Different Role of Bisulfite/Sulfite in UVC-S(IV)-O2 System for Arsenite Oxidation in Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedures
2.3. Analysis
3. Results and Discussion
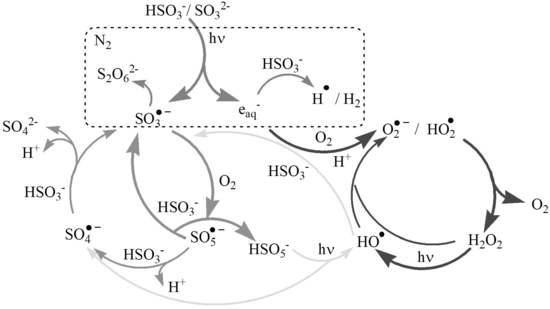
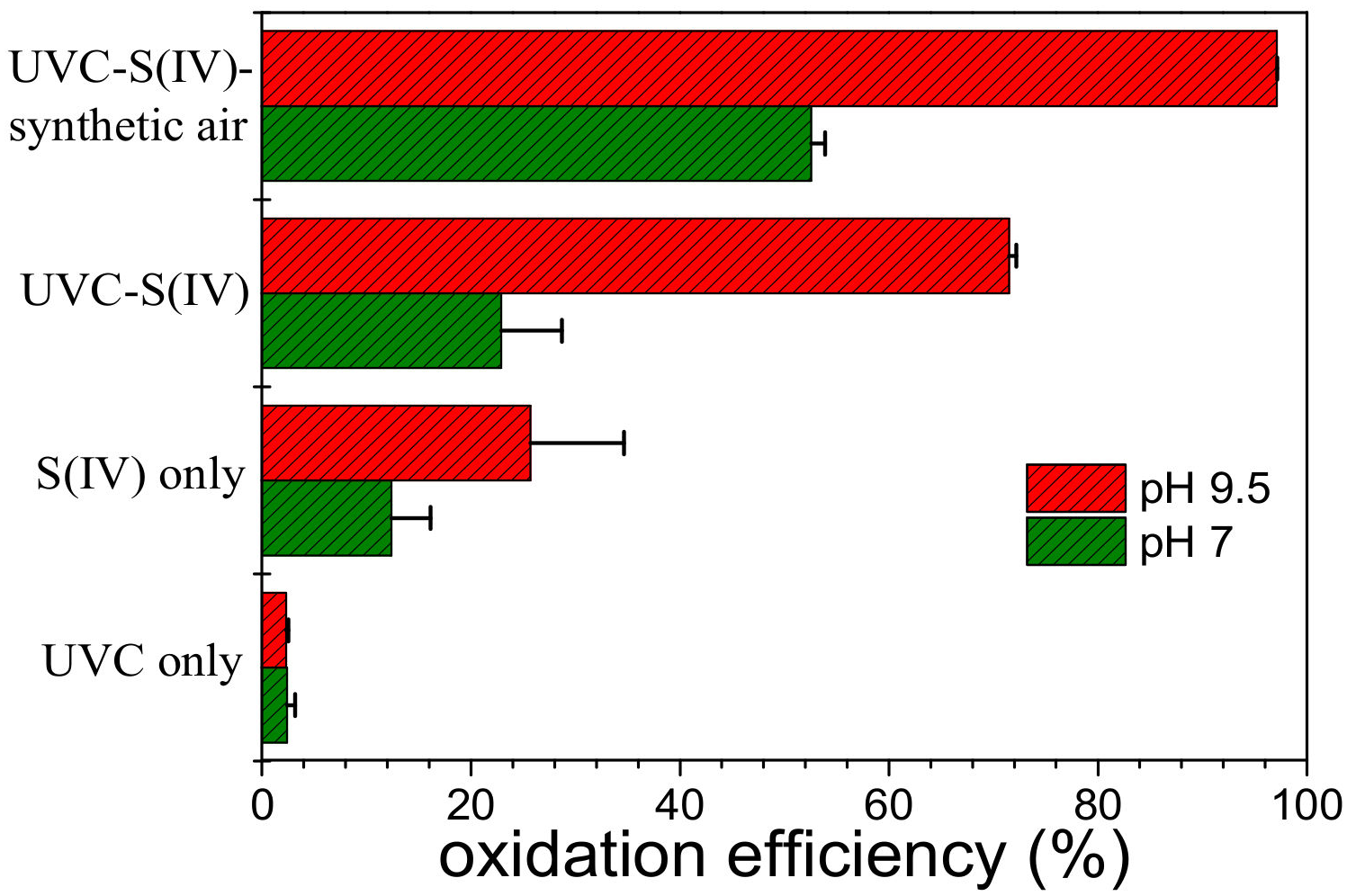
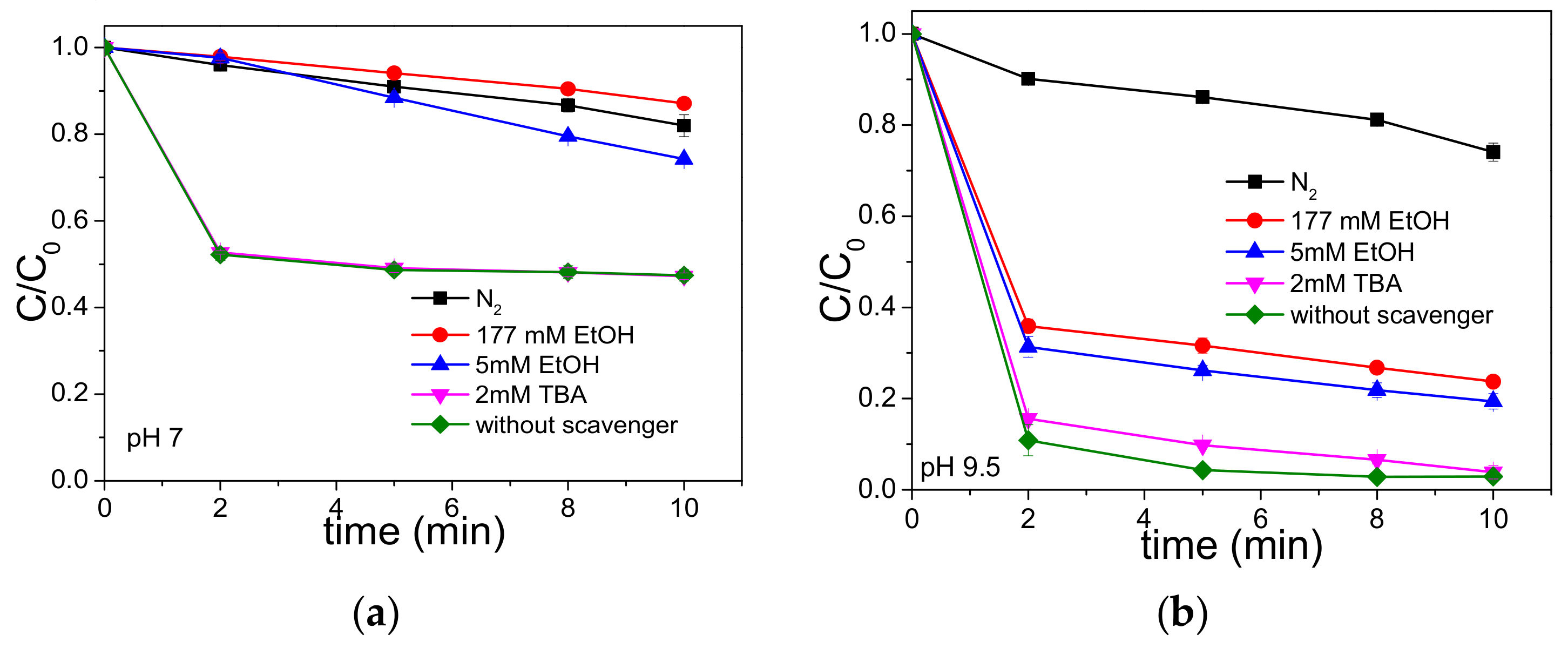
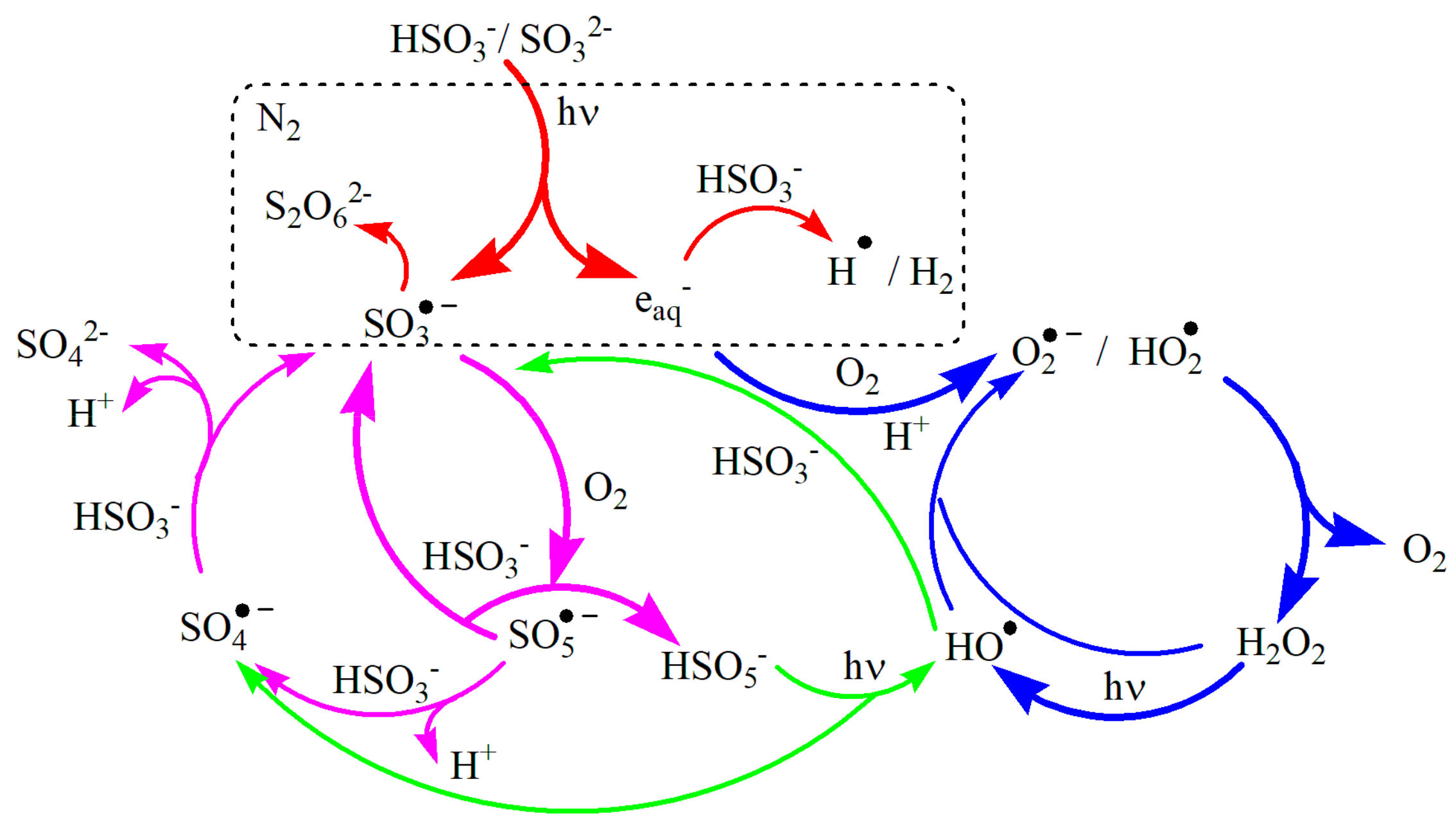
3.1. As(III) Oxidation in the UVC-S(IV)-O2 System
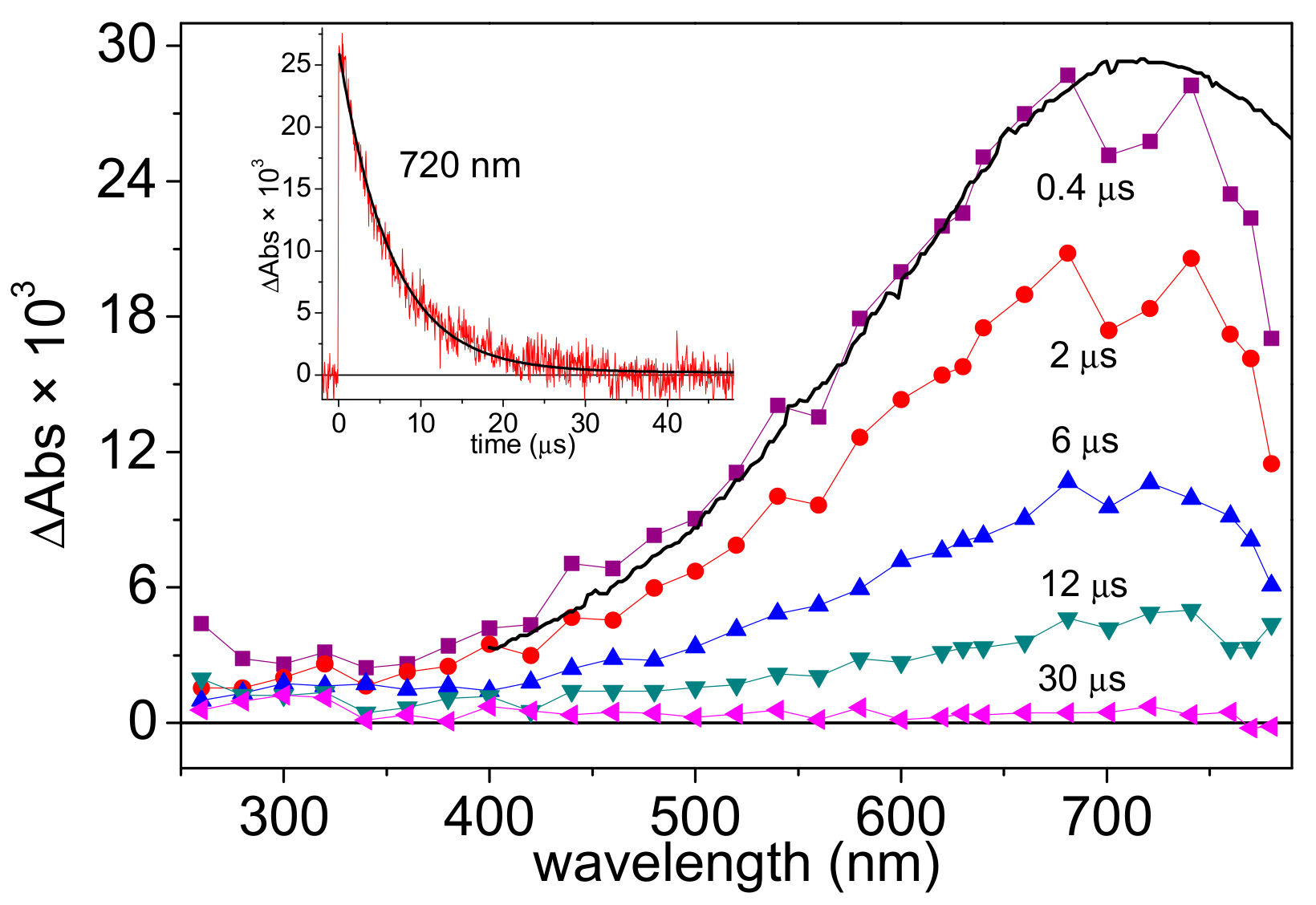
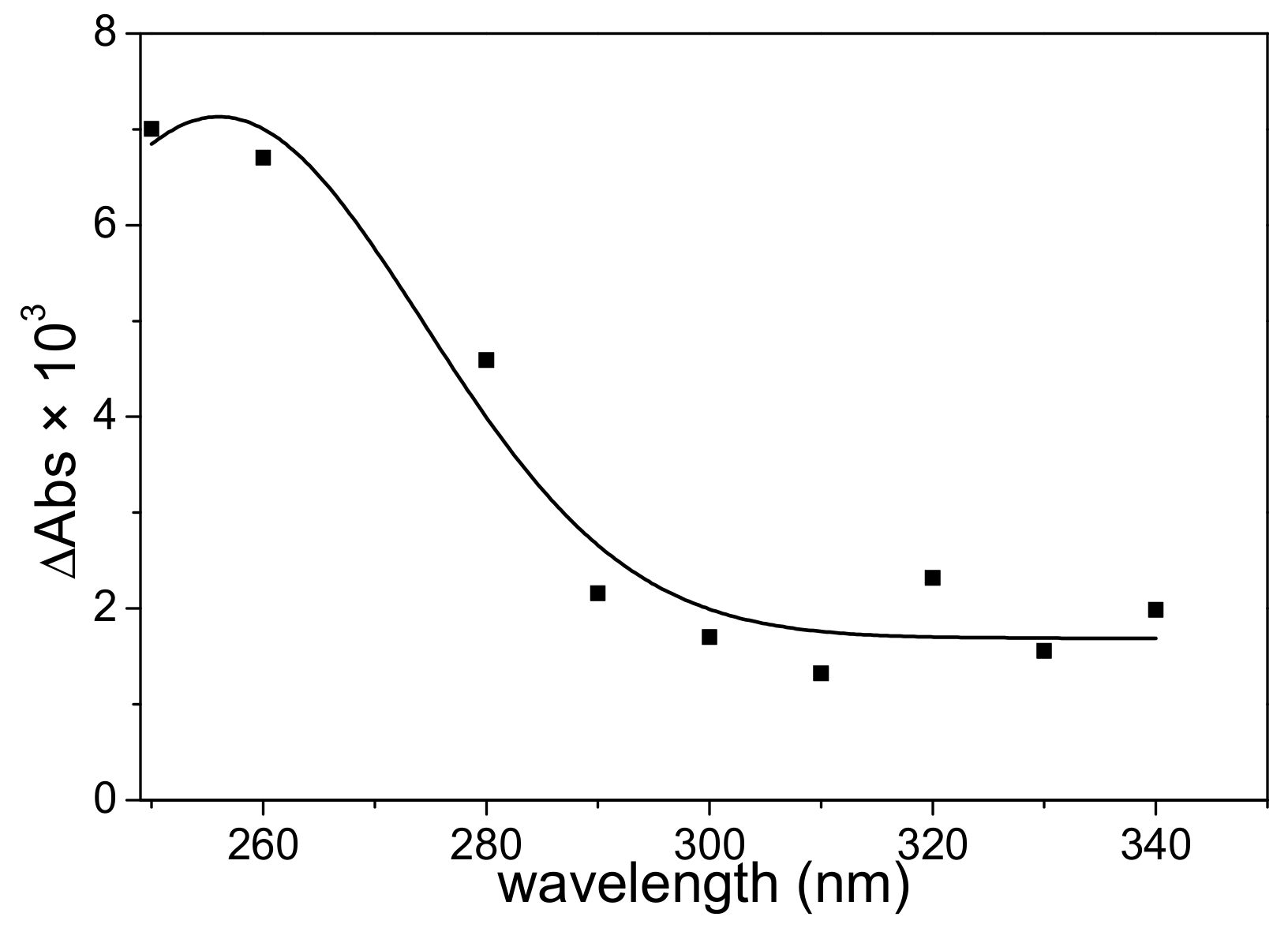
3.2. LFP Studies of SO3•− and Hydrated Electrons
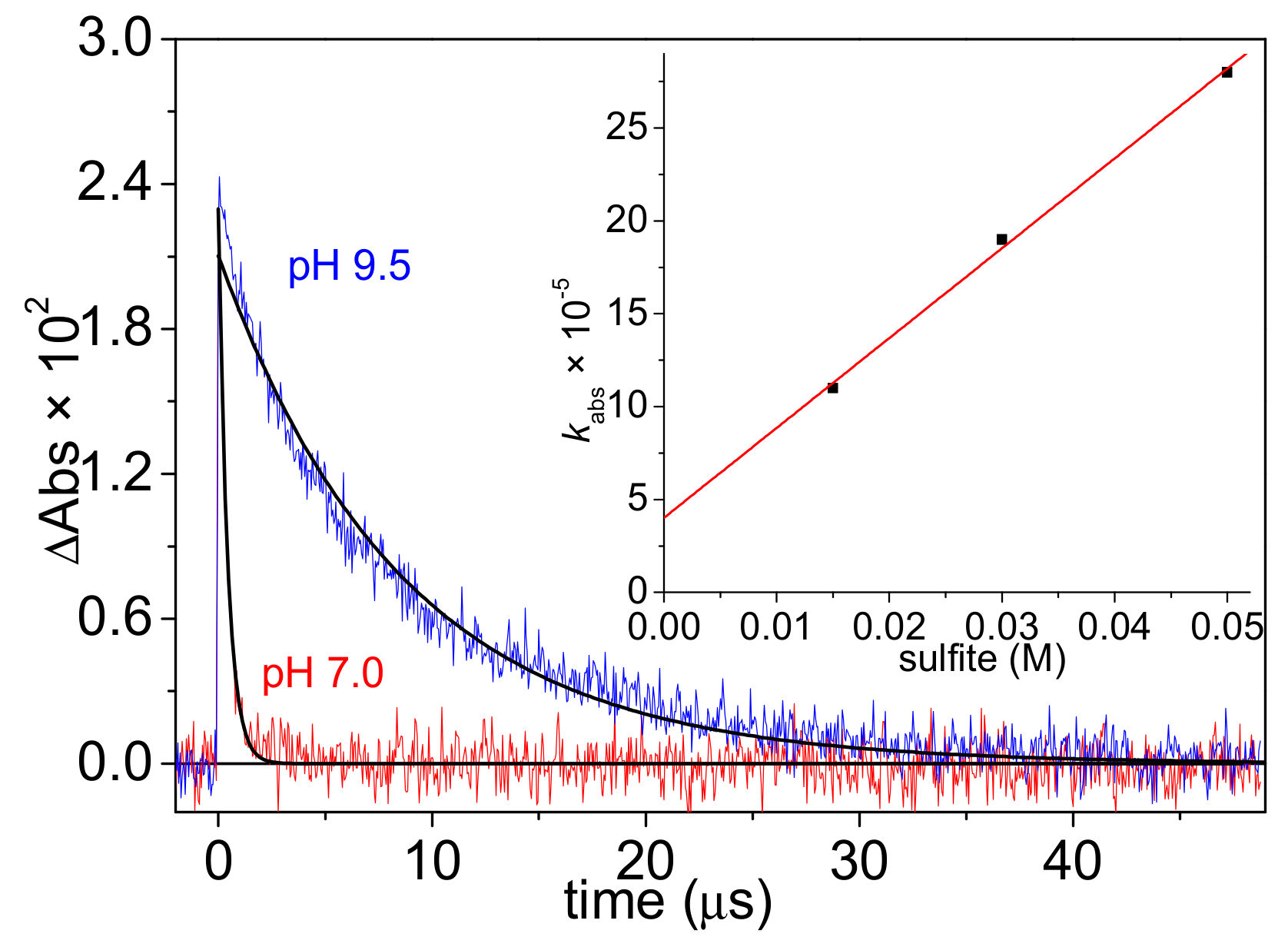
3.3. Decay of eaq− in Aqueous Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Han, D.; Wan, J.; Ma, Y.; Wang, Y.; Li, Y.; Li, D.; Guan, Z. New insights into the role of organic chelating agents in Fe(II) activated persulfate processes. Chem. Eng. J. 2015, 269, 425–433. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, Y.; Yang, S.; Gao, H.; Chen, L. Roles of oxysulfur radicals in the oxidation of acid orange 7 in the Fe(III)–sulfite system. J. Sulfur Chem. 2015, 36, 373–384. [Google Scholar] [CrossRef]
