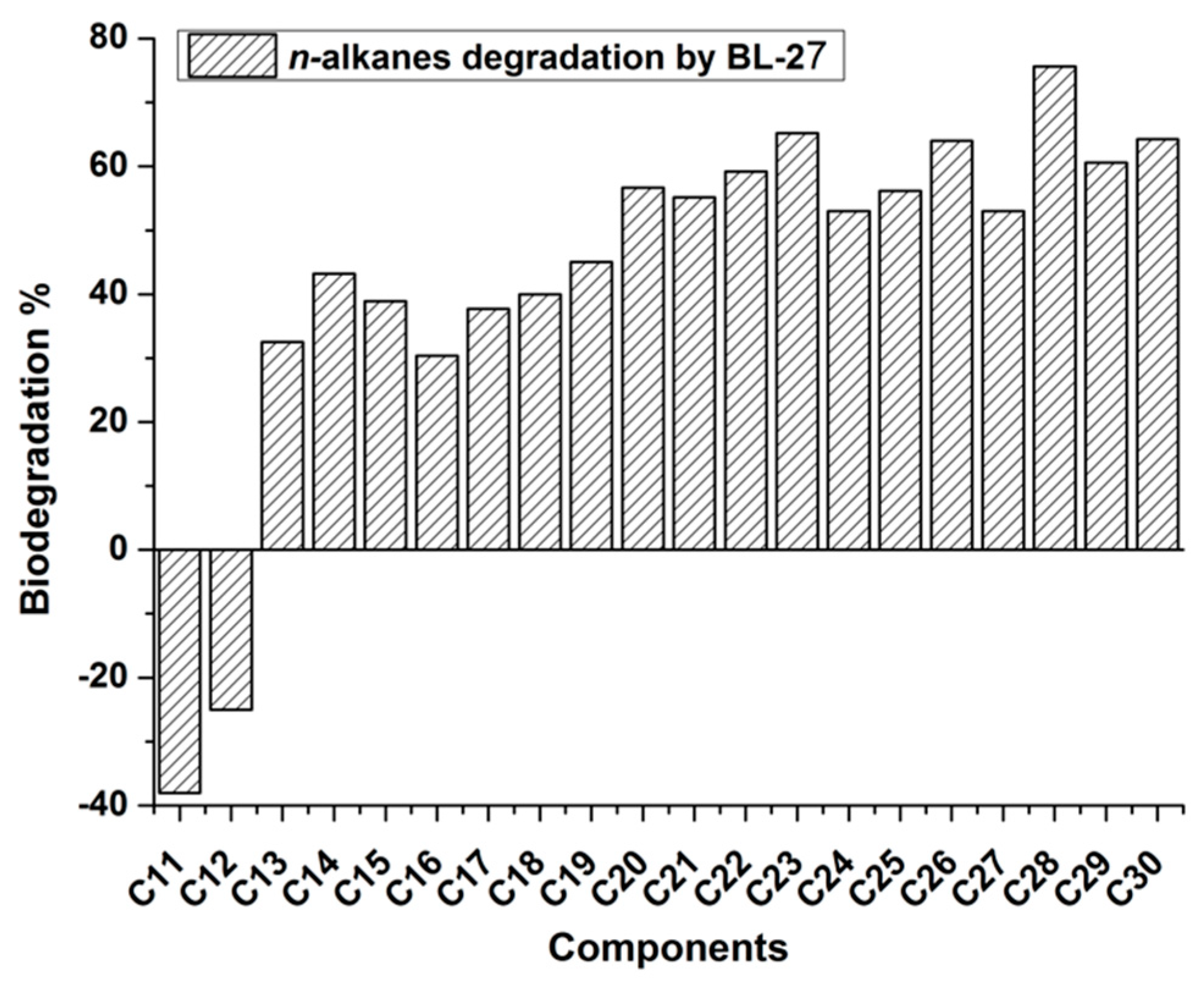
2.3. GC-MS Analysis of Residual Alkanes after Biodegradation
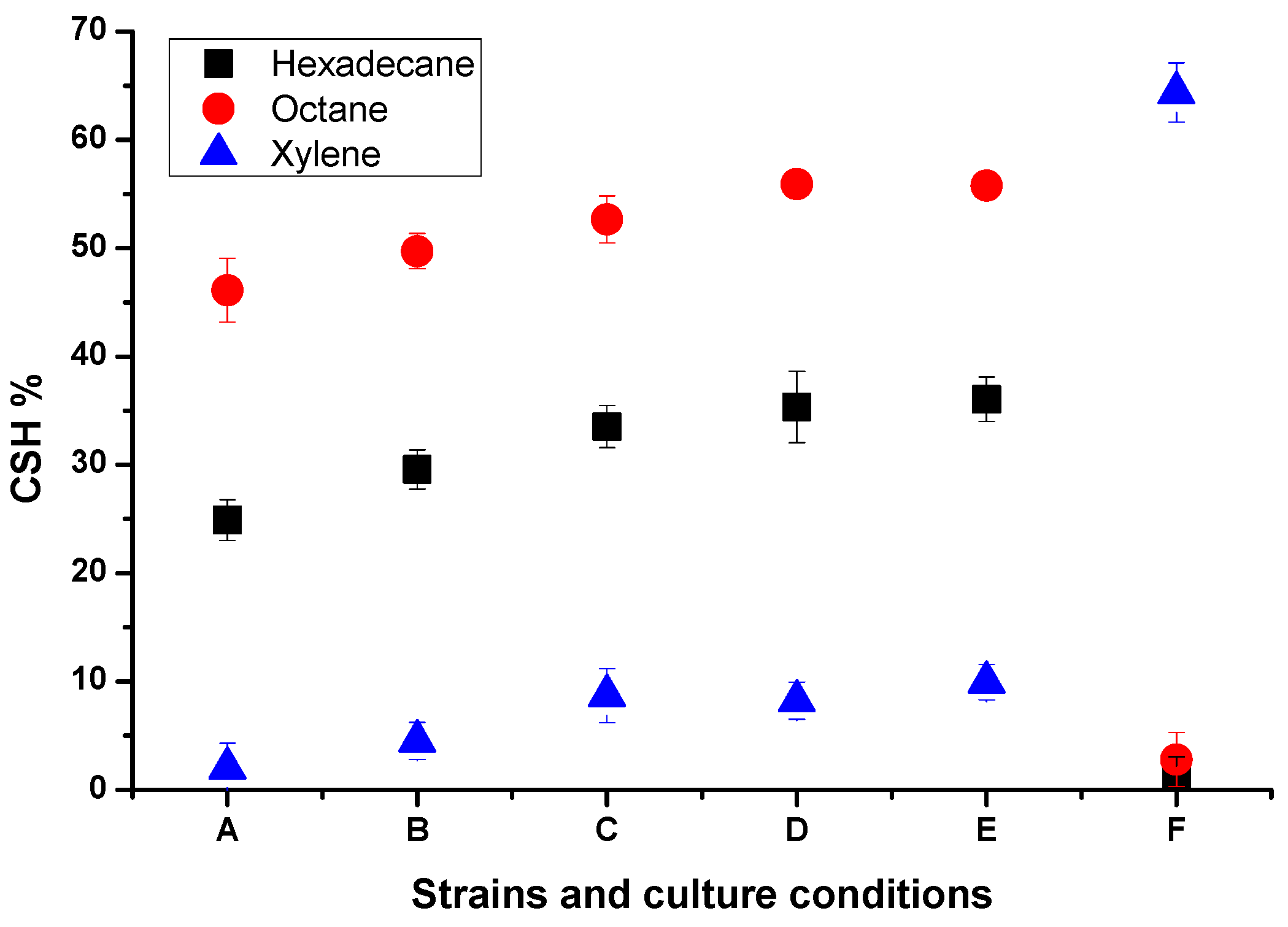
2.4. Analysis of Cell Surface Hydrophobicity (CSH)
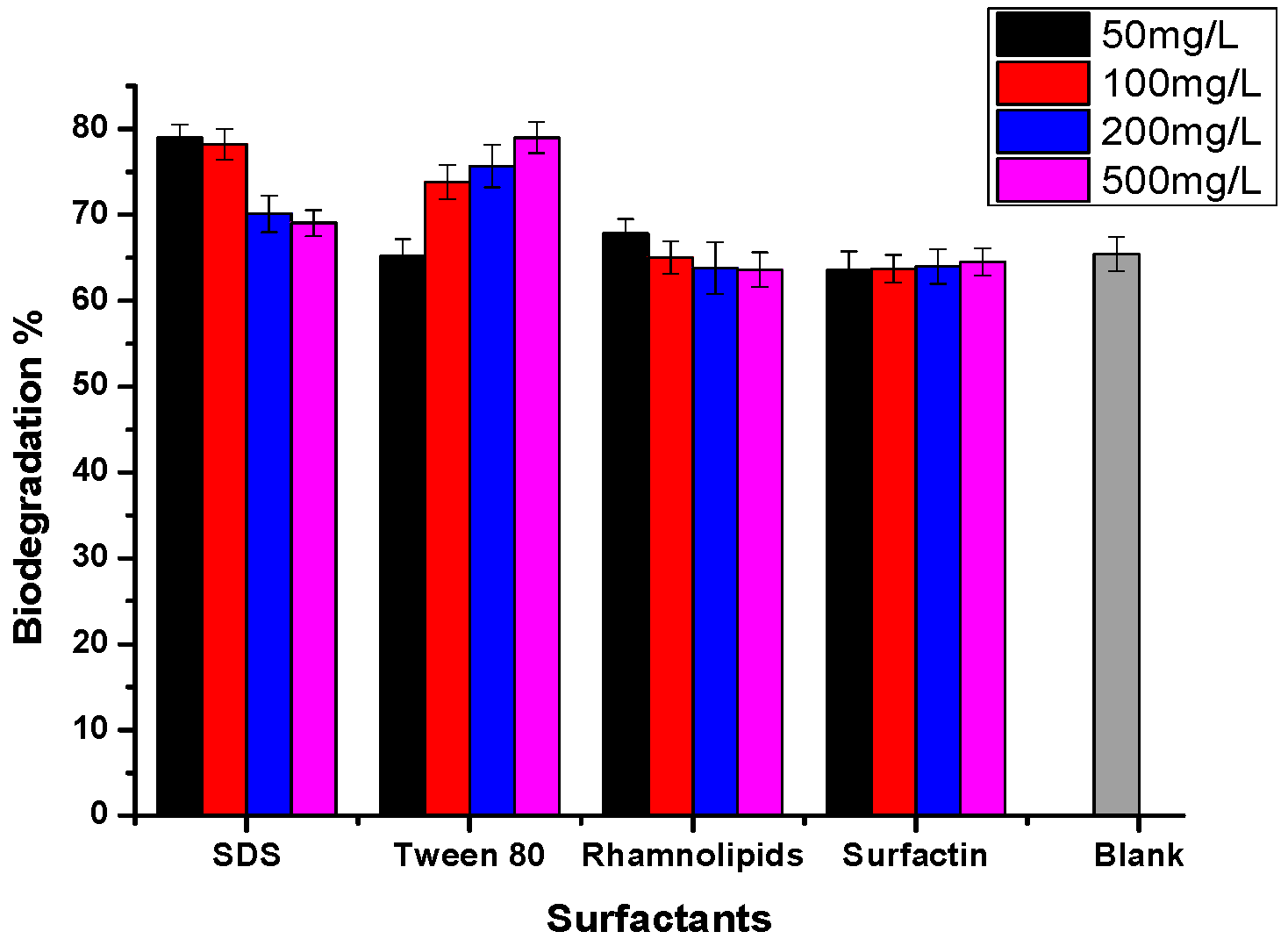
2.5. The Effects of Different Surfactants on Growth, CSH and Crude Oil Degradation by Strain BL-27
3. Discussion
4. Materials and Methods
4.1. Crude Oil, Diesel Oil, Chemicals and Culture Media
4.2. Isolation and Identification of Bacteria
4.3. Carbon Source Utilization
4.4. Optimization of Environmental Parameters for the Growth of Strain BL-27 and Degradation of Crude Oil
4.5. Analysis of Cell Surface Hydrophobicity
4.6. Cell Surface Hydrophobicity Test of Cells Cultured with Different Carbon Sources
4.7. The Effect of Surfactants on Strain Growth, CSH and the Biodegradation of Crude Oil
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Varjani, S.J.; Upasani, V.N. Carbon spectrum utilization by an indigenous strain of Pseudomonas aeruginosa NCIM 5514: Production, characterization and surface active properties of biosurfactant. Bioresour. Technol. 2016, 221, 510–516. [Google Scholar] [CrossRef]
- Varjani, S.J.; Srivastava, V.K. Green technology and sustainable development of environment. Renew. Res. J. 2015, 3, 244–249. [Google Scholar]
- Silva, R.; Almeida, D.; Rufino, R. Applications of biosurfactants in the petroleum industry and the remediation of oil spills. Int. J. Mol. Sci. 2014, 15, 12523–12542. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial degradation of petroleum hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef]
- Sakthipriya, N.; Doble, M.; Sangwai, J.S. Fast degradation and viscosity reduction of waxy crude oil and model waxy crude oil using Bacillus subtilis. J. Pet. Sci. Eng. 2015, 134, 158–166. [Google Scholar] [CrossRef]
