Antiplasmodial Activity and In Vivo Bio-Distribution of Chloroquine Molecules Released with a 4-(4-Ethynylphenyl)-Triazole Moiety from Organometallo-Cobalamins
Abstract
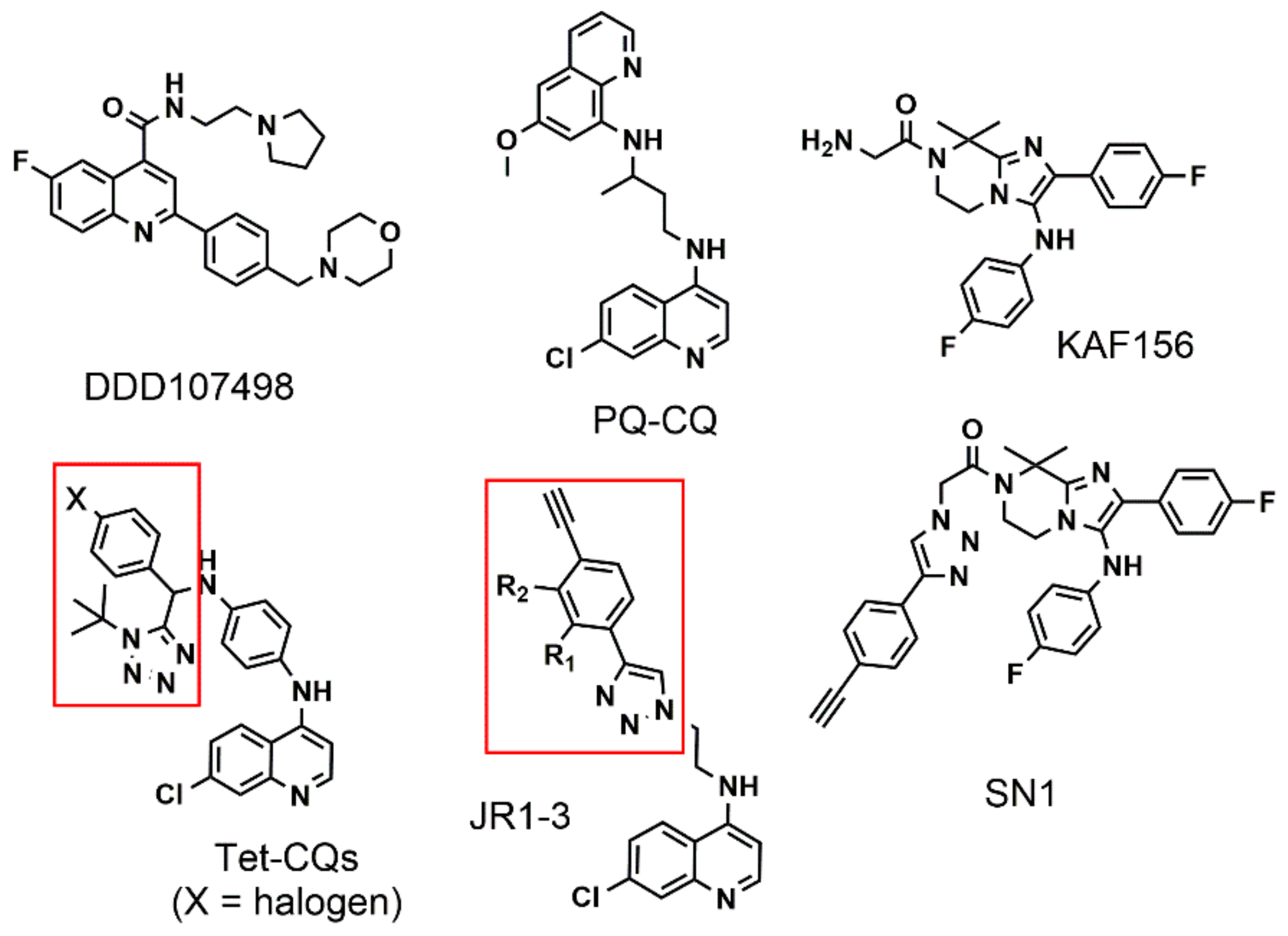
1. Introduction
2. Results and Discussion
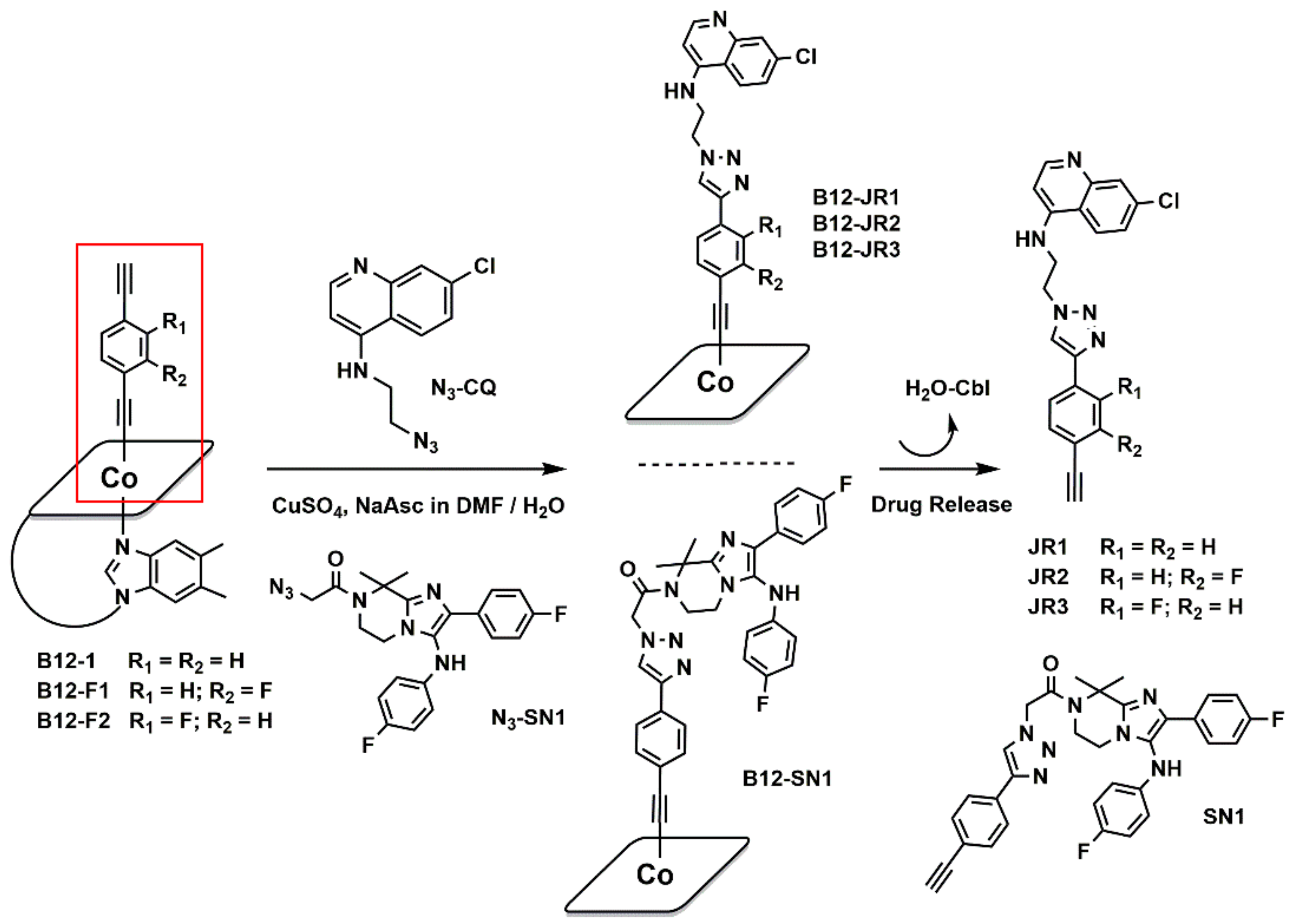
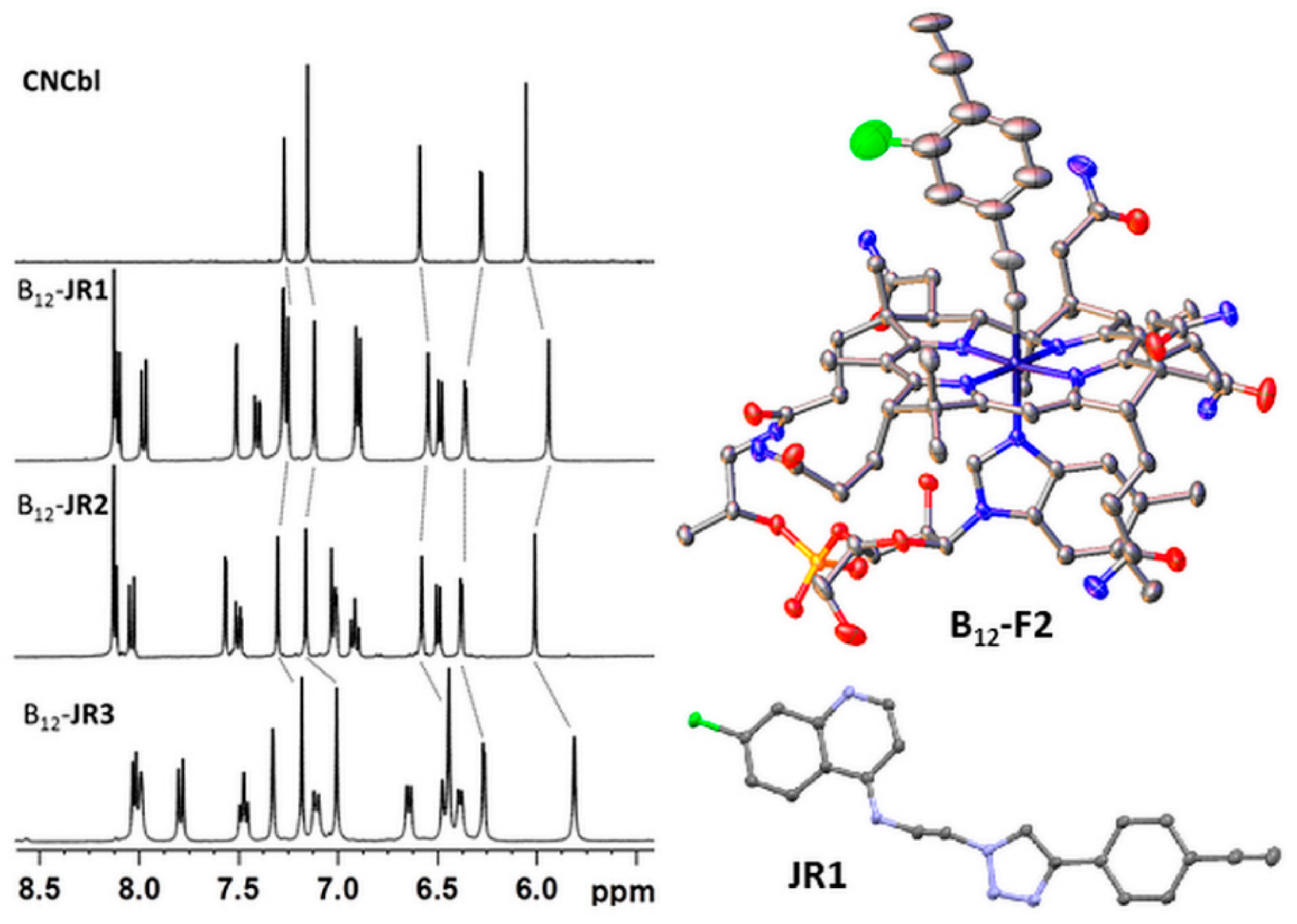
2.1. Synthesis and Characterization
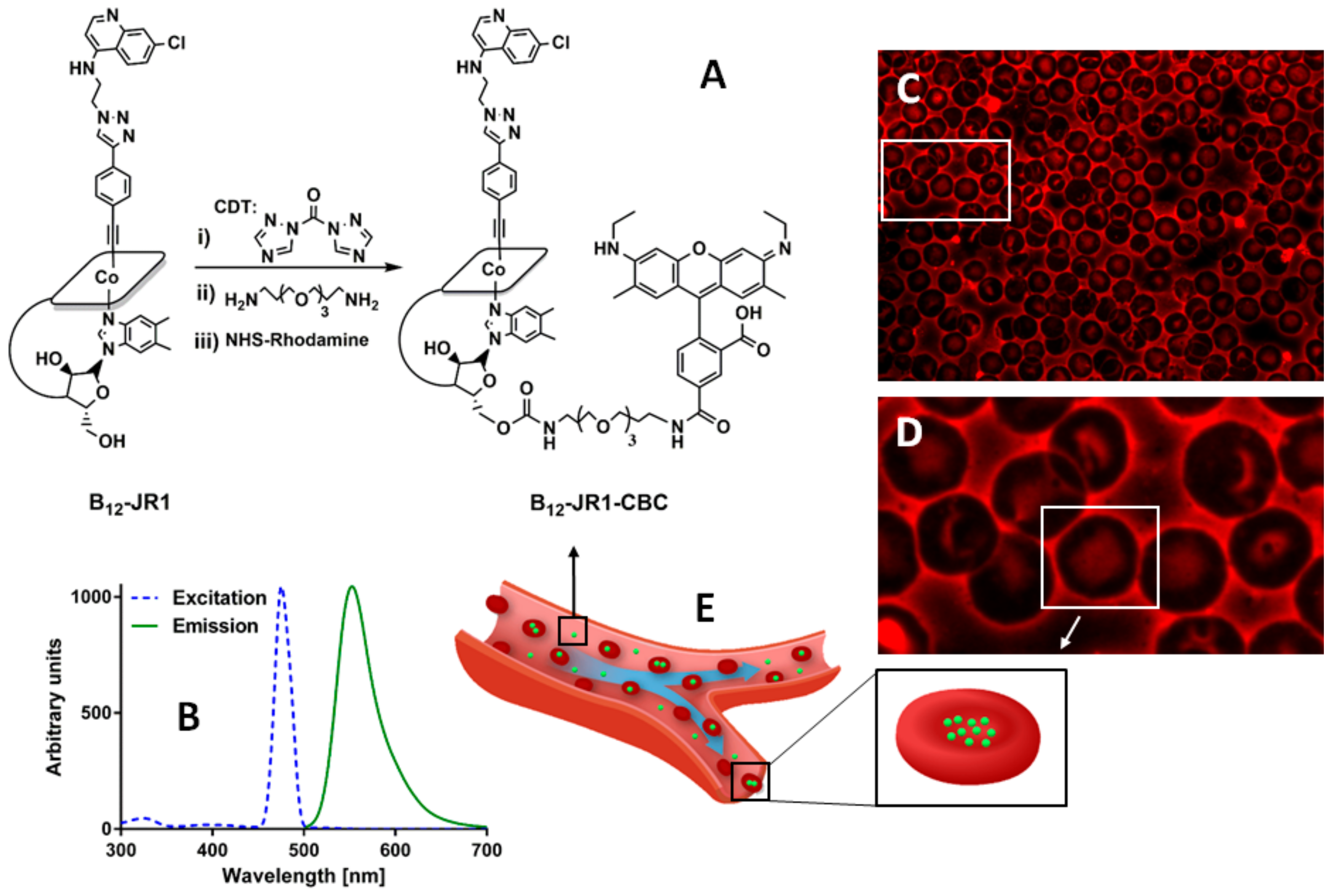
2.2. In Vitro Bio-Distribution and Antiplasmodial Activity
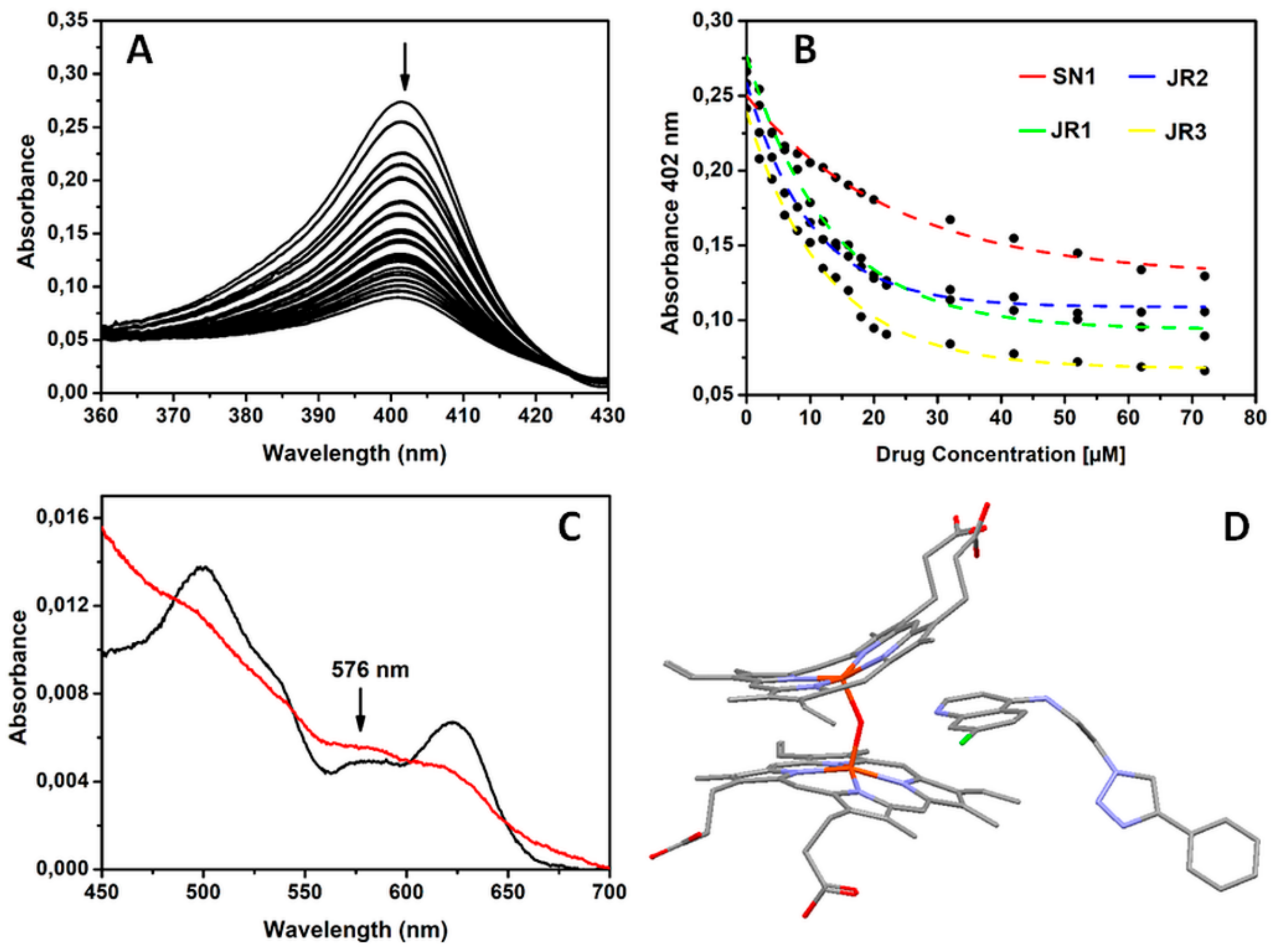
2.3. Drugs Interaction with Monomeric Ferriprotoporphyrin IX (PPIX)
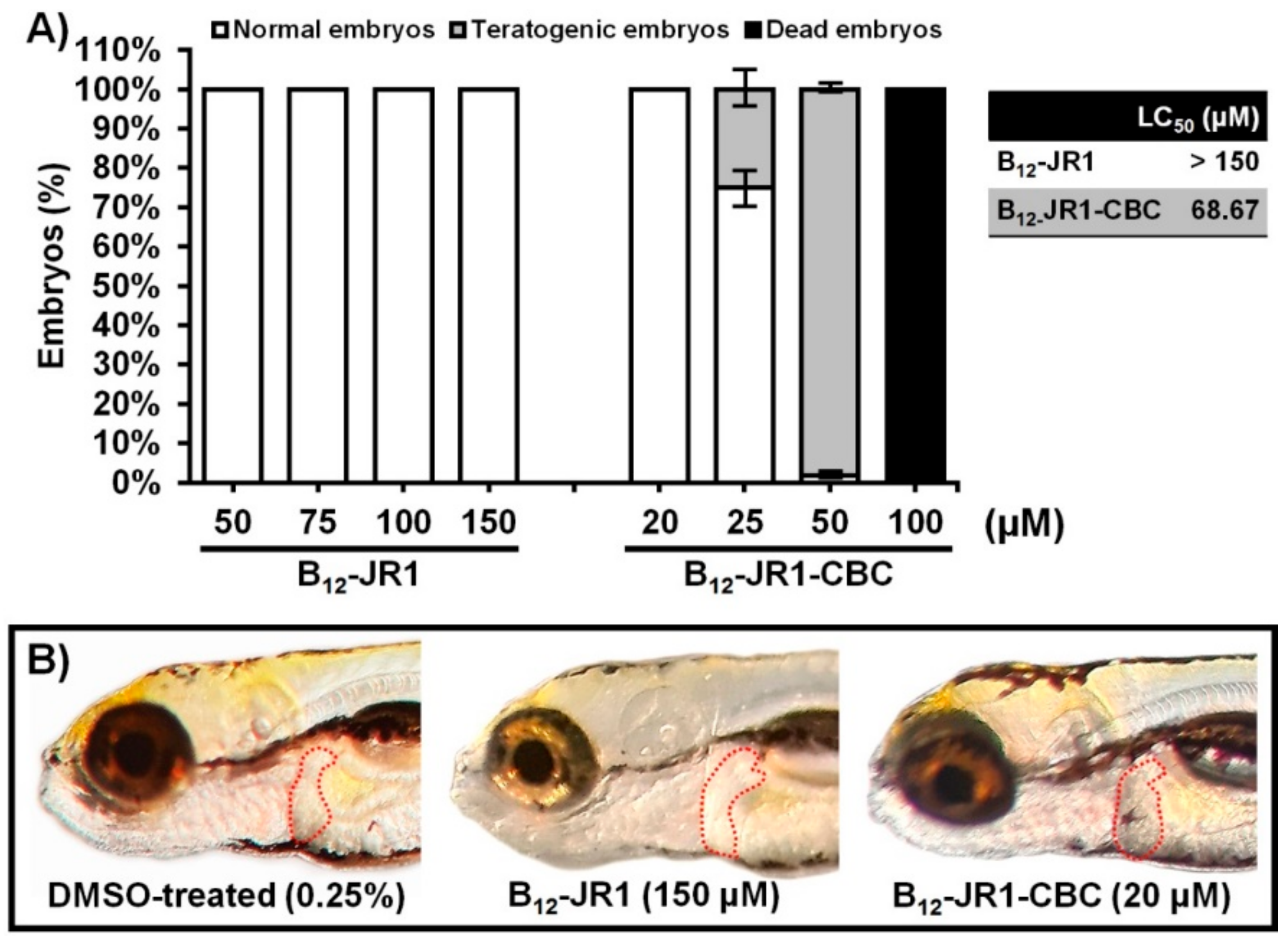
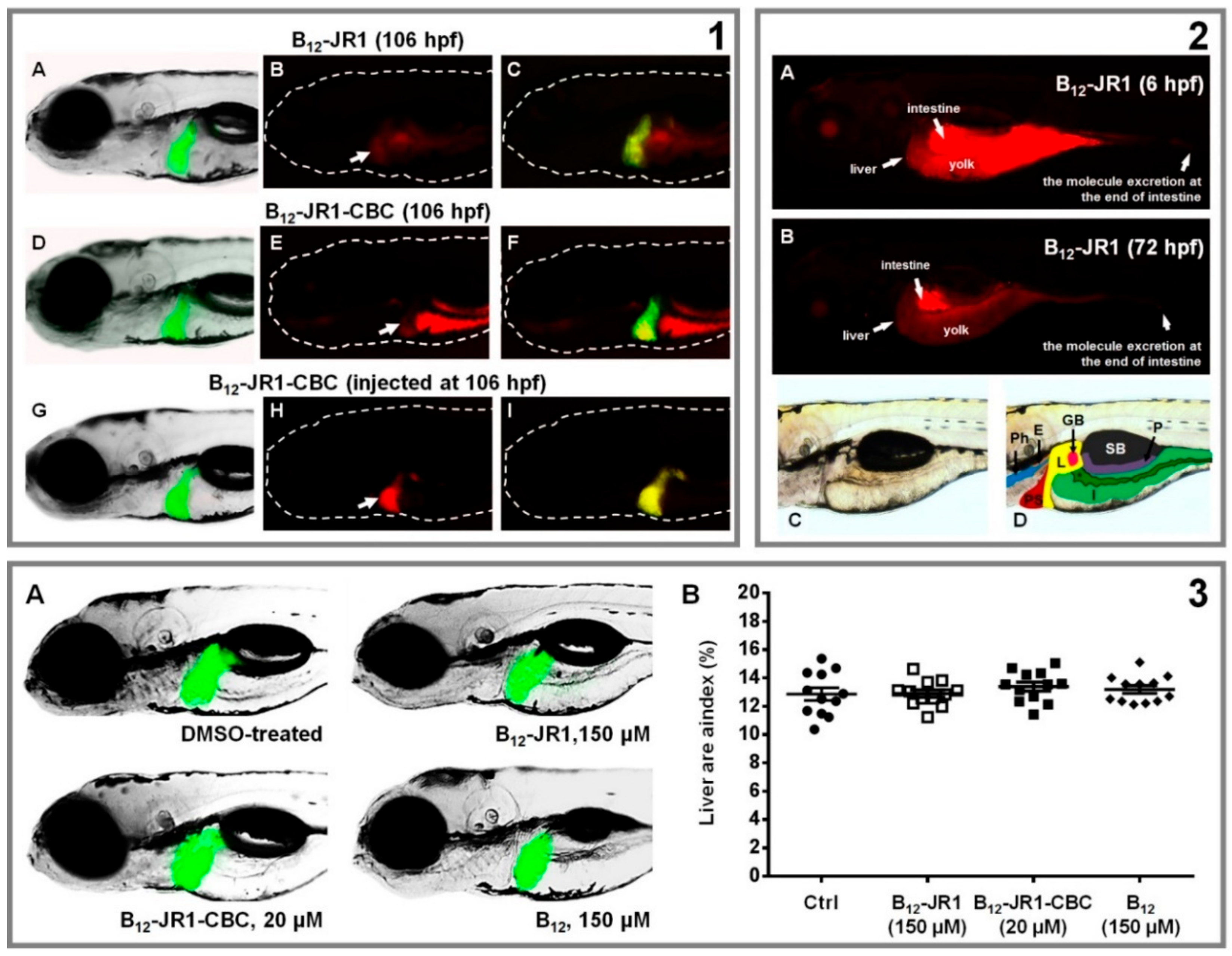
2.4. In Vivo Toxicity and Bio-Distribution
3. Materials and Methods
3.1. General Experimental Details
3.2. Instrumentation
3.3. Computational Details
3.4. General Synthesis Procedures
3.5. In Vitro Cytotoxicity Assay
3.6. In Vitro Antiplasmodial Assay
3.7. In Vitro Blood Assays
3.8. In Vivo Zebrafish Assays Toxicity
3.9. Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO. World Malaria Report 2018; World Health Organization: Geneva, Switzerland, 2018; Licence: CC BY-NC-SA 2013.2010 IGO. [Google Scholar]
- Alonso, P.L.; Brown, G.; Arevalo-Herrera, M.; Binka, F.; Chitnis, C.; Collins, F.; Doumbo, O.K.; Greenwood, B.; Hall, B.F.; Levine, M.M.; et al. A research agenda to underpin malaria eradication. PLoS Med. 2011, 8, e1000406. [Google Scholar] [CrossRef] [PubMed]
- Kappe, S.H.; Vaughan, A.M.; Boddey, J.A.; Cowman, A.F. That was then but this is now: Malaria research in the time of an eradication agenda. Science 2010, 328, 862–866. [Google Scholar] [CrossRef] [PubMed]
- Kilama, W.; Ntoumi, F. Malaria: A research agenda for the eradication era. Lancet 2009, 374, 1480–1482. [Google Scholar] [CrossRef]
- Flannery, E.L.; Chatterjee, A.K.; Winzeler, E.A. Antimalarial drug discovery - approaches and progress towards new medicines. Nat. Rev. Microbiol. 2013, 11, 849–862. [Google Scholar] [CrossRef] [PubMed]