- Deng, W.; Zhao, H.; Pan, F.; Feng, X.; Jung, B.; Abdel-wahab, A.; Batchelor, B.; Li, Y. Visible-light-driven photocatalytic degradation of organic water pollutants promoted by sulfite addition. Environ. Sci. Technol. 2017, 51, 13372–13379. [Google Scholar] [CrossRef]
- Li, X.; Ma, J.; Liu, G.; Fang, J.; Yue, S.; Guan, Y.; Chen, L.; Liu, X. Efficient reductive dechlorination of monochloroacetic acid by sulfite/UV process. Environ. Sci. Technol. 2012, 46, 7342–7349. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Neta, P.; Huie, R.E. Free-radical chemistry of sulfite. Environ. Health Perspect. 1985, 64, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Vecitis, C.D.; Cheng, J.; Choi, W.; Mader, B.T.; Hoffmann, M.R. Reductive defluorination of aqueous perfluorinated alkyl surfactants: Effects of ionic headgroup and chain length. J. Phys. Chem. A 2009, 113, 690–696. [Google Scholar] [CrossRef]
- Huie, R.E.; Neta, P. Chemical behavior of SO3•− and SO5•− radicals in aqueous solutions. J. Phys. Chem. 1984, 88, 5665–5669. [Google Scholar] [CrossRef]
- Buxton, G.V.; Mcgowan, S.; Salmon, G.A.; Willians, J.E.; Wood, N.D. A study of the spectra and reactivity of oxysulphur-radical anions involved in the chain oxidation of S(IV):a pulse and -radiolysis study. Atmos. Environ. 1996, 30, 2483–2493. [Google Scholar] [CrossRef]
- Huie, R.E.; Neta, P. Rate constants for some oxidations of S(IV) by radicals in aqueous solutions. Atmos. Environ. 1987, 21, 17431747. [Google Scholar] [CrossRef]
- Deister, U.; Warneck, P. Photooxidation of SO32− in aqueous solution. J. Phys. Chem. 1990, 94, 2191–2198. [Google Scholar] [CrossRef]
- Herrmann, H.; Reese, A.; Zellner, R. Time-resolved UV/VIS diode array absorption spectroscopy of SOx•−(x = 3, 4, 5) radical anions in aqueous solution. J. Mol. Struct. 1995, 348, 183–186. [Google Scholar] [CrossRef]
