Cytotoxic Effects of Diterpenoid Alkaloids Against Human Cancer Cells
Abstract
:1. Introduction
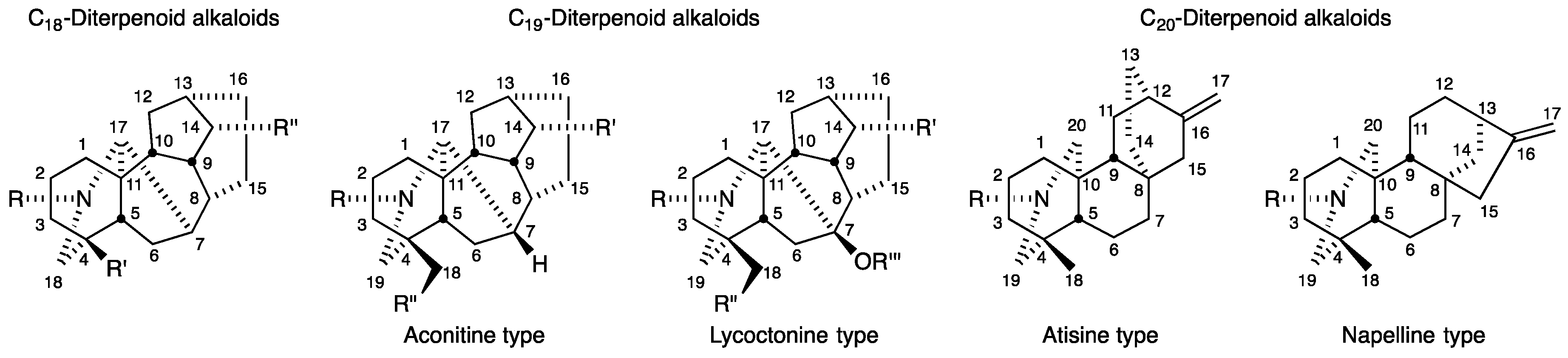
2. Antiproliferative Activity of C19-Diterpenoid Alkaloid Derivatives
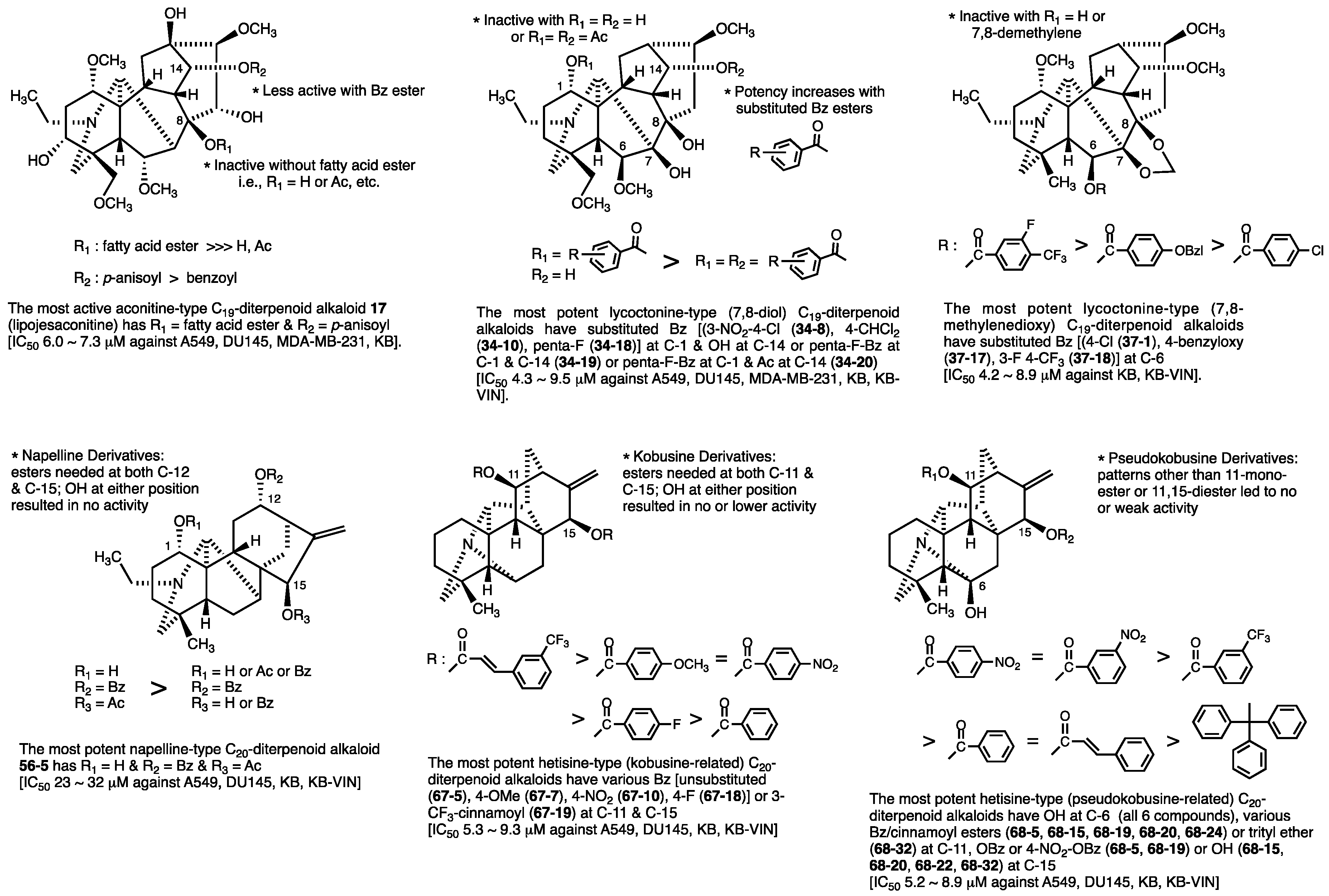
2.1. Aconitine-Type C19-Diterpenoid Alkaloids
2.2. Lycoctonine-Type (7,8-diol) C19-Diterpenoid Alkaloids
2.3. Lycoctonine-Type (7,8-methylenedioxy) C19-Diterpenoid Alkaloids
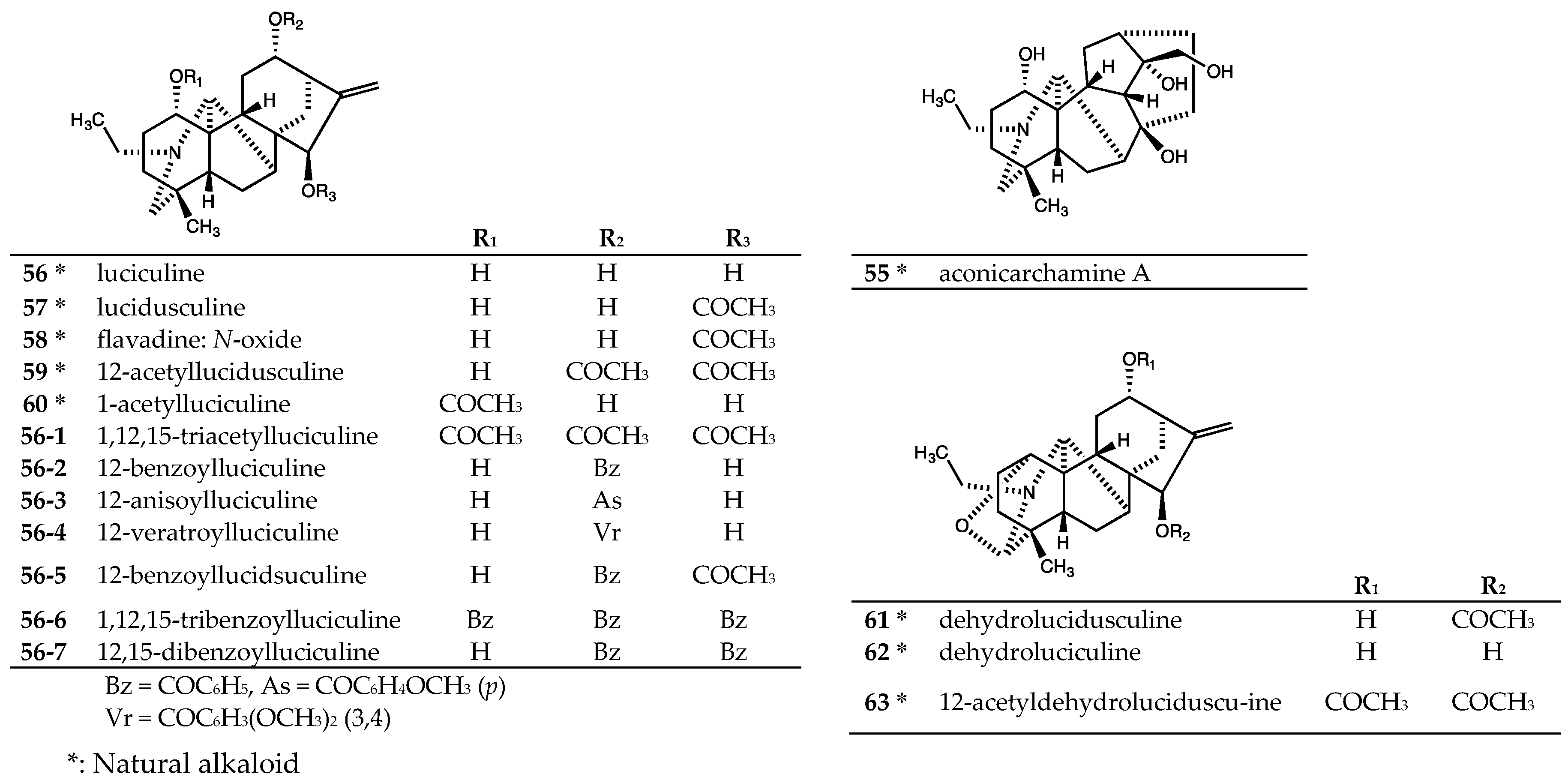
3. Antiproliferative Activity of C20-Diterpenoid Alkaloid Derivatives
3.1. Actaline and Napelline-Type C20-Diterpenoid Alkaloids
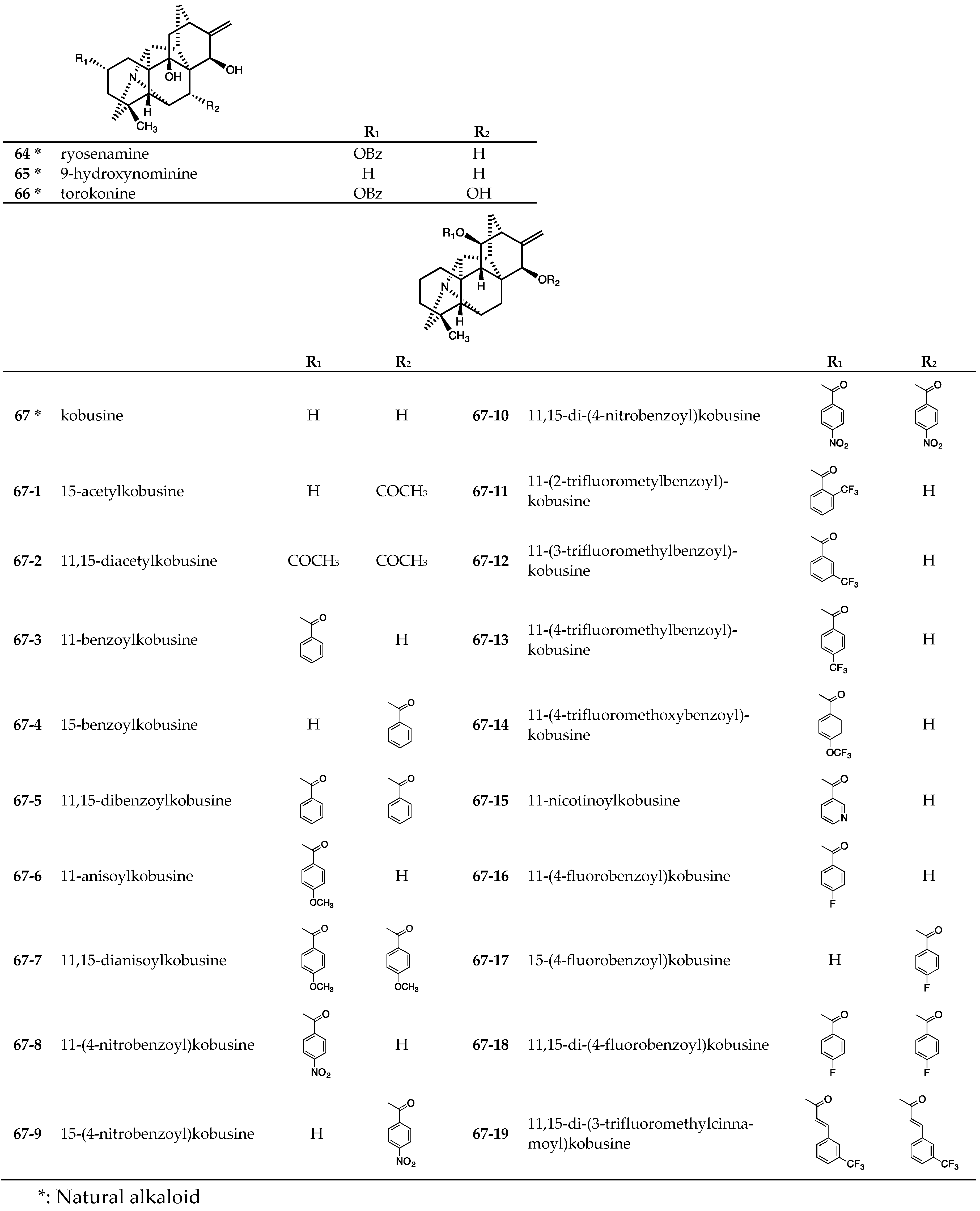
3.2. Hetisine-Type (Analogs of Kobusine) C20-Diterpenoid Alkaloids
3.3. Hetisine-Type (Analogs of Pseudokobusine) C20-Diterpenoid Alkaloids
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urban, J.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Nature: A vital source of leads for anticancer drug development. Phytochem. Rev. 2009, 8, 313–331. [Google Scholar] [CrossRef]
- Kingston, D.G. Tubulin-interactive natural products as anticancer agents. J. Nat. Prod. 2009, 72, 507–515. [Google Scholar] [CrossRef]
- Lee, K.H. Discovery and development of natural products-derived chemotherapeutic agents based on a medicinal chemistry approach. J. Nat. Prod. 2010, 73, 500–516. [Google Scholar] [CrossRef]
- Grothaus, P.G.; Cragg, G.M.; Newman, D.J. Plant natural products in anticancer drug discovery. Curr. Org. Chem. 2010, 14, 1781–1791. [Google Scholar] [CrossRef]
- Kinghorn, A.D.; Pan, L.; Fletcher, J.N.; Chai, H. The relevance of higher plants in lead compound discovery programs. J. Nat. Prod. 2011, 74, 1539–1555. [Google Scholar] [CrossRef]
- Dall’Acqua, S. Natural products as antimitotic agents. Curr. Top. Med. Chem. 2014, 14, 2272–2285. [Google Scholar] [CrossRef]
- Hussain, M.; Khera, R.A.; Iqbal, J.; Khalid, M.; Hanif, M.A. Phytochemicals: Key to effective anticancer drugs. Mini-Rev. Org. Chem. 2019, 16, 141–158. [Google Scholar] [CrossRef]
- Agarwal, G.; Carcache, P.J.B.; Addo, E.M.; Kinghorn, A.D. Current status and contemporary approaches to the discovery of antitumor agents from higher plants. Biotechnol. Adv. 2019. [Google Scholar] [CrossRef]
- Wang, F.P.; Chen, Q.H. The diterpenoid alkaloids. The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: San Diego, CA, USA, 2010; Volume 69, pp. 1–577. [Google Scholar]
- Wang, F.P.; Chen, Q.H.; Liu, X.Y. Diterpenoid alkaloids. Nat. Prod. Rep. 2010, 27, 529–570. [Google Scholar] [CrossRef]
- Benn, M.H.; Jacyno, J.M. The toxicology and pharmacology of diterpenoid alkaloids. In Alkaloids: Chemical and Biological Perspectives; Pelletier, S.W., Ed.; Wiley-Interscience: New York, NY, USA, 1983; Volume 1, pp. 153–210. [Google Scholar]
- Bock, J.H.; Norris, D.O. Introduction to forensic plant science. In Forensic Plant Science; Elsevier Academic Press: Boston, MA, USA, 2016; pp. 1–22. [Google Scholar]
- Amiya, T.; Bando, H. Aconitum alkaloids. In The Alkaloids; Brossi, A., Ed.; Academic Press: San Diego, CA, USA, 1988. [Google Scholar]
