A Novel Cold-Adapted and Salt-Tolerant RNase R from Antarctic Sea-Ice Bacterium Psychrobacter sp. ANT206
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of psrnr Gene
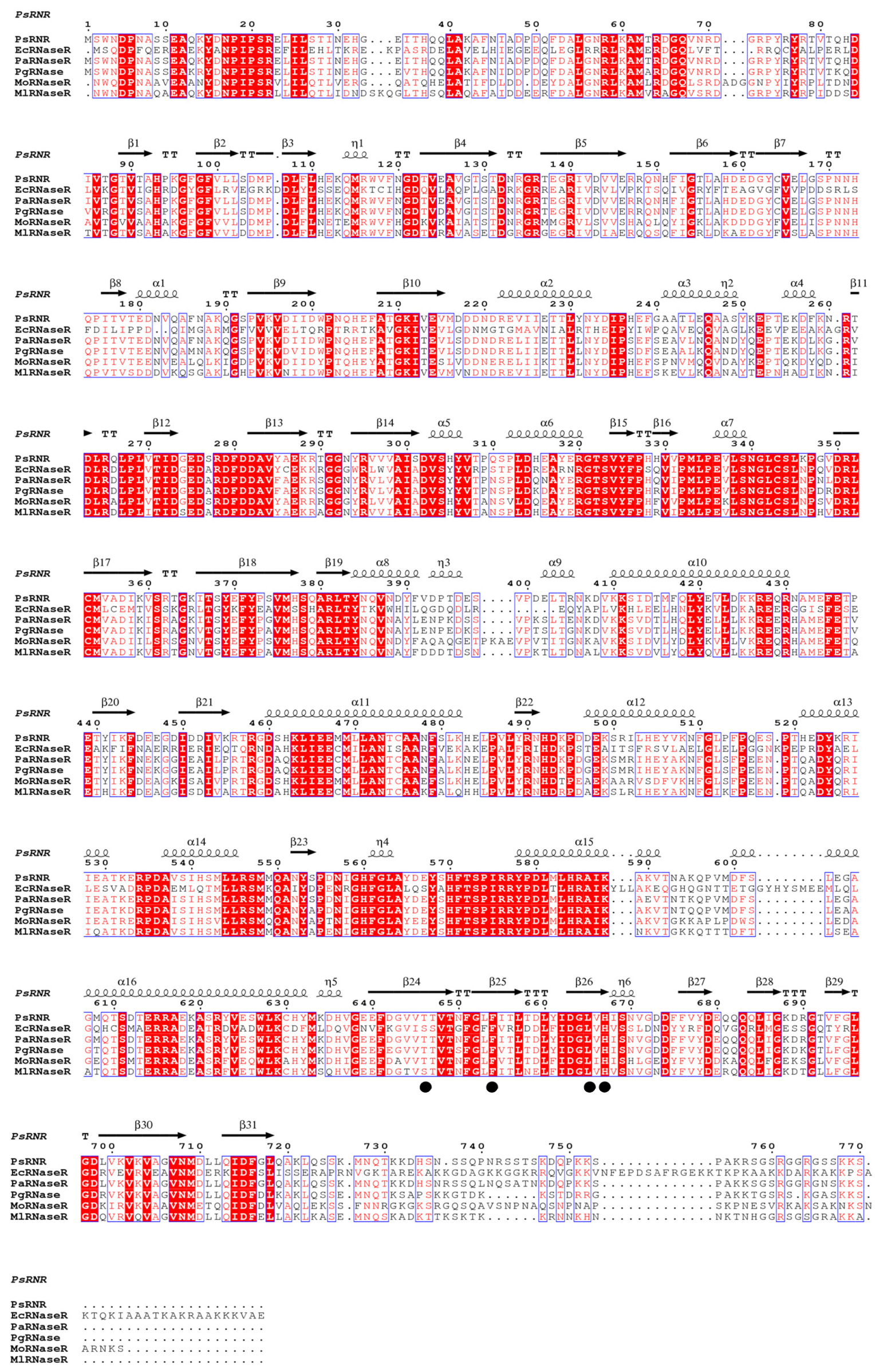
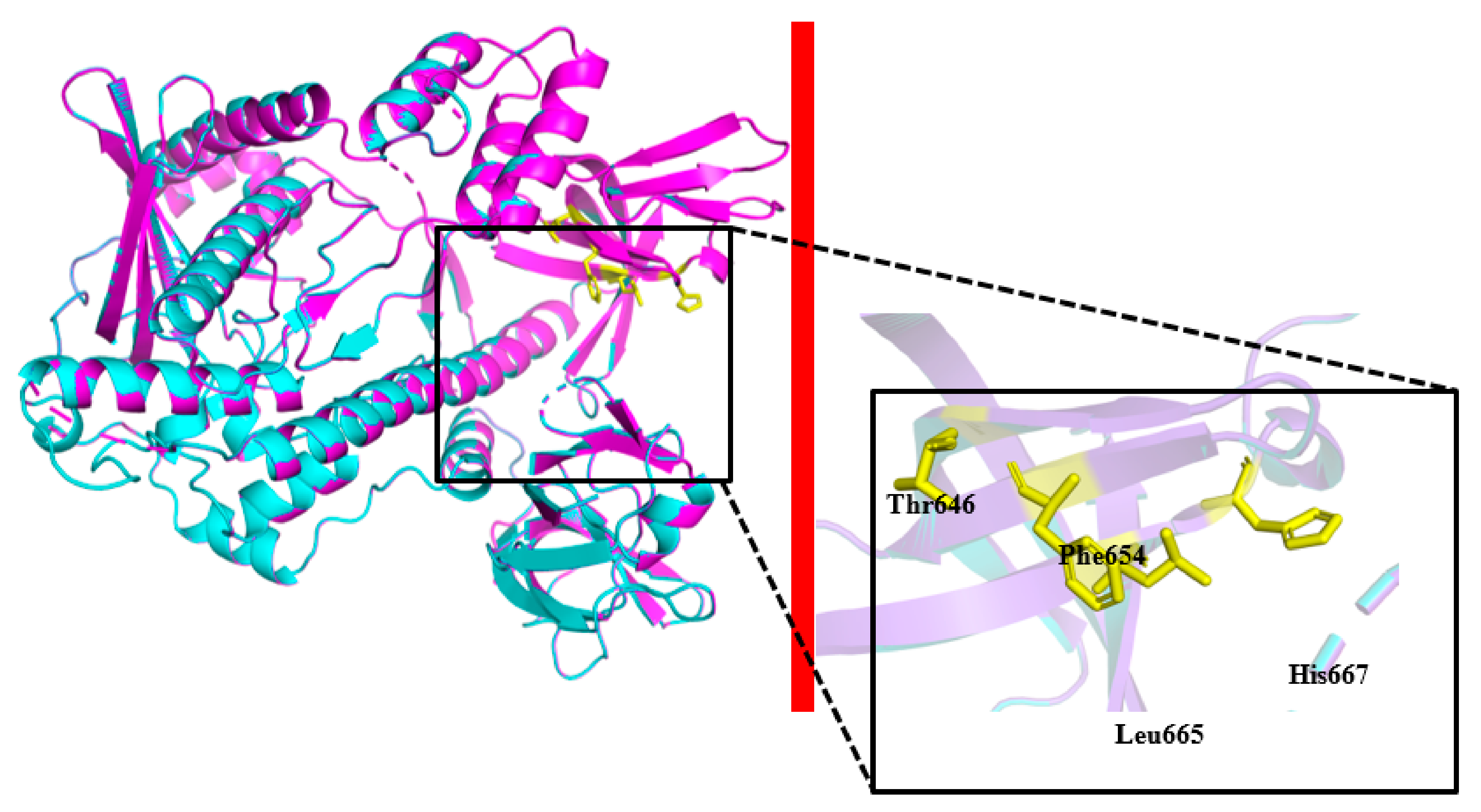
2.2. Homology 3D Modeling and Structure Analysis
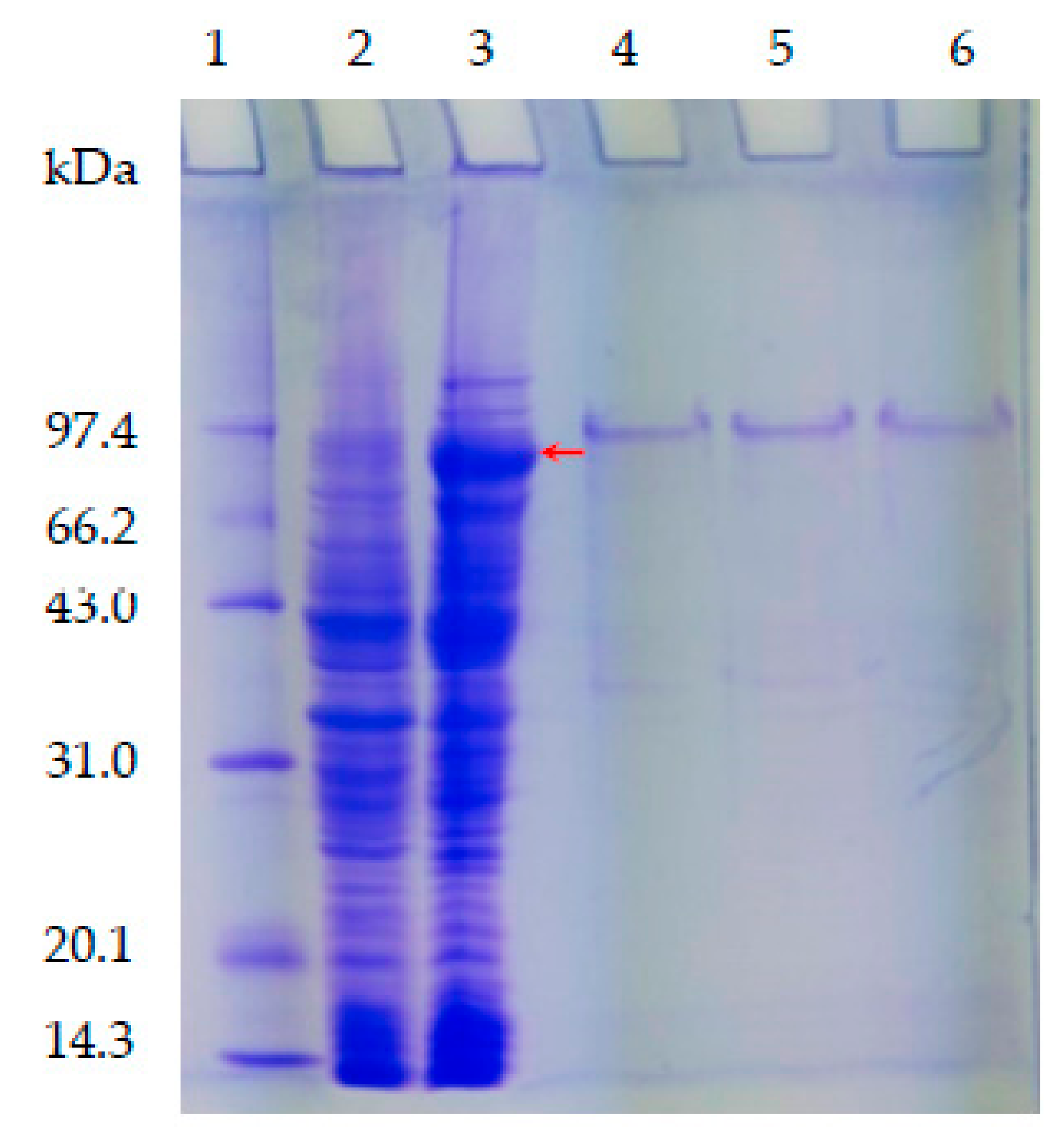
2.3. Expression, Purification and Enzyme Assays
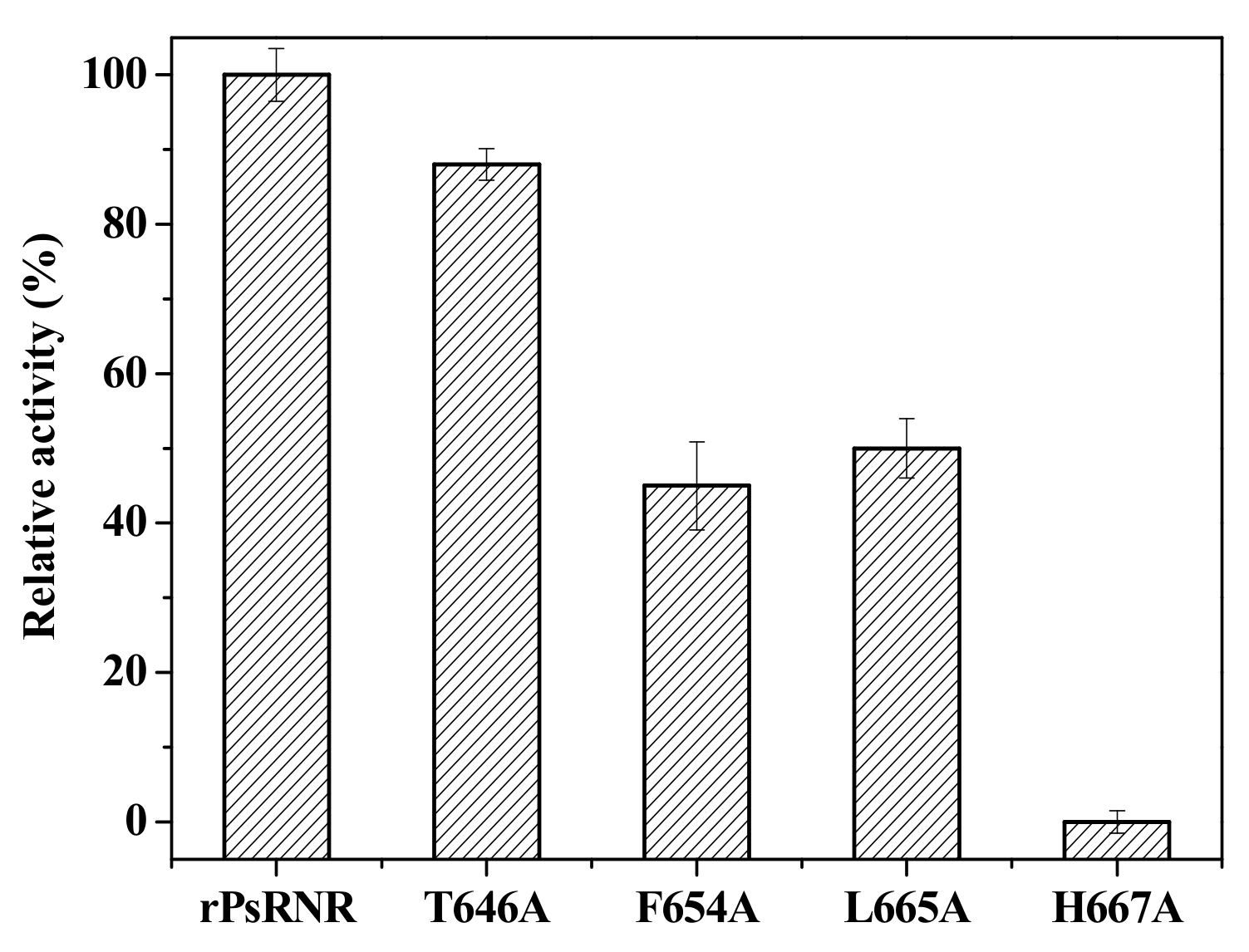
2.4. Site-Directed Mutagenesis
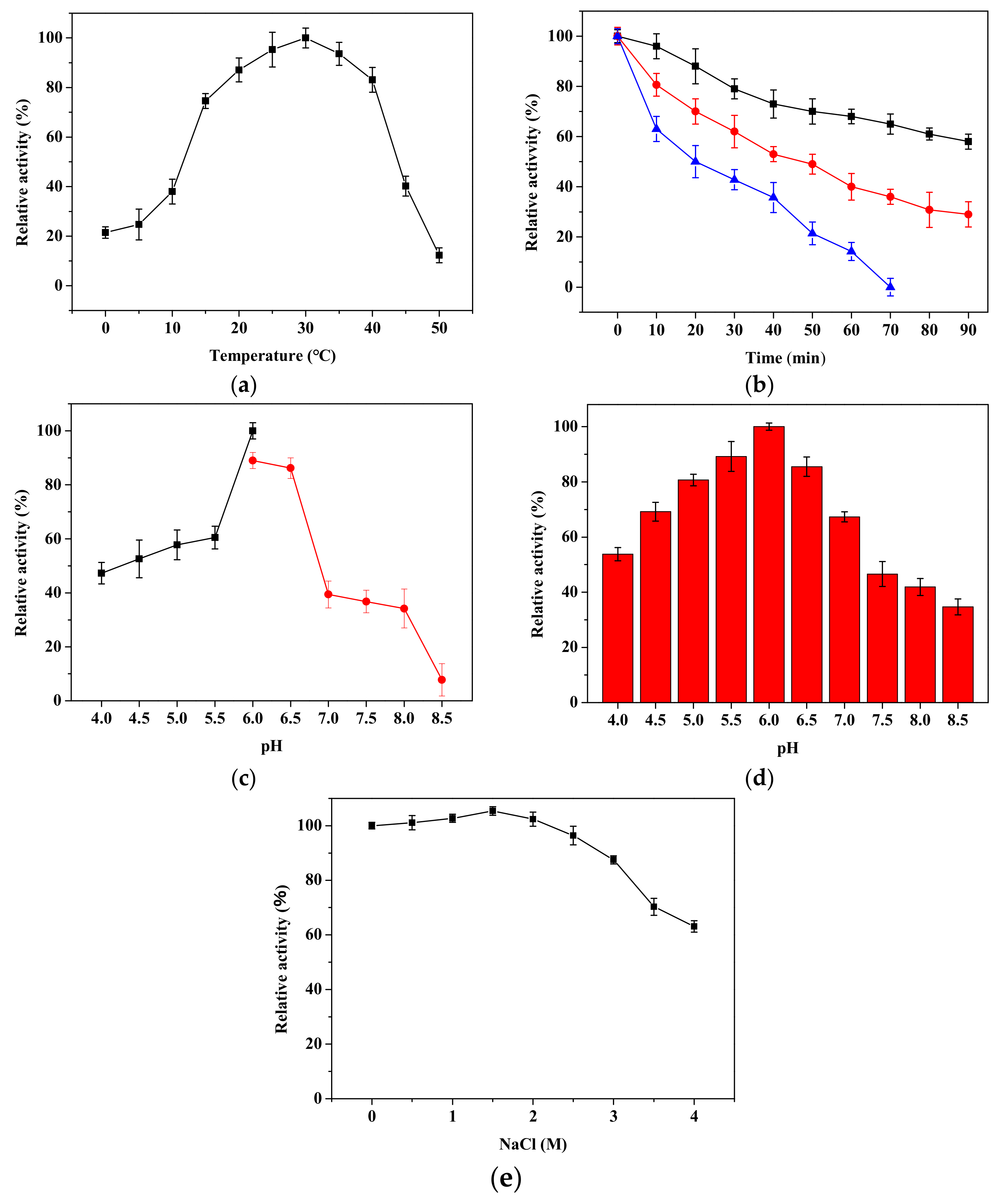
2.5. Biochemical Characteristics of rPsRNR
2.6. Enzyme Kinetics of rPsRNR
2.7. Enzyme Thermodynamics of rPsRNR
3. Materials and Methods
3.1. Strains and Bacteria Cultivation
3.2. Identification of psrnr Gene
3.3. Homology Modeling of PsRNR
3.4. Site-Directed Mutagenesis
3.5. Expression and Purification of PsRNR
3.6. Enzyme Assay
3.7. Biochemical Characteristics of rPsRNR
3.8. Kinetic Parameters and Thermodynamic Parameters of the rPsRNR
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fong, W.P.; Mock, W.Y.; Ng, T.B. Intrinsic ribonuclease activities in ribonuclease and ribosome-inactivating proteins from the seeds of bitter gourd. Int. J. Biochem. Cell Biol. 2000, 32, 571–577. [Google Scholar] [CrossRef]
- Cheng, Z.F.; Deutscher, M.P. An important role for RNase R in mRNA decay. Mol. Cell 2005, 17, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.F.; Zuo, Y.H.; Li, Z.W.; Rudd, K.E.; Deutscher, M.P. The vacB gene required for virulence in Shigella flexneri and Escherichia coli encodes the exoribonuclease RNase R. J. Biol. Chem. 1998, 273, 14077–14080. [Google Scholar] [CrossRef] [PubMed]