- Baragaña, B.; Hallyburton, I.; Lee, M.C.S.; Norcross, N.R.; Grimaldi, R.; Otto, T.D.; Proto, W.R.; Blagborough, A.M.; Meister, S.; Wirjanata, G.; et al. A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 2015, 522, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Baragaña, B.; Norcross, N.R.; Wilson, C.; Porzelle, A.; Hallyburton, I.; Grimaldi, R.; Osuna-Cabello, M.; Norval, S.; Riley, J.; Stojanovski, L.; et al. Discovery of a quinoline-4-carboxamide derivative with a novel mechanism of action, multistage antimalarial activity, and potent in vivo efficacy. J. Med. Chem. 2016, 59, 9672–9685. [Google Scholar] [CrossRef] [PubMed]
- Keough, D.T.; Rejman, D.; Pohl, R.; Zborníková, E.; Hocková, D.; Croll, T.; Edstein, M.D.; Birrell, G.W.; Chavchich, M.; Naesens, L.M.J.; et al. Design of plasmodium vivax hypoxanthine-guanine phosphoribosyltransferase inhibitors as potential antimalarial therapeutics. ACS Chem. Biol. 2018, 13, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Muregi, F.W.; Ishih, A. Next-generation antimalarial drugs: Hybrid molecules as a new strategy in drug design. Drug Dev. Res. 2010, 71, 20–32. [Google Scholar] [CrossRef]
- Lodige, M.; Lewis, M.D.; Paulsen, E.S.; Esch, H.L.; Pradel, G.; Lehmann, L.; Brun, R.; Bringmann, G.; Mueller, A.K. A primaquine-chloroquine hybrid with dual activity against Plasmodium liver and blood stages. Int. J. Med. Microbiol. 2013, 303, 539–547. [Google Scholar] [CrossRef]
- Rossier, J.; Hauser, D.; Kottelat, E.; Rothen-Rutishauser, B.; Zobi, F. Organometallic cobalamin anticancer derivatives for targeted prodrug delivery via transcobalamin-mediated uptake. Dalton Trans. 2017, 46, 2159–2164. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, P.; Konig, C.; Ferrari, S.; Alberto, R. Vitamin B(1)(2) as a carrier for targeted platinum delivery: In vitro cytotoxicity and mechanistic studies. J. Biol. Inorg. Chem. 2011, 16, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Zelder, F. Recent trends in the development of vitamin B12 derivatives for medicinal applications. Chem. Commun. 2015, 51, 14004–14017. [Google Scholar] [CrossRef] [PubMed]