- Chan, T.Y.K. Aconite poisoning. Clin. Toxicol. 2009, 47, 279–285. [Google Scholar] [CrossRef]
- Chan, T.Y.K. Aconite poisoning following the percutaneous absorption of Aconitum alkaloids. Forensic Sci. Int. 2012, 223, 25–27. [Google Scholar] [CrossRef]
- Povšnar, M.; Koželj, G.; Kreft, S.; Lumpert, M. Rare tradition of the folk medicinal use of Aconitum spp. is kept alive in Solčavsko, Slovenia. J. Ethnobiol. Ethnomed. 2017, 13, 45. [Google Scholar] [CrossRef]
- Wada, K.; Hazawa, M.; Takahashi, K.; Mori, T.; Kawahara, N.; Kashiwakura, I. Inhibitory effects of diterpenoid alkaloids on the growth of A172 human malignant cells. J. Nat. Prod. 2007, 70, 1854–1858. [Google Scholar] [CrossRef]
- Hazawa, M.; Wada, K.; Takahashi, K.; Mori, T.; Kawahara, N.; Kashiwakura, I. Suppressive effects of novel derivatives prepared from Aconitum alkaloids on tumor growth. Invest. New Drugs 2009, 27, 111–119. [Google Scholar] [CrossRef]
- Hazawa, M.; Wada, K.; Takahashi, K.; Mori, T.; Kawahara, N.; Kashiwakura, I. Structure-activity relationships between the Aconitum C20-diterpenoid alkaloid derivatives and the growth suppressive activities of non-Hodgkin’s lymphoma Raji cells and human hematopoietic stem/progenitor cells. Invest. New Drugs 2011, 29, 1–8. [Google Scholar] [CrossRef]
- Wada, K.; Hazawa, M.; Takahashi, K.; Mori, T.; Kawahara, N.; Kashiwakura, I. Structure-activity relationships and the cytotoxic effects of novel diterpenoid alkaloid derivatives against A549 human lung carcinoma cells. J. Nat. Med. 2011, 65, 43–49. [Google Scholar] [CrossRef]
- Bando, H.; Kanaiwa, Y.; Wada, K.; Mori, T.; Amiya, T. Structure of deoxyjesaconitine. A new diterpene alkaloid from Aconitum subcuneatum Nakai. Heterocycles 1981, 16, 1723–1725. [Google Scholar]
- Mori, T.; Bando, H.; Kanaiwa, Y.; Wada, K.; Amiya, T. Studies on the constituents of Aconitum Species. II. Structure of deoxyjesaconitine. Chem. Pharm. Bull. 1983, 31, 2884–2886. [Google Scholar] [CrossRef]
- Wada, K.; Bando, H.; Mori, T.; Wada, R.; Kanaiwa, Y.; Amiya, T. Studies on the constituents of Aconitum Species. III. On the components of Aconitum subcuneatum Nakai. Chem. Pharm. Bull. 1985, 33, 3658–3661. [Google Scholar] [CrossRef]
- Bando, H.; Wada, K.; Watanabe, M.; Mori, T.; Amiya, T. Studies on the constituents of Aconitum Species. IV. On the components of Aconitum japonicum Thunb. Chem. Pharm. Bull. 1985, 33, 4717–4722. [Google Scholar] [CrossRef]
- Bando, H.; Wada, K.; Amiya, T.; Fujimoto, Y.; Kobayashi, K. Structures of secojesaconitine and subdesculine, two new diterpenoid alkaloids from Aconitum japonicum Thunb. Chem. Pharm. Bull. 1988, 36, 1604–1606. [Google Scholar] [CrossRef]
- Yamashita, H.; Takeda, K.; Haraguchi, M.; Abe, Y.; Kuwahara, N.; Suzuki, S.; Terui, A.; Masaka, T.; Munakata, N.; Uchida, M.; et al. Four new diterpenoid alkaloids from Aconitum japonicum subsp. subcuneatum. J. Nat. Med. 2018, 72, 230–237. [Google Scholar] [CrossRef]
- Yamashita, H.; Miyao, M.; Hiramori, K.; Kobayashi, D.; Suzuki, Y.; Mizukami, M.; Goto, M.; Lee, K.H.; Wada, K. Cytotoxic diterpenoid alkaloid from Aconitum japonicum subsp. subcuneatum. J. Nat. Med. 2019, submitted. [Google Scholar]