- Erova, T.E.; Kosykh, V.G.; Fadl, A.A.; Sha, J.; Horneman, A.J.; Chopra, A.K. Cold shock exoribonuclease R (vacB) is involved in Aeromonas hydrophila pathogenesis. J. Bacteriol. 2008, 190, 3467–3474. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, X.; Faucher, S.P.; Kalachikov, S.; Shuman, H.A. Loss of RNase R induces competence development in Legionella pneumophila. J. Bacteriol. 2008, 190, 8126–8136. [Google Scholar] [CrossRef] [PubMed]
- Barria, C.; Domingues, S.; Arraiano, C.M. Pneumococcal RNase R globally impacts protein synthesis by regulating the amount of actively translating ribosomes. RNA Biol. 2019, 16, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.F.; Deutscher, M.P. Purification and characterization of the Escherichia coli exoribonuclease RNase R—Comparison with RNase II. J. Biol. Chem. 2002, 277, 21624–21629. [Google Scholar] [CrossRef] [PubMed]
- Malecki, M.; Barria, C.; Arraiano, C.M. Characterization of the RNase R association with ribosomes. BMC Microbiol. 2014, 14. [Google Scholar] [CrossRef]
- Domingues, S.; Moreira, R.N.; Andrade, J.M.; dos Santos, R.F.; Barria, C.; Viegas, S.C.; Arraiano, C.M. The role of RNase R in trans-translation and ribosomal quality control. Biochimie 2015, 114, 113–118. [Google Scholar] [CrossRef]
- Phadtare, S. Escherichia coli cold-shock gene profiles in response to over-expression/deletion of CsdA, RNase R and PNPase and relevance to low-temperature RNA metabolism. Genes Cells 2012, 17, 850–874. [Google Scholar] [CrossRef]