- Zheng, C.; He, J.; Wang, Y.; Wang, M.; Huang, Z. Hydrocarbon degradation and bioemulsifier production by thermophilic Geobacillus pallidus strains. Bioresour. Technol. 2011, 102, 9155–9161. [Google Scholar] [CrossRef]
- Wang, L.; Tang, Y.; Wang, S.; Liu, R.L.; Liu, M.Z.; Zhang, Y.; Liang, F.L.; Feng, L. Isolation and characterization of a novel thermophilic Bacillus strain degrading long-chain n-alkanes. Extremophiles 2006, 10, 347–356. [Google Scholar] [CrossRef]
- Elumalai, P.; Parthipan, P.; Narenkumar, J.; Anandakumar, B.; Madhavan, J.; Oh, B.T.; Rajasekar, A. Role of thermophilic bacteria (Bacillus and Geobacillus) on crude oil degradation and biocorrosion in oil reservoir environment. Biotech 2019, 9, 79. [Google Scholar] [CrossRef]
- Watkinson, R.; Morgan, P. Physiology of aliphatic hydrocarbon-degrading microorganisms. Biodegradation 1990, 1, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Jia, L.; Wang, S. Biodegradation of beta-cypermethrin by two Serratia spp. with different cell surface hydrophobicity. Bioresour. Technol. 2010, 101, 3423–3429. [Google Scholar] [CrossRef] [PubMed]
- Pacwa-Płociniczak, M.; Płaza, G.A.; Piotrowska-Seget, Z. Environmental applications of biosurfactants: Recent advances. Int. J. Mol. Sci. 2011, 12, 633–654. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, S.; Mukherji, S. Alteration in cell surface properties of Burkholderia spp. during surfactant-aided biodegradation of petroleum hydrocarbons. Appl. Microbiol. Biot. 2012, 94, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.; Ramya, J.; Kumar, S.; Vasanthi, N.S.; Chandran, P.; Khan, S. Diesel biodegradation capacities of indigenous bacterial species isolated from diesel contaminated soil. J. Environ. Health Sci. Eng. 2014, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Varjani, S.J. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat, India. Int. Biodeterior. Biodegrad. 2015, 103, 116–124. [Google Scholar] [CrossRef]