- Wine, P.H.; Tang, Y.; Thorn, R.P.; Wells, J.R.; Davis, D.D. Kinetics of aqueous phase reactions of the SO4•− radical with potential importance in cloud chemistry. J. Geophys. Res. Atmos. 1989, 94, 1085–1094. [Google Scholar] [CrossRef]
- Rodríguez-lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater arsenic contamination throughout China. Science 2013, 341, 866–869. [Google Scholar] [CrossRef] [PubMed]
- Bissen, M.; Frimmel, F.H. Arsenic-A review. Part II: Oxidation of arsenic and its removal in water treatment. Acta Hydrochim. Hydrobiol. 2003, 31, 97–107. [Google Scholar] [CrossRef]
- Ryu, J.; Monllor-Satoca, D.; Kim, D.H.; Yeo, J.; Choi, W. Photooxidation of arsenite under 254 nm irradiation with a quantum yield higher than unity. Environ. Sci. Technol. 2013, 47, 9381–9387. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Choi, W. Iodide-mediated photooxidation of arsenite under 254 nm irradiation. Environ. Sci. Technol. 2009, 43, 3784–3788. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Yang, S.; Zhou, D.; Wu, F. A simple Cr(VI)–S(IV)–O2 system for rapid and simultaneous reduction of Cr(VI) and oxidative degradation of organic pollutants. J. Hazard. Mater. 2016, 307, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, S.; Yuan, Y.; Xu, J.; Zhu, Y.; Li, J. A novel heterogeneous system for sulfate radical generation through sulfite activation on a CoFe2O4 nanocatalyst surface. J. Hazard. Mater. 2017, 324, 583–592. [Google Scholar] [CrossRef]
- Chen, L.; Luo, T.; Yang, S.; Xu, J.; Liu, Z.; Wu, F. Efficient metoprolol degradation by heterogeneous copper ferrite/sulfite reaction. Environ. Chem. Lett. 2018, 16, 599–603. [Google Scholar] [CrossRef]
- Cullen, W.R.; McBride, B.C.; Reglinski, J. The reduction of trimethylarsine oxide to trimethylarsine by thiols: a mechanistic model for the biological reduction of arsenicals. J. Inorg. Biochem. 1984, 21, 45–60. [Google Scholar] [CrossRef]