- Moreira, R.N.; Domingues, S.; Viegas, S.C.; Amblar, M.; Arraiano, C.M. Synergies between RNA degradation and trans-translation in Streptococcus pneumoniae: Cross regulation and co-transcription of RNase R and SmpB. BMC Microbiol. 2012, 12. [Google Scholar] [CrossRef]
- Sulthana, S.; Rajyaguru, P.I.; Mittal, P.; Ray, M.K. rnr Gene from the Antarctic Bacterium Pseudomonas syringae Lz4W, Encoding a Psychrophilic RNase R. Appl. Environ. Microbiol. 2011, 77, 7896–7904. [Google Scholar] [CrossRef]
- Purusharth, R.I.; Madhuri, B.; Ray, M.K. Exoribonuclease R in Pseudomonas syringae is essential for growth at low temperature and plays a novel role in the 3′ end processing of 16 and 5S ribosomal RNA. J. Biol. Chem. 2007, 282, 16267–16277. [Google Scholar] [CrossRef]
- Piette, F.; D’Amico, S.; Struvay, C.; Mazzucchelli, G.; Renaut, J.; Tutino, M.L.; Danchin, A.; Leprince, P.; Feller, G. Proteomics of life at low temperatures: Trigger factor is the primary chaperone in the Antarctic bacterium Pseudoalteromonas haloplanktis TAC125. Mol. Microbiol. 2010, 76, 120–132. [Google Scholar] [CrossRef]
- Chu, L.Y.; Hsieh, T.J.; Golzarroshan, B.; Chen, Y.P.; Agrawal, S.; Yuan, H.N.S. Structural insights into RNA unwinding and degradation by RNase R. Nucleic Acids Res. 2017, 45, 12015–12024. [Google Scholar] [CrossRef]
- Matos, R.G.; Barbas, A.; Arraiano, C.M. RNase R mutants elucidate the catalysis of structured RNA: RNA-binding domains select the RNAs targeted for degradation. Biochem. J. 2009, 423, 291–301. [Google Scholar] [CrossRef]