- Nagle, A.; Wu, T.; Kuhen, K.; Gagaring, K.; Borboa, R.; Francek, C.; Chen, Z.; Plouffe, D.; Lin, X.; Caldwell, C.; et al. Imidazolopiperazines: Lead optimization of the second-generation antimalarial agents. J. Med. Chem. 2012, 55, 4244–4273. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Agarwal, P.; Srivastava, K.; Rajakumar, S.; Puri, S.K.; Verma, P.; Saxena, J.K.; Sharma, A.; Lal, J.; Chauhan, P.M. Synthesis and bioevaluation of novel 4-aminoquinoline-tetrazole derivatives as potent antimalarial agents. Eur. J. Med. Chem. 2013, 66, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Staines, H.M.; Krishna, S. Treatment and Prevention of Malaria: Antimalarial Drug Chemistry, Action and Use; Springer: Basel, Switzerland, 2012. [Google Scholar]
- Mushtaque, M.; Shahjahan. Reemergence of chloroquine (CQ) analogs as multi-targeting antimalarial agents: A review. Eur. J. Med. Chem. 2015, 90, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, M.; Barteselli, A.; Basilico, N.; Parapini, S.; Taramelli, D.; Sparatore, A. Synthesis and antiplasmodial activity of new heteroaryl derivatives of 7-chloro-4-aminoquinoline. Bioorg. Med. Chem. 2012, 20, 5965–5979. [Google Scholar] [CrossRef] [PubMed]
- Chromiński, M.; Lewalska, A.; Karczewski, M.; Gryko, D. Vitamin B12 Derivatives for Orthogonal Functionalization. J. Org. Chem. 2014, 79, 7532–7542. [Google Scholar] [CrossRef]
- Chemaly, S.M.; Chen, C.T.; van Zyl, R.L. Naturally occurring cobalamins have antimalarial activity. J. Inorg. Biochem. 2007, 101, 764–773. [Google Scholar] [CrossRef]
- Retief, F.P.; Gottlieb, C.W.; Herbert, V. Mechanism of Vitamin B12 uptake by erythrocytes. J. Clin. Invest. 1966, 45, 1907–1915. [Google Scholar] [CrossRef]
- Herbert, V.; Sullivan, L.W. Activity of coenzyme B12 in man. Ann. N. Y. Acad. Sci. 1964, 112, 855–870. [Google Scholar] [CrossRef]