- Wada, K.; Bando, H.; Kawahara, N.; Mori, T.; Murayama, M. Determination and quantitative analysis of Aconitum japonicum by liquid chromatography atomospheric pressure chemical ionization mass spectrometry. Biol. Mass Spectrom. 1994, 23, 97–102. [Google Scholar] [CrossRef]
- Wada, K.; Ohkoshi, E.; Zhao, Y.; Goto, M.; Morris-Natschke, S.L.; Lee, K.H. Evaluation of Aconitum diterpenoid alkaloids as antiproliferative agents. Bioorg. Med. Chem. Lett. 2015, 25, 1525–1531. [Google Scholar] [CrossRef]
- Wada, K.; Bando, H.; Amiya, T. Two new C20-diterpenoid alkaloids from Aconitum yesoense var. macroyesoense (NAKAI) TAMURA. Structures of dehydrolucidusculine and N-deethyldehydrolucidusculine. Heterocycles 1985, 23, 2473–2477. [Google Scholar]
- Bando, H.; Wada, K.; Amiya, T.; Fujimoto, Y.; Kobayashi, K.; Sakurai, T. Studies on Aconitum Species. V. Constituents of Aconitum yesoense var. macroyesoense (Nakai) Tamura. Heterocycles 1987, 26, 2623–2637. [Google Scholar]
- Wada, K.; Bando, H.; Kawahara, N. Studies on Aconitum species. XI. Two new diterpenoid alkaloids from Aconitum yesoense var. macroyesoense (Nakai) Tamura V. Heterocycles 1989, 29, 2141–2148. [Google Scholar]
- Wada, K.; Kawahara, N. Diterpenoid and norditerpenoid alkaloids from the roots of Aconitum yesoense var. macroyesoense. Helvetica Chimica Acta 2009, 92, 629–637. [Google Scholar] [CrossRef]
- Wada, K.; Yamamoto, T.; Bando, H.; Kawahara, N. Four diterpenoid alkaloids from Delphinium elatum. Phytochemistry 1992, 31, 2135–2138. [Google Scholar] [CrossRef]
- Wada, K.; Ishizuki, S.; Mori, T.; Bando, H.; Murayama, M.; Kawahara, N. Effects of alkaloids from Aconitum yesoense var. macroyesoense on cutaneous blood flow in mice. Biol. Pharm. Bull. 1997, 20, 978–982. [Google Scholar]
- Wada, K.; Mori, T.; Kawahara, N. Application of liquid chromatography−atmospheric pressure chemical ionization mass spectrometry to the differentiation of stereoisomeric C19-norditerpenoid alkaloids. Chem. Pharm. Bull. 2000, 48, 660–668. [Google Scholar] [CrossRef]
- Wada, K.; Goto, M.; Shimizu, T.; Kusanagi, N.; Lee, K.-H.; Yamashita, H. Structure-activity relationships and evaluation of esterified diterpenoid alkaloid derivatives as antiproliferative agents. J. Nat. Med. 2019. [Google Scholar] [CrossRef]
- Bando, H.; Wada, K.; Tanaka, J.; Kimura, S.; Hasegawa, E.; Amiya, T. Two new alkaloids from Delphinium pacific giant and revised 13C-NMR assignment of delpheline. Heterocycles 1989, 29, 1293–1300. [Google Scholar] [CrossRef]
- Wada, K.; Chiba, R.; Kanazawa, R.; Matsuoka, K.; Suzuki, M.; Ikuta, M.; Goto, M.; Yamashita, H.; Lee, K.H. Six new norditerpenoid alkaloids from Delphinium elatum. Phytochem. Lett. 2015, 12, 79–83. [Google Scholar] [CrossRef]
- Wada, K.; Asakawa, E.; Tosho, Y.; Nakata, A.; Hasegawa, Y.; Kaneda, K.; Goto, M.; Yamashita, H.; Lee, K.H. Four new norditerpenoid alkaloids from Delphinium elatum. Phytochem. Lett. 2016, 17, 190–193. [Google Scholar] [CrossRef]