- Awano, N.; Rajagopal, V.; Arbing, M.; Patel, S.; Hunt, J.; Inouye, M.; Phadtare, S. Escherichia coli RNase R Has Dual Activities, Helicase and RNase. J. Bacteriol. 2010, 192, 1344–1352. [Google Scholar] [CrossRef]
- Zheng, Y.Y.; Li, Y.J.; Liu, W.D.; Chen, C.C.; Ko, T.P.; He, M.; Xu, Z.X.; Liu, M.X.; Luo, H.Y.; Guo, R.T.; et al. Structural insight into potential cold adaptation mechanism through a psychrophilic glycoside hydrolase family 10 endo-beta-1,4-xylanase. J. Struct. Biol. 2016, 193, 206–211. [Google Scholar] [CrossRef]
- Kumar, S.; Tsai, C.J.; Nussinov, R. Factors enhancing protein thermostability. Protein Eng. 2000, 13, 179–191. [Google Scholar] [CrossRef]
- Ramli, A.N.M.; Mahadi, N.M.; Shamsir, M.S.; Rabu, A.; Joyce-Tan, K.H.; Murad, A.M.A.; Illias, R.M. Structural prediction of a novel chitinase from the psychrophilic Glaciozyma antarctica PI12 and an analysis of its structural properties and function. J. Comput. Aided Mol. Des. 2012, 26, 947–961. [Google Scholar] [CrossRef]
- Siddiqui, K.S.; Cavicchioli, R. Cold-adapted enzymes. Annu. Rev. Biochem. 2006, 75, 403–433. [Google Scholar] [CrossRef]
- Feller, G.; Gerday, C. Psychrophilic enzymes: Hot topics in cold adaptation. Nat. Rev. Microbiol. 2003, 1, 200–208. [Google Scholar] [CrossRef]
- Fu, J.; Leiros, H.K.S.; de Pascale, D.; Johnson, K.A.; Blencke, H.M.; Landfald, B. Functional and structural studies of a novel cold-adapted esterase from an Arctic intertidal metagenomic library. Appl. Microbiol. Biotechnol. 2013, 97, 3965–3978. [Google Scholar] [CrossRef]
- Hossain, S.T.; Malhotra, A.; Deutscher, M.P. How RNase R Degrades Structured RNA: Role of the helicase activity and the S1 domain. J. Biol. Chem. 2016, 291, 7877–7887. [Google Scholar] [CrossRef]