- Gong, H.Y.; Bao, M.T.; Pi, G.L.; Li, Y.M.; Wang, A.Q.; Wang, Z.N. Dodecanol-Modified Petroleum Hydrocarbon Degrading Bacteria for Oil Spill Remediation: Double Effect on Dispersion and Degradation. ACS Sustain. Chem. Eng. 2016, 4, 169–176. [Google Scholar] [CrossRef]
- Huang, L.; Xie, J.; Wang, F.M.; Sun, T.T.; Shi, X.F.; Lian, J.Y.; Liang, F.L. Cell-surface hydrophobicity and emulsification of a hydrocarbon-degrading strain. Microbiol. China 2013, 40, 1610–1617. [Google Scholar]
- Liu, T.; Wang, F.H.; Guo, L.P.; Li, X.L.; Yang, X.J.; Lin, A.J. Biodegradation of n-hexadecane by bacterial strains B1 and B2 isolated from petroleum-contaminated soil. Sci. China Chem. 2012, 55, 1968–1975. [Google Scholar] [CrossRef]
- Yuan, X.Z.; Ren, F.Y.; Zeng, G.M.; Zhong, H.; Fu, H.Y.; Liu, J.; Xu, X.M. Adsorption of surfactants on a Pseudomonas aeruginosa strain and the effect on cell surface lypohydrophilic property. Appl. Microbiol. Biot. 2007, 76, 1189–1198. [Google Scholar] [CrossRef]
- Zhao, Z.Y.; Selvam, A.; Wong, J.W.C. Effects of rhamnolipids on cell surface hydrophobicity of PAH degrading bacteria and the biodegradation of phenanthrene. Bioresour. Technol. 2011, 102, 3999–4007. [Google Scholar] [CrossRef]
- Kumari, S.; Regar, R.K.; Manicham, N. Improved polycyclic aromatic hydrocarbon degradation in a crude oil by individual and a consortium of bacteria. Bioresour. Technol. 2018, 254, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Q.; Xu, L.; Tang, Y.Q.; Chen, F.M.; Wu, X.L. Simultaneous degradation of phenol and n-hexadecane by Acinetobacter strains. Bioresour. Technol. 2012, 123, 664–668. [Google Scholar] [CrossRef]