- Yamashita, H.; Katoh, M.; Kokubun, A.; Uchimura, A.; Mikami, S.; Takeuchi, A.; Kaneda, K.; Suzuki, Y.; Mizukami, M.; Goto, M.; et al. Four new C19-diterpenoid alkaloids from Delphinium elatum. Phytochem. Lett. 2018, 24, 6–9. [Google Scholar] [CrossRef]
- Wada, K.; Ohkoshi, E.; Morris-Natschke, S.L.; Bastow, K.F.; Lee, K.H. Cytotoxic esterified diterpenoid alkaloid derivatives with increased selectivity against a drug-resistant cancer cell line. Bioorg. Med. Chem. Lett. 2012, 22, 249–252. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.P.; Liang, X.T. C20-Diterpenoid alkaloids. In The Alkaloids: Chemistry and Biology; Cordell, G.A., Ed.; Academic Press: San Diego, CA, USA, 2002; Volume 59, pp. 1–280. [Google Scholar]
- Wada, K.; Mori, T.; Kawahara, N. Application of liquid chromatography-atmospheric pressure chemical ionization mass spectrometry to the differentiation of stereoisomeric diterpenoid alkaloids. Chem. Pharm. Bull. 2000, 48, 1065–1074. [Google Scholar] [CrossRef]
- Wada, K.; Ishizuki, S.; Mori, T.; Fujihira, E.; Kawahara, N. Effects of Aconitum alkaloid kobusine and pseudokobusine derivatives on cutaneous blood flow in mice. Biol. Pharm. Bull. 1998, 21, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Ishizuki, S.; Mori, T.; Fujihira, E.; Kawahara, N. Effects of Aconitum alkaloid kobusine and pseudokobusine derivatives on cutaneous blood flow in mice; II. Biol. Pharm. Bull. 2000, 23, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Wada, K.; Bando, H.; Kawahara, N. Studies on Aconitum Species. XIII. Two new diterpenoid alkaloids from Aconitum yesoense var. macroyesoense (Nakai) Tamura VI. Heterocycles 1990, 31, 1081–1088. [Google Scholar] [CrossRef]
- Wada, K.; Bando, H.; Wada, R.; Amiya, T. Analgesic activity of main components from Aconitum yesoense var. macroyesoense (Nakai) Tamura and pseudokobusine derivatives. Shouyakugaku Zasshi 1989, 43, 50–54. [Google Scholar]
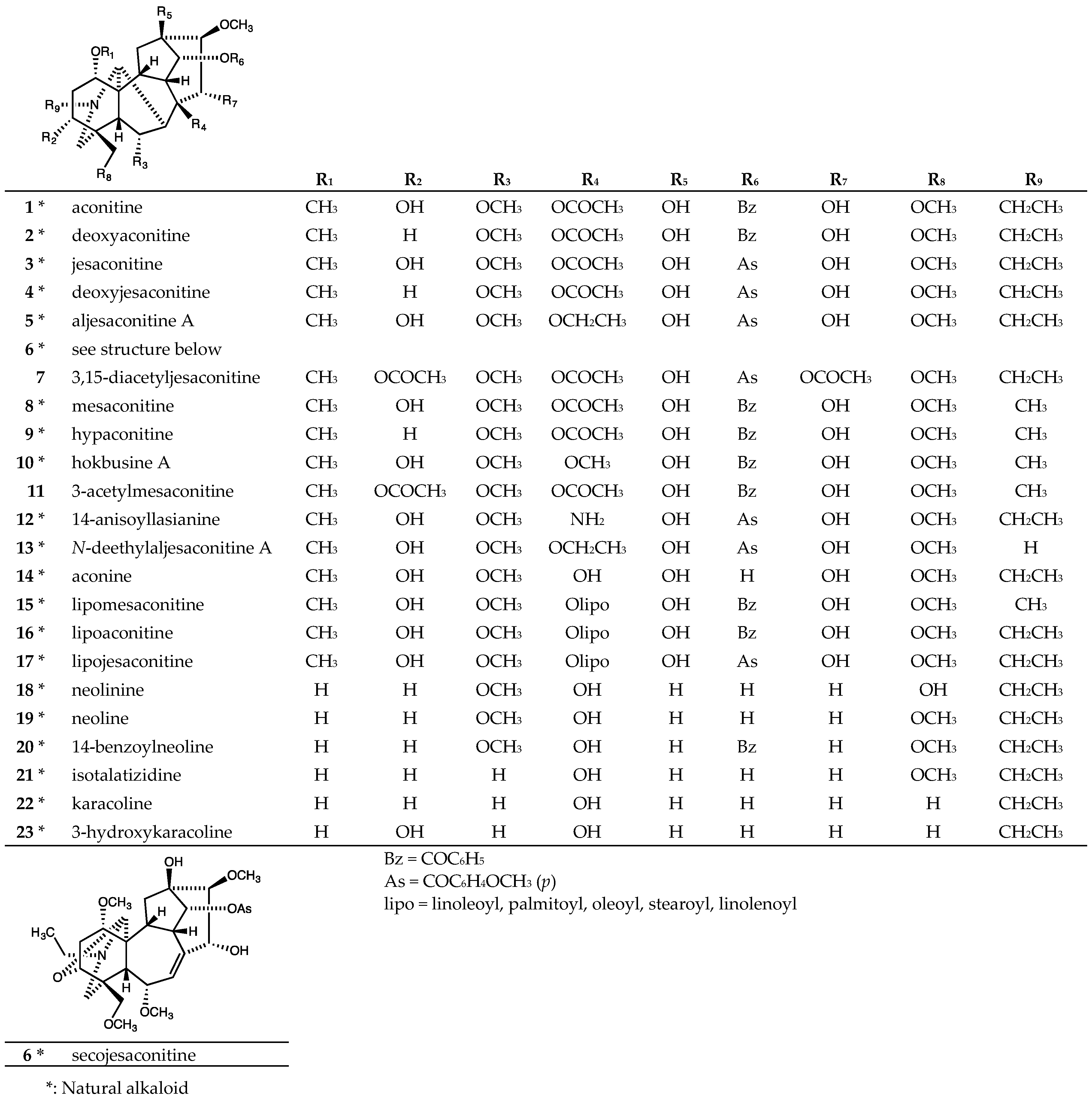




| Cell Line/IC50 (μM) 1 | ||||||
|---|---|---|---|---|---|---|
| Alkaloids | A549 | DU145 | MDA-MB-231 | MCF-7 | KB | KB-VIN |
| Aconitine (1) | >20 | >20 | - | - | >20 | >20 |
| Deoxyaconitine (2) | >20 | >20 | - | - | >20 | >20 |
| Jesaconitine (3) | >20 | >20 | - | - | >20 | >20 |
| Deoxyjesaconitine (4) | >20 | >20 | - | - | >20 | >20 |
| Aljesaconitine A (5) | >20 | >20 | - | - | >20 | >20 |
| Secojesaconitine (6) | >20 | >20 | - | - | >20 | >20 |
| 7 | >20 | >20 | - | - | >20 | >20 |
| Mesaconitine (8) | >20 | >20 | - | - | >20 | >20 |
| Hypaconitine (9) | >20 | >20 | - | - | >20 | >20 |
| Hokbusine A (10) | >20 | >20 | - | - | >20 | >20 |
| 11 | >20 | >20 | - | - | >20 | >20 |
| 14-Anisoyllasianine (12) | >40 | - | >40 | >40 | >40 | >40 |
| N-Deethylaljesaconitine A (13) | >40 | - | >40 | >40 | >40 | >40 |
| Aconine (14) | >40 | - | >40 | >40 | >40 | >40 |
| Lipomesaconitine (15) | 17.2 ± 2.3 | - | 20.0 ± 0.2 | 19.0 ± 1.0 | 10.0 ± 3.3 | 21.5 ± 0.9 |
| Lipoaconitine (16) | 17.4 ± 1.1 | - | 15.5 ± 0.5 | 16.0 ± 0.3 | 13.7 ± 1.3 | 20.3 ± 1.1 |
| Lipojesaconitine (17) | 7.3 ± 0.3 | - | 6.0 ± 0.2 | 6.7 ± 0.2 | 6.0 ± 0.2 | 18.6 ± 0.9 |
| Neolinine (18) | >40 | - | >40 | >40 | >40 | >40 |
| Neoline (19) | >20 | >20 | - | - | >20 | >20 |