- Zhang, W.N.; Singh, P.; Muir, D. Kinetics of oxidation of As(III) with SO2/O2 and UV light. In Minor Elements 2000: Processing and Environmental Aspects of As, Sb, Se, Te, and Bi; Young, C., Ed.; Society for Mining, Metallurgy, and Exploration: Littleton, Co, USA, 2000; pp. 333–343. [Google Scholar]
- Yang, J.Q.; Chai, L.Y.; Li, Q.Z.; Shu, Y. De Redox behavior and chemical species of arsenic in acidic aqueous system. Trans. Nonferrous Met. Soc. China 2017, 27, 2063–2072. [Google Scholar] [CrossRef]
- Müller, K.; Ciminelli, V.S.T.; Dantas, M.S.S.; Willscher, S. A comparative study of As(III) and As(V) in aqueous solutions and adsorbed on iron oxy-hydroxides by Raman spectroscopy. Water Res. 2010, 44, 5660–5672. [Google Scholar] [CrossRef] [PubMed]
- Hayon, E.; Treinin, A.; Wilf, J. Electronic Spectra, Photochemistry, and Autoxidation Mechanism of the Sulfite-Bisulfite-Pyrosulfite Systems. the SO2•−, SO3•−, SO4•−, and SO5•− Radicals. J. Am. Chem. Soc. 1972, 94, 47–57. [Google Scholar] [CrossRef]
- Xu, J.; Ding, W.; Wu, F.; Mailhot, G.; Zhou, D.; Hanna, K. Rapid catalytic oxidation of arsenite to arsenate in an iron(III)/sulfite system under visible light. Appl. Catal. B Environ. 2016, 186, 56–61. [Google Scholar] [CrossRef]