- Borah, D.; Yadav, R.N.S. Biodegradation of Diesel, Crude Oil, Kerosene and Used Engine Oil by a Newly Isolated Bacillus cereus strain DRDU1 from an Automobile Engine in Liquid Culture. Arab. J. Sci. Eng. 2014, 39, 5337–5345. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, Z.; Yang, C.; Ma, C.; Tao, F.; Xu, P. Degradation of n-alkanes and polycyclic aromatic hydrocarbons in petroleum by a newly isolated Pseudomonas aeruginosa DQ8. Bioresour. Technol. 2011, 102, 4111–4116. [Google Scholar] [CrossRef]
- Zeinali, M.; Vossoughi, M.; Ardestani, S.K. Degradation of phenanthrene and anthracene by Nocardia otitidiscaviarum strain TSH1, a moderately thermophilic bacterium. J. Appl. Microbiol. 2008, 105, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.T.; Pi, Y.R.; Wang, L.N.; Sun, P.Y.; Li, Y.M.; Cao, L.X. Lipopeptide biosurfactant production bacteria Acinetobacter sp. D3-2 and its biodegradation of crude oil. Environ. Sci. Process. Impacts. 2014, 16, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Li, G.Y.; Li, L.; Tarozo, R.; Longo, W.M.; Wang, K.J.; Dong, H.L.; Huang, Y.S. Microbial production of long-chain n-alkanes: Implication for interpreting sedimentary leaf wax signals. Org. Geochem. 2018, 115, 24–31. [Google Scholar] [CrossRef]
- Ding, C.; Ma, T.T.; Hu, A.Y.; Dai, L.R.; He, Q.; Cheng, L.; Zhang, H. Enrichment and Characterization of a Psychrotolerant Consortium Degrading Crude Oil Alkanes Under Methanogenic Conditions. Microb. Ecol. 2015, 70, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.J.; Yu, L.; Wang, P.; Xiu, J.L.; Dong, H.P. Characterization of a thermophilic and halotolerant Geobacillus pallidus H9 and its application in microbial enhanced oil recovery (MEOR). Ann. Microbiol. 2012, 62, 1779–1789. [Google Scholar]
- She, Y.H.; Zhang, F.; Xia, J.J.; Kong, S.Q.; Wang, Z.L.; Shu, F.C.; Hu, J.M. Investigation of Biosurfactant-Producing Indigenous Microorganisms that Enhance Residue Oil Recovery in an Oil Reservoir After Polymer Flooding. Appl. Biochem. Biotechnol. 2011, 163, 223–234. [Google Scholar] [CrossRef]