| 14-benzoylneoline (20) | >20 | >20 | - | - | >20 | >20 |
| Isotalatizidine (21) | >40 | - | >40 | >40 | >40 | >40 |
| Karacoline (22) | >20 | >20 | - | - | >20 | >20 |
| 3-Hydroxykaracoline (23) | >40 | - | >40 | >40 | >40 | >40 |
| PXL2 (nM) | 4.8 ± 0.6 | 5.9 ± 1.9 | 8.4 ± 0.8 | 10.2 ± 0.9 | 5.8 ± 0.2 | 2405.4 ± 44.8 |
| Cell Line/IC50 (μM) 1 | ||||||
|---|---|---|---|---|---|---|
| Alkaloids | A549 | DU145 | MDA-MB-231 | MCF-7 | KB | KB-VIN |
| Nevadensine (24) | >40 | - | >40 | >40 | >40 | >40 |
| N-Deethylnevadensine (25) | >40 | - | >40 | >40 | >40 | >40 |
| 18-Methoxygadesine (26) | >20 | >20 | - | - | >20 | >20 |
| Virescenine (27) | >40 | - | >40 | >40 | >40 | >40 |
| Delphinifoline (28) | >20 | >20 | - | - | >20 | >20 |
| N-Deethyldelsoline (29) | >20 | >20 | - | - | >20 | >20 |
| Andersonidine (30) | >20 | >20 | - | - | >20 | >20 |
| Pacifiline (31) | >20 | >20 | - | - | >20 | >20 |
| Pacifinine (32) | >20 | >20 | - | - | >20 | >20 |
| Pacifidine (33) | >20 | >20 | - | - | >20 | >20 |
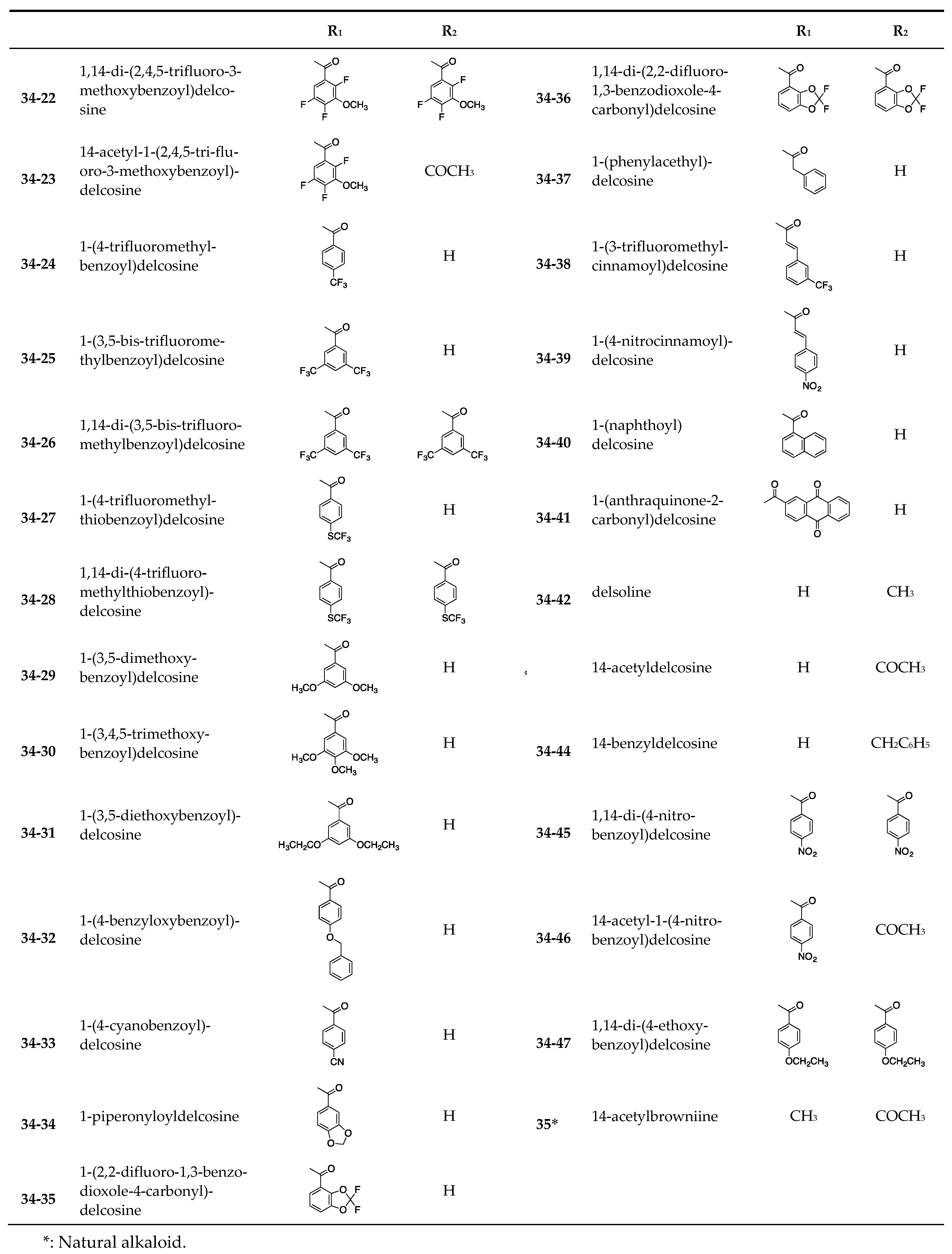
| Delcosine (34) | >20 | >20 | - | - | >20 | >20 |
| 34-1 | >20 | >20 | - | - | >20 | >20 |
| 34-2 | >20 | >20 | - | - | >20 | >20 |
| 34-3 | 20.6 ± 0.3 | - | 19.4± 1.0 | 17.9 ± 0.3 | 14.6 ± 0.6 | 17.1 ± 0.8 |
| 34-4 | >40 | - | >40 | >40 | >40 | >40 |
| 34-5 | 18.7 ± 0.1 | - | 29.1 ± 1.6 | 25.8 ± 1.4 | 19.6 ± 0.3 | 21.1 ± 1.5 |
| 34-6 | 7.7 ± 0.9 | - | 8.6 ± 6.0 | 15.8 ± 4.2 | 5.6 ± 1.2 | 8.6 ± 1.9 |
| 34-7 | >40 | - | >40 | >40 | >40 | >40 |
| 34-8 | 4.5 ± 0.5 | - | 5.0 ± 0.1 | 5.9 ± 0.3 | 5.4 ± 0.3 | 5.6 ± 0.4 |
| 34-9 | 24.8 ± 0.1 | - | 4.7 ± 0.1 | 12.2 ± 0.3 | 5.8 ± 0.4 | >40 |
| 34-10 | 4.8 ± 0.3 | - | 4.8 ± 0.7 | 5.7 ± 0.4 | 4.3 ± 0.5 | 5.3 ± 0.4 |
| 34-11 | >40 | - | >40 | >40 | >40 | >40 |
| 34-12 | 26.5 ± 0.3 | - | >40 | 40.6 ± 2.5 | 27.8 ± 1.7 | 28.1 ± 3.0 |
| 34-13 | 20.8 ± 1.7 | - | 32.4 ± 1.8 | 25.9 ± 2.4 | 23.0 ± 2.4 | 21.5 ± 1.3 |
| 34-14 | >40 | - | >40 | >40 | >40 | >40 |
| 34-15 | 21.7 ± 1.6 | - | 30.2 ± 2.7 | 26.9 ± 1.4 | 20.7 ± 1.2 | 21.5 ± 3.6 |
| 34-16 | 14.4 ± 2.1 | - | 20.1 ± 0.7 | 16.4 ± 2.1 | 13.6 ± 1.1 | 15.7 ± 0.8 |
| 34-17 | 11.4 ± 1.4 | - | 10.4 ± 1.7 | 22.5 ± 1.5 | 10. 8 ± 1.9 | 11.8 ± 3.2 |
| 34-18 | 4.7 ± 0.1 | - | 5.3 ± 0.2 | 9.2 ± 0.4 | 5.8 ± 0.6 | 9.5 ± 0.5 |
| 34-19 | 4.9 ± 0.1 | - | 4.9 ± 0.1 | 5.3 ± 0.3 | 4.7 ± 0.1 | 4.9 ± 0.1 |
| 34-20 | 4.8 ± 0.1 | - | 4.6 ± 0.3 | 6.0 ± 0.1 | 4.8 ± 0.4 | 4.9 ± 0.4 |
| 34-21 | 20.8 ± 2.1 | - | 21.5 ± 0.6 | 21.4± 0.3 | 18.6 ± 1.7 | 15.0 ± 0.1 |
| 34-22 | >40 | - | >40 | >40 | >40 | 39.1 ± 2.0 |
| 34-23 | 23.8 ± 2.0 | - | 25.2 ± 1.0 | 23.3 ± 1.1 | 23.7 ± 1.1 | 22. 6 ± 0.3 |
| 34-24 | >20 | >20 | - | - | >20 | >20 |
| 34-25 | 20.6 ± 1.2 | - | 21.3 ± 1.3 | 22.4 ± 1.2 | 20. 8 ± 2.1 | 18.0 ± 1.0 |
| 34-26 | >40 | - | >40 | >40 | >40 | >40 |
| 34-27 | 18.6 ± 2.6 | - | 19.7 ± 2.0 | 20.6 ± 1.2 | 22.2± 1.8 | 19.8 ± 1.9 |
| 34-28 | 33.0 ± 2.1 | - | 32.4 ± 1.7 | 31.1 ± 0.8 | 23.2 ± 1.1 | 40.0 ± 1.0 |
| 34-29 | 23.8 ± 2.6 | - | 33.4 ± 1.7 | 29.8 ± 1.2 | 22.8 ± 1.7 | 22.6 ± 2.4 |
| 34-30 | >40 | - | >40 | >40 | >40 | >40 |