- Zhou, W.W.; Niu, T.G. Purification and some properties of an extracellular ribonuclease with antiviral activity against tobacco mosaic virus from Bacillus cereus. Biotechnol. Lett. 2009, 31, 101–105. [Google Scholar] [CrossRef]
- Landry, K.S.; Levin, R.E. Purification and Characterization of Iso-Ribonucleases from a Novel Thermophilic Fungus. Int. J. Mol. Sci. 2014, 15, 944–957. [Google Scholar] [CrossRef]
- Lalonde, M.S.; Zuo, Y.; Zhang, J.; Gong, X.; Wu, S.; Malhotra, A.; Li, Z. Exoribonuclease R in Mycoplasma genitalium can carry out both RNA processing and degradative functions and is sensitive to RNA ribose methylation. RNA 2007, 13, 1957–1968. [Google Scholar] [CrossRef]
- Hou, J.L.; Liu, Y.F.; Lu, Z.; Liu, X.P.; Liu, J.H. Biochemical characterization of RNase HII from Aeropyrum pernix. Acta Biochim. Biophys. Sin. 2012, 44, 339–346. [Google Scholar] [CrossRef]
- Jongruja, N.; You, D.J.; Angkawidjaja, C.; Kanaya, E.; Koga, Y.; Kanaya, S. Structure and characterization of RNase H3 from Aquifex aeolicus. FEBS J. 2012, 15, 2737–2753. [Google Scholar] [CrossRef]
- Altermark, B.; Niiranen, L.; Willassen, N.P.; Smalas, A.O.; Moe, E. Comparative studies of endonuclease I from cold-adapted Vibrio salmonicida and mesophilic Vibrio cholerae. FEBS J. 2007, 274, 252–263. [Google Scholar] [CrossRef]