- Zhou, J.F.; Gao, P.K.; Dai, X.H.; Cui, X.Y.; Tian, H.M.; Xie, J.J.; Li, G.Q.; Ma, T. Heavy hydrocarbon degradation of crude oil by a novel thermophilic Geobacillus stearothermophilus strain A-2. Int. Biodeter. Biodegr. 2018, 126, 224–230. [Google Scholar] [CrossRef]
- Chandankere, R.; Yao, J.; Cai, M.M.; Masakorala, K.; Jain, A.K.; Choi, M.M.F. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel 2014, 122, 140–148. [Google Scholar] [CrossRef]
- Kumari, B.; Singh, S.N.; Singh, D.P. Characterization of two biosurfactant producing strains in crude oil degradation. Process Biochem. 2012, 47, 2463–2471. [Google Scholar] [CrossRef]
- Lawniczak, L.; Marecik, R.; Chrzanowski, L. Contributions of biosurfactants to natural or induced bioremediation. Appl. Biochem. Biotechnol. 2013, 97, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Pi, Y.R.; Bao, M.T.; Liu, Y.Q.; Lu, T.Y.; He, R. The contribution of chemical dispersants and biosurfactants on crude oil biodegradation by Pseudomonas sp. LSH-7′. J. Clean. Prod. 2017, 153, 74–82. [Google Scholar] [CrossRef]
- Thavasi, R.; Jayalakshmi, S.; Banat, I.M. Effect of biosurfactant and fertilizer on biodegradation of crude oil by marine isolates of Bacillus megaterium, Corynebacterium kutscheri and Pseudomonas aeruginosa. Bioresour. Technol. 2011, 102, 772–778. [Google Scholar] [CrossRef]
- Kaczorek, E.; Urbanowicz, M.; Olszanowski, A. The influence of surfactants on cell surface properties of Aeromonas hydrophila during diesel oil biodegradation. Colloids Surf. B Biointerfaces 2010, 81, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Tachibana, S. Characterization of phenanthrene degradation by strain Polyporus sp. S133. J. Environ. Sci. 2010, 22, 142–149. [Google Scholar] [CrossRef]
- Matsuura, T.; Shinohara, H.; Inoue, Y. Erwinia isolates from the bacterial shoot blight of pear in Japan are closely related to Erwinia pyrifoliae based on phylogenetic analyses of gyrB and rpoD genes. J. Gen. Plant Pathol. 2007, 73, 53–58. [Google Scholar] [CrossRef]