| 34-31 | 17. 3 ± 2.2 | - | 23. 1 ± 0.5 | 20.0 ± 0.7 | 16.2 ± 1.8 | 17.4 ± 1.9 |
| 34-32 | 16.5 ± 1.3 | - | 22.5 ± 0.8 | - | 8.71 ± 0.7 | 15.8 ± 0.8 |
| 34-33 | 40.9 ± 5.3 | - | >40 | >40 | 36.3 ± 1.0 | 29.3 ± 0.6 |
| 34-34 | >40 | - | >40 | >40 | >40 | >40 |
| 34-35 | 21.2 ± 0.1 | - | 24.8 ± 1.6 | 24.6 ± 1.0 | 18.7 ± 1.2 | 21.7± 0.6 |
| 34-36 | >40 | - | >40 | >40 | >40 | >40 |
| 34-37 | 23.8 ± 0.5 | - | 32.9 ± 1.0 | 22.6 ± 1.5 | 21.2 ± 0.1 | 19.2 ± 0.1 |
| 34-38 | 11.2 ± 0.7 | >20 | - | - | 21.1 ± 3.9 | 19.5 ± 8.2 |
| 34-39 | 29.7 ± 0.7 | - | 43.2 ± 1.8 | 32.0 ± 0.6 | 36.0 ± 0.4 | 45.1 ± 3.4 |
| 34-40 | 18.5 ± 0.5 | - | 17.9 ± 0.5 | 15.5± 0.6 | 13.7± 0.1 | 14.2 ± 0.5 |
| 34-41 | 22.9± 0.5 | - | 20.7 ± 2.1 | 20.5 ± 1.0 | 21.6 ± 0.1 | 24.4 ± 0.5 |
| 34-42 | >20 | >20 | - | - | >20 | >20 |
| 14-Acetyldelcosine (34-43) | >20 | >20 | - | - | >20 | >20 |
| 34-44 | >20 | >20 | - | - | >20 | >20 |
| 34-45 | >20 | >20 | - | - | >20 | >20 |
| 34-46 | >20 | >20 | - | - | >20 | >20 |
| 34-47 | >20 | >20 | - | >20 | >20 | >20 |
| 14-Acetylbrowniine (35) | >20 | >20 | - | - | >20 | >20 |
| PXL2 (nM) | 4.8 ± 0.6 | 5.9 ± 1.9 | 8.4 ± 0.8 | 10.2 ± 0.9 | 5.8 ± 0.2 | 2405.4 ± 44.8 |
| Cell Line/IC50 (μM) 1 | KB/KB-VIN Ratio | |||||
|---|---|---|---|---|---|---|
| Alkaloids | A549 | DU145 | MDA-MB-231 | KB | KB-VIN | |
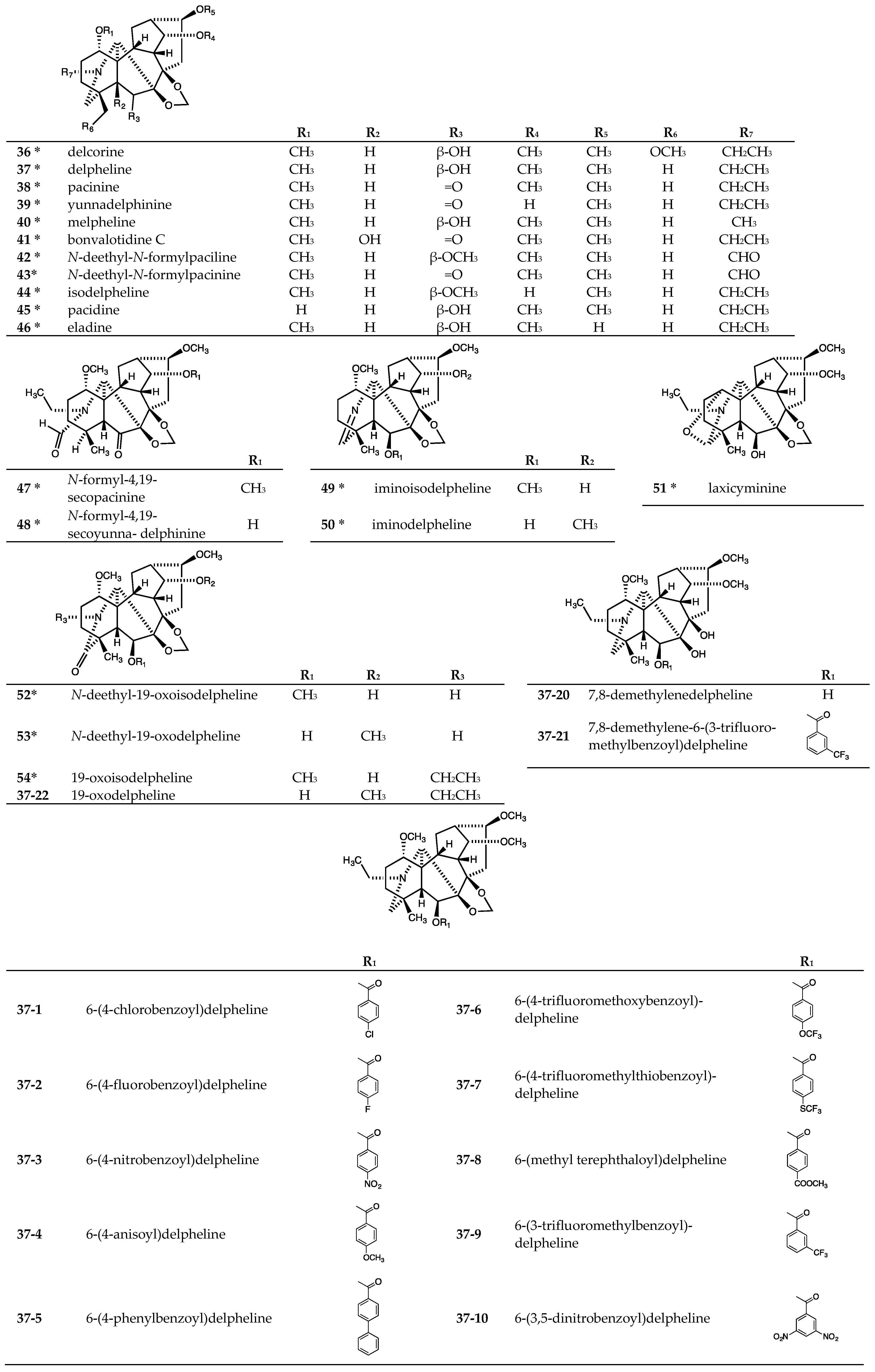
| Delcorine (36) | >40 | - | >40 | >40 | >40 | - |
| Delpheline (37) | >20 | >20 | - | >20 | >20 | - |
| Pacinine (38) | >20 | >20 | - | >20 | >20 | - |
| Yunnadelphinine (39) | >20 | >20 | - | >20 | >20 | - |
| Melpheline (40) | >40 | - | >40 | >40 | >40 | - |
| Bonvalotidine C (41) | >40 | - | >40 | >40 | >40 | - |
| N-Deethyl-N-formylpaciline (42) | >40 | - | >40 | >40 | >40 | - |
| N-Deethyl-N-formylpacinine (43) | >40 | - | >40 | >40 | >40 | - |
| Isodelpheline (44) | >40 | - | >40 | >40 | >40 | - |
| Pacidine (45) | >40 | - | >40 | >40 | >40 | - |
| Eladine (46) | >40 | - | >40 | >40 | >40 | - |
| N-Formyl-4,19-secopacinine (47) | >40 | - | >40 | >40 | >40 | - |
| N-Formyl-4,19-secoyunnadelphinine (48) | >40 | - | >40 | >40 | >40 | - |
| Iminoisodelpheline (49) | >40 | - | >40 | >40 | >40 | - |
| Iminodelpheline (50) | >40 | - | >40 | >40 | >40 | - |
| Laxicyminine (51) | >40 | - | >40 | >40 | >40 | - |
| N-Deethyl-19-oxoisodelpheline (52) | >40 | - | >40 | >40 | >40 | - |
| N-Deethyl-19-oxo-delpheline (53) | >40 | - | >40 | >40 | >40 | - |
| 19-Oxoisodelpheline (54) | >40 | - | >40 | >40 | >40 | - |
| 37-1 | 14.8 ± 3.8 | 7.4 ± 1.2 | - | 8.9 ± 2.0 | 8.3 ± 1.6 | 1.07 |
| 37-2 | 38.1 ± 11.8 | 15.6 ± 5.4 | - | 23.3 ± 3.9 | 15.0 ± 6.5 | 1.55 |
| 37-3 | 22.7 ± 0.3 | 17.2 ± 3.3 | - | 20.7 ± 0.9 | 17.7 ± 3.5 | 1.17 |
| 37-4 | >20 | >20 | - | >20 | >20 | - |
| 37-5 | 24.1 ± 2.7 | 17.1 ± 11.4 | - | 23.6 ± 0.4 | 17.4 ± 7.4 | 1.36 |
| 37-6 | 18.7 ± 6.6 | 20.3 ± 7.1 | - | 20.1 ± 7.6 | 18.9 ± 5.0 | 1.06 |
| 37-7 | 21.1 ± 9.2 | 16.6 ± 12.7 | - | 21.7 ± 11.6 | 17.9 ± 4.2 | 1.21 |
| 37-8 | 28.7 ± 13.6 | 28.7 ± 7.2 | - | 24.3 ± 5.7 | 23.3 ± 3.7 | 1.04 |
| 37-9 | 21.2 ± 4.7 | 12.6 ± 3.0 | - | 14.9 ± 4.9 | 11.9 ± 3.3 | 1.25 |