- Tisman, G.; Vu, T.; Amin, J.; Luszko, G.; Brenner, M.; Ramos, M.; Flener, V.; Cordts, V.; Bateman, R.; Malkin, S.; et al. Measurement of red blood cell-vitamin B12: A study of the correlation between intracellular B12 content and concentrations of plasma holotranscobalamin II. Am. J. Hematol. 1993, 43, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Schuster, F.L. Cultivation of pathogenic and opportunistic free-living amebas. Clin. Microbiol. Rev. 2002, 15, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Krungkrai, J.; Webster, H.K.; Yuthavong, Y. Characterization of cobalamin-dependent methionine synthase purified from the human malarial parasite, Plasmodium falciparum. Parasitol. Res. 1989, 75, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Kirk, K. Membrane transport in the malaria-infected erythrocyte. Physiol. Rev. 2001, 81, 495–537. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, H.; Stein, W.D. How many functional transport pathways does Plasmodium falciparum induce in the membrane of its host erythrocyte? Trends Parasitol. 2005, 21, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Saliba, K.J.; Kirk, K. Nutrient acquisition by intracellular apicomplexan parasites: Staying in for dinner. Int. J. Parasitol. 2001, 31, 1321–1330. [Google Scholar] [CrossRef]
- Chrominski, M.; Gryko, D. “Clickable” vitamin B12 derivative. Chem. Eur. J. 2013, 19, 5141–5148. [Google Scholar] [CrossRef] [PubMed]
- Chrominski, M.; Lewalska, A.; Gryko, D. Reduction-free synthesis of stable acetylide cobalamins. Chem. Commun. 2013, 49, 11406–11408. [Google Scholar] [CrossRef]
- Njogu, P.M.; Gut, J.; Rosenthal, P.J.; Chibale, K. Design, synthesis, and antiplasmodial activity of hybrid compounds Based on (2R,3S)-N-Benzoyl-3-phenylisoserine. ACS Med. Chem. Lett. 2013, 4, 637–641. [Google Scholar] [CrossRef]
- Ruetz, M.; Salchner, R.; Wurst, K.; Fedosov, S.; Kräutler, B. Phenylethynylcobalamin: A light-stable and thermolysis-resistant organometallic Vitamin B12 derivative prepared by radical synthesis. Angew. Chem. Int. Ed. 2013, 52, 11406–11409. [Google Scholar] [CrossRef]