- Wang, L.T.; Lee, F.L.; Tai, C.J.; Kasai, H. Comparison of gyrB gene sequences, 16S rRNA gene sequences and DNA–DNA hybridization in the Bacillus subtilis group. Int. J. Syst. Evol. Microbiol. 2007, 57, 1846–1850. [Google Scholar] [CrossRef] [PubMed]
- Antonio, J.M.M.; Arturo, M.; Saavedra, M.J.; Remedios, O.; Monserrate, L.A.; Erica, L.; Figueras, M.J. Multilocus phylogenetic analysis of the genus Aeromonas. Syst. Appl. Microbiol. 2011, 34, 189–199. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Moubasher, H.A.; Hegazy, A.K.; Mohamed, N.H.; Moustafa, Y.M.; Kabiel, H.F.; Hamad, A.A. Phytoremediation of soils polluted with crude petroleum oil using Bassia scoparia and its associated rhizosphere microorganisms. Int. Biodeterior. Biodegrad. 2015, 98, 113–120. [Google Scholar] [CrossRef]
- Yan, S.P.; Wang, Q.Y.; Qu, L.N.; Li, C. Characterization of Oil-Degrading Bacteria from Oil-Contaminated Soil and Activity of their Enzymes. Biotechnol. Biotechnol. Equip. 2013, 27, 3932–3938. [Google Scholar] [CrossRef]
- Wang, J.X.; Wang, J.M.; Zhang, Z.Z.; Li, Y.F.; Zhang, B.Y.; Zhang, Z.Y.; Zhang, G.Q. Cold-adapted bacteria for bioremediation of crude oil-contaminated soil. J. Chem. Technol. Biotechnol. 2016, 91, 2286–2297. [Google Scholar] [CrossRef]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Tian, W.; Yao, J.; Liu, R.P.; Zhu, M.J.; Wang, F.; Wu, X.Y.; Liu, H.J. Effect of natural and synthetic surfactants on crude oil biodegradation by indigenous strains. Ecotoxicol. Environ. Saf. 2016, 129, 171–179. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds (crude oil and diesel oil) are available from the authors. |
| Carbon Source | Growth a, b | Carbon Source | Growth a, b |
|---|---|---|---|
| C7 | + | C23 | ++ |
| C8 | + | C24 | ++ |
| C9 | + | C25 | ++ |