- Zhou, J.P.; He, L.M.; Gao, Y.J.; Han, N.Y.; Zhang, R.; Wu, Q.; Li, J.J.; Tang, X.H.; Xu, B.; Ding, J.M.; et al. Characterization of a novel low-temperature-active, alkaline and sucrose-tolerant invertase. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Shruti, G.; Sukhdev, S.; Singh, K.S. Purification and characterization of an extracellular ribonuclease from a Bacillus sp. RNS3 (KX966412). Int. J. Biol. Macromol. 2017, 97, 440–446. [Google Scholar] [CrossRef]
- Hoyoux, A.; Jennes, I.; Dubois, P.; Genicot, S.; Dubail, F.; Francois, J.M.; Baise, E.; Feller, G.; Gerday, C. Cold-adapted beta-galactosidase from the Antarctic psychrophile Pseudoalteromonas haloplanktis. Appl. Environ. Microbiol. 2001, 67, 1529–1535. [Google Scholar] [CrossRef]
- Pawlak-Szukalska, A.; Wanarska, M.; Popinigis, A.T.; Kur, J. A novel cold-active β-D-galactosidase with transglycosylation activity from the Antarctic Arthrobacter sp. 32cB-Gene cloning, purification and characterization. Process Biochem. 2014, 49, 2122–2133. [Google Scholar] [CrossRef]
- Zhou, J.P.; Liu, Y.; Shen, J.D.; Zhang, R.; Tang, X.H.; Li, J.J.; Wang, Y.Y.; Huang, Z. X Kinetic and thermodynamic characterization of a novel low-temperature-active xylanase from Arthrobacter sp. GN16 isolated from the feces of Grus nigricollis. Bioengineered 2015, 6, 111–114. [Google Scholar] [CrossRef][Green Version]
- Xue, D.S.; Zeng, X.H.; Gong, C.J.; Lin, D.Q.; Yao, S.J. A cold adapt and ethanol tolerant endoglucanase from a marine Bacillus subtilis. Chin. J. Chem. Eng. 2018, 26, 2601–2606. [Google Scholar] [CrossRef]
- Kunitz, M. A spectrophotometric method for the measurement of ribonuclease activity. J. Biol. Chem. 1946, 164, 563–568. [Google Scholar]