- Vagianou, C.-D.; Stuhr-Hansen, N.; Moll, K.; Bovin, N.; Wahlgren, M.; Blixt, O. ABO blood group antigen decorated giant unilamellar vesicles exhibit distinct interactions with plasmodium falciparum infected red blood cells. ACS Chem. Biol. 2018, 13, 2421–2426. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N.; Grissom, C.B.; Fedosova, N.U.; Moestrup, S.K.; Nexø, E.; Petersen, T.E. Application of a fluorescent cobalamin analogue for analysis of the binding kinetics. FEBS J. 2006, 273, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Slater, A.F.G.; Cerami, A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature 1992, 355, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.J.; Ross, D.C.; Adams, P.A. Quinoline anti-malarial drugs inhibit spontaneous formation of β-haematin (malaria pigment). FEBS Lett. 1994, 352, 54–57. [Google Scholar] [CrossRef]
- Dorn, A.; Stoffel, R.; Matile, H.; Bubendorf, A.; Ridley, R.G. Malarial haemozoin/β-haematin supports haem polymerization in the absence of protein. Nature 1995, 374, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Combrinck, J.M.; Mabotha, T.E.; Ncokazi, K.K.; Ambele, M.A.; Taylor, D.; Smith, P.J.; Hoppe, H.C.; Egan, T.J. Insights into the Role of heme in the mechanism of action of antimalarials. ACS Chem. Biol. 2013, 8, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Mehrotra, S.; Sharma, A.; Chugh, M.; Pandey, R.; Kaushik, A.; Khurana, S.; Srivastava, N.; Srivastava, T.; Deshmukh, A.; et al. Exploring heme and hemoglobin binding regions of plasmodium heme detoxification protein for new antimalarial discovery. J. Med. Chem. 2017, 60, 8298–8308. [Google Scholar] [CrossRef] [PubMed]
- Dorn, A.; Vippagunta, S.R.; Matile, H.; Jaquet, C.; Vennerstrom, J.L.; Ridley, R.G. An assessment of drug-haematin binding as a mechanism for inhibition of haematin polymerisation by quinoline antimalarials. Biochem. Pharmacol. 1998, 55, 727–736. [Google Scholar] [CrossRef]
- De Dios, A.C.; Tycko, R.; Ursos, L.M.B.; Roepe, P.D. NMR studies of chloroquine−ferriprotoporphyrin IX complex. J. Phys. Chem. A 2003, 107, 5821–5825. [Google Scholar] [CrossRef]
- Crespo, M.P.; Tilley, L.; Klonis, N. Solution behavior of hematin under acidic conditions and implications for its interactions with chloroquine. J. Biol. Inorg. Chem. 2010, 15, 1009–1022. [Google Scholar] [CrossRef]