| C10 | + | Cyclohexane | + |
| C11 | + | Xylene | + |
| C12 | + | Phenol | - |
| C13 | + | Naphthalene | + |
| C14 | + | Phenanthrene | + |
| C15 | + | Styrene | - |
| C16 | + | Ethanol | ++ |
| C17 | ++ | Acetonitrile | ++ |
| C18 | ++ | Trichloromethane | + |
| C19 | ++ | Isoamylol | - |
| C20 | ++ | Ethyl acetate | + |
| C21 | ++ | Liquid paraffin | + |
| C22 | ++ | Diesel oil | ++ |
| Surfactant Concentration (mg/L) | Relative Growth % | CSH * % | ||||||
|---|---|---|---|---|---|---|---|---|
| 50 | 100 | 200 | 500 | 0 | 50 | 200 | 0 | |
| SDS | 103 ± 1.2 | 102 ± 1.5 | 100 ± 1.4 | 100 ± 1.3 | 100 | 16.4 ± 1.9 | 18.7 ± 2.3 | 24.9 ± 1.9 |
| Tween 80 | 99 ± 1.6 | 101 ± 1.6 | 101 ± 1.7 | 103 ± 1.8 | 100 | 22.6 ± 1.8 | 12.6 ± 1.6 | 24.9 ± 1.9 |
| Rhamnolipids | 97 ± 1.6 | 85 ± 2.0 | 83 ± 1.3 | 85 ± 2.1 | 100 | 16.9 ± 1.4 | 9.5 ± 2.1 | 24.9 ± 1.9 |
| Surfactin | 89 ± 1.6 | 87 ± 1.3 | 88 ± 1.1 | 85 ± 1.6 | 100 | 22.1 ± 2.1 | 19.4 ± 1.7 | 24.9 ± 1.9 |
| Triton X-100 | 48 ± 2.2 | 41 ± 4.2 | 37 ± 3.9 | 27 ± 3.1 | 100 | ND | ND | ND |
| CTAB | 6.2 ± 1.3 | 6.2 ± 1.2 | 5.7 ± 1.3 | 5.3 ± 1.3 | 100 | ND | ND | ND |
| TTAB | 3.9 ± 1.3 | 3.9 ± 1.2 | 3.5 ± 1.3 | 3.3 ± 1.4 | 100 | ND | ND | ND |
| Strains | Temperature (°C) | Salinity (g/L) | Preferential Degradation Components | Biodegradation Rate (g/L/d) | Substrates of CSH | CSH % | Reference |
|---|---|---|---|---|---|---|---|
| Geobacillus stearothermophilus A-2 | 60 | 0 | C22-C33 | 0.164 | hexadecane | 83.9% a | [30] |
| Bacillus subtilis YB7 | 50 | 0.5 | n-alkanes | 0.633 | hexadecane | 72–95% b | [5] |
| Bacillus methylotrophicus USTBa | 35 | 0 | n-alkanes | 1.314 | crude oil | 62.0% b | [31] |
| Pseudomonas sp. BP10 | 35 | 0 | C10-C28 | 0.404 | crude oil | 70.0% b | [32] |
| Bacillus subtilis BL-27 | 45 | 10 | C17-C30 | 0.387 | hexadecane | 24.9% a 33.5% b | This work |