- Lonhienne, T.; Gerday, C.; Feller, G. Psychrophilic enzymes: Revisiting the thermodynamic parameters of activation may explain local flexibility. Biochim. Biophys. Acta 2000, 1543, 1–10. [Google Scholar] [CrossRef]
Sample Availability: Samples of the recombinant strain is available from the authors. |

| Parameters | PsRNR | EcRNase R | Expected Effect on PsRNR |
|---|---|---|---|
| Salt Bridges | 17 | 27 | Stability |
| Hydrogen Bonds | 478 | 492 | |
| Cation-pi interactions | 11 | 14 | |
| Aromatic | 22 | 23 | |
| Hydrophobic interactions | 447 | 543 | Thermolability |
| Glycine residues | 46 | 59 | Flexibility |
| Proline residues | 37 | 35 | |
| Arginine residues | 44 | 74 | |
| Arg/(Arg + Lys) | 0.47 | 0.56 |
| Reagent | Concentration | Relative Activity (%) | Reagent | Concentration | Relative Activity (%) |
|---|---|---|---|---|---|
| None | -- | 100.0 | None | -- | 100.0 |
| Mg2+ | 1 mM | 121.4 ± 2.6 | Mg2+ | 5 mM | 131.2 ±4.8 |
| Ca2+ | 1 mM | 102.3 ± 4.3 | Ca2+ | 5 mM | 86.0 ± 3.5 |
| Zn2+ | 1 mM | 81.8 ± 2.3 | Zn2+ | 5 mM | 75.8 ± 1.5 |
| Fe2+ | 1 mM | 90.9 ± 6.2 | Fe2+ | 5 mM | 85.9 ± 4.1 |
| Cu2+ | 1 mM | 40.9 ± 1.9 | Cu2+ | 5 mM | 20.5 ± 2.5 |
| Pb2+ | 1 mM | 72.7 ± 5.6 | Pb2+ | 5 mM | 65.3 ± 3.7 |
| Cr2+ | 1 mM | 63.2 ± 3.7 | Cr2+ | 5 mM | 62.7 ± 5.5 |
| Ba2+ | 1 mM | 2.1 ± 2.9 | Ba2+ | 5 mM | ND |
| EDTA | 1 mM | ND | EDTA | 5 mM | ND |
| Temperature (°C) | Vm (μmol/min/mg) | Km (μM) | kcat (s−1) |
|---|---|---|---|
| 0 | 44.932 | 1.008 | 27.126 |
| 5 | 52.856 | 0.828 | 31.910 |
| 10 | 79.288 | 0.798 | 47.867 |
| 15 | 84.250 | 0.684 | 50.863 |
| 20 | 89.864 | 0.533 | 54.252 |
| 25 | 103.69 | 0.472 | 62.600 |
| 30 | 117.220 | 0.339 | 70.767 |