- Sullivan, D.J., Jr.; Gluzman, I.Y.; Russell, D.G.; Goldberg, D.E. On the molecular mechanism of chloroquine’s antimalarial action. Proc. Natl. Acad. Sci. USA 1996, 93, 11865–11870. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, D.J., Jr.; Matile, H.; Ridley, R.G.; Goldberg, D.E. A common mechanism for blockade of heme polymerization by antimalarial quinolines. J. Biol. Chem. 1998, 273, 31103–31107. [Google Scholar] [CrossRef] [PubMed]
- Leed, A.; DuBay, K.; Ursos, L.M.B.; Sears, D.; de Dios, A.C.; Roepe, P.D. Solution structures of antimalarial drug−heme complexes. Biochemistry 2002, 41, 10245–10255. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, J.K.; Alumasa, J.N.; Yearick, K.; Ekoue-Kovi, K.A.; Casabianca, L.B.; de Dios, A.C.; Wolf, C.; Roepe, P.D. 4-N-, 4-S-, and 4-O-Chloroquine analogues: influence of side chain length and quinolyl nitrogen pka on activity vs chloroquine resistant malaria. J. Med. Chem. 2008, 51, 3466–3479. [Google Scholar] [CrossRef] [PubMed]
- Buller, R.; Peterson, M.L.; Almarsson, O.; Leiserowitz, L. Quinoline binding site on malaria pigment crystal: A rational pathway for antimalaria drug design. Cryst. Growth Des. 2002, 2, 553–562. [Google Scholar] [CrossRef]
- Egan, T.J.; Hunter, R.; Kaschula, C.H.; Marques, H.M.; Misplon, A.; Walden, J. Structure−function relationships in aminoquinolines: Effect of amino and chloro groups on quinoline−hematin complex formation, inhibition of β-hematin formation, and antiplasmodial activity. J. Med. Chem. 2000, 43, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Egan, T.J.; Mavuso, W.W.; Ross, D.C.; Marques, H.M. Thermodynamic factors controlling the interaction of quinoline antimalarial drugs with ferriprotoporphyrin IX. J. Inorg. Biochem. 1997, 68, 137–145. [Google Scholar] [CrossRef]
- O’Keeffe, D.H.; Barlow, C.H.; Smythe, G.A.; Fuchsman, W.H.; Moss, T.H.; Lilienthal, H.R.; Caughey, W.S. Magnetic and spectroscopic probes for FeOFe linkages in hemin systems. Bioinorg. Chem. 1975, 5, 125–147. [Google Scholar] [CrossRef]