| Surfactant | Concentration | Strains | CSH % | Degradation % | Reference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Substrates | Without Surfactant | With Surfactant | Substrates | Without Surfactant | With Surfactant | ||||
| Rhamnolipids | 600 mg/L | Pseudomonas LSH-7 | crude oil | - | - | crude oil | 77% | 87% | [34] |
| Rhamnolipids | 400 mg/L | Bacillus subtilis BUM | phenanthrene | 23% | 25% | phenanthrene | 82% | 32% | [35] |
| Rhamnolipids | 120 mg/L | Aeromonas hydrofila | diesel oil | 7% | 12% | diesel oil | 58% | 60% | [36] |
| Tween 80 | 1000 mg/L | Polyporus sp. S133 | phenanthrene | - | - | phenanthrene | 30% | 47% | [37] |
| 5000 mg/L | 72% | ||||||||
| 10000 mg/L | 61% | ||||||||
| SDS | 50 mg/L | Bacillus subtilis BL-27 | n-hexadecane | 25% | 16% | crude oil | 65% | 79% | This work |
| 200 mg/L | 19% | 70% | |||||||
| Tween 80 | 50 mg/L | 23% | 65% | ||||||
| 200 mg/L | 13% | 76% | |||||||
| Rhamnolipids | 50 mg/L | 17% | 68% | ||||||
| 200 mg/L | 10% | 64% | |||||||
| Surfactin | 50 mg/L | 22% | 64% | ||||||
| 200 mg/L | 19% | 64% | |||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, D.; Lin, J.; Lin, J.; Wang, W.; Li, S. Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity. Molecules 2019, 24, 3021. https://doi.org/10.3390/molecules24173021
Wang D, Lin J, Lin J, Wang W, Li S. Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity. Molecules. 2019; 24(17):3021. https://doi.org/10.3390/molecules24173021
Chicago/Turabian StyleWang, Dan, Jiahui Lin, Junzhang Lin, Weidong Wang, and Shuang Li. 2019. "Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity" Molecules 24, no. 17: 3021. https://doi.org/10.3390/molecules24173021
APA StyleWang, D., Lin, J., Lin, J., Wang, W., & Li, S. (2019). Biodegradation of Petroleum Hydrocarbons by Bacillus subtilis BL-27, a Strain with Weak Hydrophobicity. Molecules, 24(17), 3021. https://doi.org/10.3390/molecules24173021