| 37-10 | 20.9 ± 4.3 | 22.7 ± 6.0 | - | 19.1 ± 4.8 | 20.3 ± 2.7 | 0.94 |
| 37-11 | 30.8 ± 13.3 | 28.9 ± 4.7 | - | 29.5 ± 3.5 | 27.5 ± 3.1 | 1.07 |
| 37-12 | 19.9 ± 10.1 | 16.9 ± 6.7 | - | 14.6 ± 7.1 | 6.80 ± 5.0 | 2.15 |
| 37-13 | 10.2 ± 2.6 | 15.1 ± 6.0 | - | 21.0 ± 9.4 | 9.10 ± 1.5 | 2.31 |
| 37-14 | 22.4 ± 7.1 | 22.8 ± 8.5 | - | 25.9 ± 9.3 | 24.2 ± 4.4 | 1.07 |
| 37-15 | 29.7 ± 11.6 | 29.0 ± 5.4 | - | 21.8 ± 1.4 | 18.7 ± 5.2 | 1.17 |
| 37-16 | 20.0 ± 0.9 | 15.6 ± 2.6 | - | 14.8 ± 3.3 | 6.5 ± 2.2 | 2.28 |
| 37-17 | 14.1 ± 2.9 | 13.2 ± 5.7 | - | 6.8 ± 1.7 | 4.2 ± 1.1 | 1.62 |
| 37-18 | 16.5 ± 2.2 | 11.3 ± 7.9 | - | 5.4 ± 1.8 | 4.4 ± 0.8 | 1.23 |
| 37-19 | 25.6 ± 1.2 | 19.8 ± 4.6 | - | 12.1 ± 7.8 | 4.7 ± 1.4 | 2.57 |
| 37-20 | >20 | >20 | - | >20 | >20 | - |
| 37-21 | >20 | >20 | - | >20 | >20 | - |
| 37-22 | >20 | >20 | - | >20 | >20 | - |
| PXL2 (nM) | 4.8 ± 0.6 | 5.9 ± 1.9 | 8.4 ± 0.8 | 5.8 ± 0.2 | 2405.4 ± 44.8 | - |
| Cell Line/IC50 (μM) 1 | ||||||
|---|---|---|---|---|---|---|
| Alkaloids | A549 | DU145 | MDA-MB-231 | MCF-7 | KB | KB-VIN |
| Aconicarchamine A (55) | >40 | - | >40 | >40 | >40 | >40 |
| Lucidusculine (57) | >20 | >20 | - | - | >20 | >20 |
| Flavadine (58) | >20 | >20 | - | - | >20 | >20 |
| 12-Acetyllucidusculine (59) | >20 | >20 | - | - | >20 | >20 |
| 1-Acetylluciculine (60) | >20 | >20 | - | - | >20 | >20 |
| Dehydrolucidusculine (61) | >20 | >20 | - | - | >20 | >20 |
| Dehydroluciculine (62) | >20 | >20 | - | - | >20 | >20 |
| 12-Acetyldehydrolucidusculine (63) | >20 | >20 | - | - | >20 | >20 |
| 56-1 | >20 | >20 | - | - | >20 | >20 |
| 56-2 | >20 | >20 | - | - | >20 | >20 |
| 56-3 | >20 | >20 | - | - | >20 | >20 |
| 56-4 | >20 | >20 | - | - | >20 | >20 |
| 56-5 | 23.3 ± 6.1 | 28.1 ± 11.1 | - | - | 31.8 ± 10.5 | 27.8 ± 1.9 |
| 56-6 | >20 | >20 | - | - | >20 | >20 |
| 56-7 | >20 | >20 | - | - | >20 | >20 |
| PXL2 (nM) | 4.8 ± 0.6 | 5.9 ± 1.9 | 8.4 ± 0.8 | 10.2 ± 0.9 | 5.8 ± 0.2 | 2405.4 ± 44.8 |
| Cell Line/IC50 (μM) 1 | KB/KB-VIN Ratio | ||||||
|---|---|---|---|---|---|---|---|
| Alkaloids | A549 | DU145 | MDA-MB-231 | MCF-7 | KB | KB-VIN | |
| Ryosenamine (64) | >40 | - | >40 | >40 | >40 | >40 | |
| 9-Hydroxynominine (65) | >40 | - | >40 | >40 | >40 | >40 | |
| Torokonine (66) | >40 | - | >40 | >40 | >40 | >40 | |
| Kobusine (67) | >20 | >20 | - | - | >20 | >20 | |
| 67-1 | >20 | >20 | - | - | >20 | >20 | |
| 67-2 | >20 | >20 | - | - | >20 | >20 | |
| 67-3 | >20 | >20 | - | - | >20 | >20 | |
| 67-4 | >20 | >20 | - | - | >20 | >20 | |
| 67-5 | 8.4 ± 1.4 | 9.3 ± 3.0 | - | - | 6.0 ± 0.8 | 7.5 ± 3.7 | 0.80 |
| 67-6 | >20 | >20 | - | - | >20 | >20 | |
| 67-7 | 6.7 ± 2.4 | 7.1 ± 2.0 | - | - | 5.3 ± 0.3 | 5.2 ± 1.2 | 1.02 |
| 67-8 | 19.5 ± 3.3 | 15.3 ± 5.6 | - | - | 13.9 ± 2.8 | 17.9 ± 1.8 | 0.78 |
| 67-9 | >20 | >20 | - | - | >20 | >20 | |
| 67-10 | 6.9 ± 1.7 | 7.0 ± 2.2 | - | - | 5.3 ± 0.6 | 5.5 ± 0.7 | 0.96 |
| 67-11 | >20 | >20 | - | - | >20 | >20 | |
| 67-12 | >20 | >20 | - | - | >20 | >20 | |
| 67-13 | 17.2 ± 0.9 | 13.2 ± 2.8 | - | - | 12.7 ± 1.1 | 14.1 ± 1.0 | 0.90 |
| 67-14 | 14.1 ± 0.7 | 9.6 ± 2.4 | - | - | 11.7 ± 0.6 | 10.9 ± 0.7 | 1.07 |