- Bowman, T.V.; Zon, L.I. Swimming into the future of drug discovery: in vivo chemical screens in zebrafish. ACS Chem. Biol. 2010, 5, 159–161. [Google Scholar] [CrossRef]
- Selakovic, Z.; Tran, J.P.; Kota, K.P.; Lazic, M.; Retterer, C.; Besch, R.; Panchal, R.G.; Soloveva, V.; Sean, V.A.; Jay, W.B.; et al. Second generation of diazachrysenes: Protection of Ebola virus infected mice and mechanism of action. Eur. J. Med. Chem. 2019, 162, 32–50. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. Density functionals with broad applicability in chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals. J. Chem. Phys. 1985, 82, 299–310. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab initio effective core potentials for molecular calculations. Potentials for main group elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- Hansen, M.B.; Nielsen, S.E.; Berg, K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J. Immunol. Methods 1989, 119, 203–210. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malaria parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar] [CrossRef]
- Makler, M.T.; Ries, J.M.; Williams, J.A.; Bancroft, J.E.; Piper, R.C.; Gibbins, B.L.; Hinrichs, D.J. Parasite lactate dehydrogenase as an assay for Plasmodium falciparum drug sensitivity. Am. J. Trop. Med. Hyg. 1993, 48, 739–741. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Zhang, Y.; Han, L.W.; He, Q.X.; Chen, W.Y.; Sun, C.; Wang, X.; Chen, X.Q.; Wang, R.C.; Hsiao, C.D.; Liu, K.C. A rapid assessment for predicting drug-induced hepatotoxicity using zebrafish. J. Pharmacol. Tox. Met. 2017, 84, 102–110. [Google Scholar] [CrossRef] [PubMed]
- He, J.H.; Guo, S.Y.; Zhu, F.; Zhu, J.J.; Chen, Y.X.; Huang, C.J.; Gao, J.M.; Dong, Q.X.; Xuan, Y.X.; Li, C.Q. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J. Pharmacol. Tox. Met. 2013, 67, 25–32. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | NF54 IC50 ± SD (µM) | K1 IC50 ± SD (µM) | Resistance Index (RI) a |
|---|---|---|---|