- Yuan, Y.; Luo, T.; Xu, J.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Enhanced oxidation of aniline using Fe(III)-S(IV) system: Role of different oxysulfur radicals. Chem. Eng. J. 2019, 362, 183–189. [Google Scholar] [CrossRef]
- Mcelroy, W.J.; Waygood, S.J. Oxidation of Formaldehyde by the Hydroxyl Radical in Aqueous Solution. J. Chem. Soc. Faraday Trans. 1991, 87, 1513–1521. [Google Scholar] [CrossRef]
- Hart, J.; Radical, H.; Michael, B.D.; Hart, E.J. The Rate Constants of Hydrated Electron, Hydrogen Atom, and Hydroxyl Radical Reactions with Benzene, 1,5-Cyclohexadiene, 1,4-Cyclohexadiene, and Cyclohexene. J. Phys. Chem. 1969, 74, 2878–2884. [Google Scholar]
- Waygood, S.J.; Mcelroy, W.J. Spectroscopy and Decay Kinetics of the Sulfite Radical Anion. J. Chem. Soc. Faraday Trans. 1992, 88, 1525–1530. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds mentioned in this paper are available from the authors. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, T.; Wang, Z.; Wang, Y.; Liu, Z.; P. Pozdnyakov, I. Different Role of Bisulfite/Sulfite in UVC-S(IV)-O2 System for Arsenite Oxidation in Water. Molecules 2019, 24, 2307. https://doi.org/10.3390/molecules24122307
Luo T, Wang Z, Wang Y, Liu Z, P. Pozdnyakov I. Different Role of Bisulfite/Sulfite in UVC-S(IV)-O2 System for Arsenite Oxidation in Water. Molecules. 2019; 24(12):2307. https://doi.org/10.3390/molecules24122307
Chicago/Turabian StyleLuo, Tao, Zhenhua Wang, Yi Wang, Zizheng Liu, and Ivan P. Pozdnyakov. 2019. "Different Role of Bisulfite/Sulfite in UVC-S(IV)-O2 System for Arsenite Oxidation in Water" Molecules 24, no. 12: 2307. https://doi.org/10.3390/molecules24122307
APA StyleLuo, T., Wang, Z., Wang, Y., Liu, Z., & P. Pozdnyakov, I. (2019). Different Role of Bisulfite/Sulfite in UVC-S(IV)-O2 System for Arsenite Oxidation in Water. Molecules, 24(12), 2307. https://doi.org/10.3390/molecules24122307