| 67-15 | >20 | >20 | - | - | >20 | >20 | |
| 67-16 | >20 | >20 | - | - | >20 | >20 | |
| 67-17 | >20 | >20 | - | - | >20 | >20 | |
| 67-18 | 8.1 ± 4.7 | 6.8 ± 2.0 | - | - | 5.2 ± 0.6 | 7.1 ± 2.6 | 0.73 |
| 67-19 | 5.5 ± 1.9 | 6.2 ± 3.1 | - | - | 4.1 ± 0.7 | 3.1 ± 1.6 | 1.32 |
| PXL2 (nM) | 4.8 ± 0.6 | 5.9 ± 1.9 | 8.4 ± 0.8 | 10.2 ± 0.9 | 5.8 ± 0.2 | 2405.4 ± 44.8 | 0.0067 |
| Cell Line/IC50 (μM) 1 | KB/KB-VIN Ratio | ||||
|---|---|---|---|---|---|
| Alkaloids | A549 | DU145 | KB | KB-VIN | |
| Pseudokobusine (68) | >20 | >20 | >20 | >20 | |
| 68-1 | >20 | >20 | >20 | >20 | |
| 68-2 | >20 | >20 | >20 | >20 | |
| 68-3 | >20 | >20 | >20 | >20 | |
| 68-4 | 19.3 ± 4.5 | 15.3 ± 4.3 | 12.8 ± 1.7 | 10.2 ± 0.9 | 1.25 |
| 68-5 | 8.8 ± 4.5 | 7.6 ± 2.5 | 5.2 ± 1.3 | 6.3 ± 0.6 | 0.83 |
| 68-6 | >20 | >20 | >20 | >20 | |
| 68-7 | 15.4 ± 3.7 | 13.2 ± 2.0 | 11.1 ± 5.5 | 15.7 ± 1.5 | 0.70 |
| 68-8 | >20 | >20 | >20 | >20 | |
| 68-9 | >20 | >20 | >20 | >20 | |
| 68-10 | 8.0 ± 5.1 | 15.3 ± 2.9 | 14.9 ± 3.6 | 20.1 ± 13.5 | 0.74 |
| 15-Veratroylpseudokobusine (68-11) | >20 | >20 | >20 | >20 | |
| 68-12 | 16.0 ± 5.5 | 16.9 ± 7.8 | 19.7 ± 3.1 | 14.7 ± 7.0 | 1.34 |
| 68-13 | 15.2 ± 6.4 | 16.6 ± 7.9 | 18.1 ± 4.3 | 12.2 ± 5.6 | 1.48 |
| 68-14 | >20 | >20 | >20 | >20 | |
| 68-15 | 5.8 ± 0.7 | 7.2 ± 1.9 | 6.4 ± 0.8 | 6.4 ± 1.8 | 1.00 |
| 68-16 | >20 | >20 | >20 | >20 | |
| 68-17 | >20 | >20 | >20 | >20 | |
| 68-18 | >20 | >20 | >20 | >20 | |
| 68-19 | 5.0 ± 1.1 | 5.2 ± 1.8 | 5.6 ± 1.2 | 5.6 ± 2.9 | 1.00 |
| 68-20 | 6.8 ± 0.7 | 7.7 ± 3.8 | 8.9 ± 3.7 | 6.2 ± 1.3 | 1.44 |
| 68-21 | >20 | >20 | >20 | >20 | |
| 68-22 | 17.9 ± 7.2 | 14.5 ± 7.2 | 15.7 ± 4.1 | 13.9 ± 3.3 | 1.13 |
| 68-23 | >20 | >20 | >20 | >20 | |
| 68-24 | 8.4 ± 1.7 | 6.5 ± 0.5 | 7.0 ± 1.3 | 6.4 ± 0.9 | 1.09 |
| 68-25 | >20 | >20 | >20 | >20 | |
| 68-26 | >20 | >20 | >20 | >20 | |
| 68-27 | >20 | >20 | >20 | >20 | |
| 68-28 | >20 | >20 | >20 | >20 | |
| 68-29 | >20 | >20 | >20 | >20 | |
| 68-30 | >20 | >20 | >20 | >20 | |
| 68-31 | >20 | >20 | >20 | >20 | |
| 68-32 | 6.4 ± 1.2 | 6.0 ± 3.3 | 6.6 ± 3.1 | 5.2 ± 1.0 | 1.27 |
| 68-33 | >20 | >20 | >20 | >20 | |
| 68-34 | >20 | >20 | >20 | >20 | |
| 68-35 | >20 | >20 | >20 | >20 | |
| 68-36 | >20 | >20 | >20 | >20 | |
| 68-37 | >20 | >20 | >20 | >20 | |
| PXL2 (nM) | 4.8 ± 0.6 | 5.9 ± 1.9 | 5.8 ± 0.2 | 2405.4 ± 44.8 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, K.; Yamashita, H. Cytotoxic Effects of Diterpenoid Alkaloids Against Human Cancer Cells. Molecules 2019, 24, 2317. https://doi.org/10.3390/molecules24122317
Wada K, Yamashita H. Cytotoxic Effects of Diterpenoid Alkaloids Against Human Cancer Cells. Molecules. 2019; 24(12):2317. https://doi.org/10.3390/molecules24122317
Chicago/Turabian StyleWada, Koji, and Hiroshi Yamashita. 2019. "Cytotoxic Effects of Diterpenoid Alkaloids Against Human Cancer Cells" Molecules 24, no. 12: 2317. https://doi.org/10.3390/molecules24122317
APA StyleWada, K., & Yamashita, H. (2019). Cytotoxic Effects of Diterpenoid Alkaloids Against Human Cancer Cells. Molecules, 24(12), 2317. https://doi.org/10.3390/molecules24122317