| JR1 | 0.14 ± 0.01 | 0.18 ± 0.02 | 1.3 |
| JR2 | 0.15 ± 0.01 | 0.16 ± 0.01 | 1.1 |
| JR3 | 0.19 ± 0.02 | 0.21 ± 0.02 | 1.1 |
| B12-JR1 | 6.27 ± 0.37 | 9.18 ± 0.85 | 1.5 |
| B12-JR2 | 4.73 ± 0.32 | 6.02 ± 0.71 | 1.3 |
| B12-JR3 | 9.68 ± 0.73 | >15 | >1.6 |
| SN1 | na b | nd c | nd |
| B12-SN1 | na | nd | nd |
| CQ | 0.013 ± 0.001 | 0.40 ± 0.04 | 30.8 |
| IC50 (µM) a | LC50 (µM) b | Ti (LC50/IC50) | |||
|---|---|---|---|---|---|
| NF54 | K1 | NF54 | K1 | ||
| B12-JR1 | 6.3 | 9.2 | >150 | >23.9 | >16.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossier, J.; Nasiri Sovari, S.; Pavic, A.; Vojnovic, S.; Stringer, T.; Bättig, S.; Smith, G.S.; Nikodinovic-Runic, J.; Zobi, F. Antiplasmodial Activity and In Vivo Bio-Distribution of Chloroquine Molecules Released with a 4-(4-Ethynylphenyl)-Triazole Moiety from Organometallo-Cobalamins. Molecules 2019, 24, 2310. https://doi.org/10.3390/molecules24122310
Rossier J, Nasiri Sovari S, Pavic A, Vojnovic S, Stringer T, Bättig S, Smith GS, Nikodinovic-Runic J, Zobi F. Antiplasmodial Activity and In Vivo Bio-Distribution of Chloroquine Molecules Released with a 4-(4-Ethynylphenyl)-Triazole Moiety from Organometallo-Cobalamins. Molecules. 2019; 24(12):2310. https://doi.org/10.3390/molecules24122310
Chicago/Turabian StyleRossier, Jeremie, Sara Nasiri Sovari, Aleksandar Pavic, Sandra Vojnovic, Tameryn Stringer, Sarah Bättig, Gregory S. Smith, Jasmina Nikodinovic-Runic, and Fabio Zobi. 2019. "Antiplasmodial Activity and In Vivo Bio-Distribution of Chloroquine Molecules Released with a 4-(4-Ethynylphenyl)-Triazole Moiety from Organometallo-Cobalamins" Molecules 24, no. 12: 2310. https://doi.org/10.3390/molecules24122310
APA StyleRossier, J., Nasiri Sovari, S., Pavic, A., Vojnovic, S., Stringer, T., Bättig, S., Smith, G. S., Nikodinovic-Runic, J., & Zobi, F. (2019). Antiplasmodial Activity and In Vivo Bio-Distribution of Chloroquine Molecules Released with a 4-(4-Ethynylphenyl)-Triazole Moiety from Organometallo-Cobalamins. Molecules, 24(12), 2310. https://doi.org/10.3390/molecules24122310