| Temperature (°C) | ∆H (KJ/mol) | ∆S (J/mol·K) | ∆G (KJ/mol) |
|---|---|---|---|
| 0 | 19.24 | −146.30 | 59.20 |
| 5 | 19.20 | −146.52 | 59.95 |
| 10 | 19.16 | −144.66 | 60.12 |
| 15 | 19.12 | −145.62 | 61.08 |
| 20 | 19.08 | −146.50 | 62.02 |
| 25 | 19.03 | −146.68 | 62.77 |
| 30 | 18.99 | −146.99 | 63.55 |
| Mutagenesis | Primers Sequences |
|---|---|
| T646A | 5′-CAAAGTTTGTGACGGTAGCTACGACACCATCGAACTC-3′ |
| 5′-GAGTTCGATGGTGTCGTAGCTACCGTCACAAACTTTG-3′ | |
| F654A | 5′-CAAATCCGTCAGAGTAATAGCTAAACCAAAGTTTGTGACGG-3′ |
| 5′-CCGTCACAAACTTTGGTTTAGCTATTACTCTGACGGATTTG-3′ | |
| L665A | 5′-GTTTGAGATATGCACCGCACCATCGATATACAAATCCG-3′ |
| 5′-CGGATTTGTATATCGATGGTGCGGTGCATATCTCAAAC-3′ | |
| H667A | 5′-CACCAACGTTTGAGATAGCCACCAAACCATCGATATAC-3′ |
| 5′-GTATATCGATGGTTTGGTGGCTATCTCAAACGTTGGTG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hou, Y.; Nie, P.; Wang, Y.; Ren, X.; Wei, Q.; Wang, Q. A Novel Cold-Adapted and Salt-Tolerant RNase R from Antarctic Sea-Ice Bacterium Psychrobacter sp. ANT206. Molecules 2019, 24, 2229. https://doi.org/10.3390/molecules24122229
Wang Y, Hou Y, Nie P, Wang Y, Ren X, Wei Q, Wang Q. A Novel Cold-Adapted and Salt-Tolerant RNase R from Antarctic Sea-Ice Bacterium Psychrobacter sp. ANT206. Molecules. 2019; 24(12):2229. https://doi.org/10.3390/molecules24122229
Chicago/Turabian StyleWang, Yatong, Yanhua Hou, Ping Nie, Yifan Wang, Xiulian Ren, Qifeng Wei, and Quanfu Wang. 2019. "A Novel Cold-Adapted and Salt-Tolerant RNase R from Antarctic Sea-Ice Bacterium Psychrobacter sp. ANT206" Molecules 24, no. 12: 2229. https://doi.org/10.3390/molecules24122229
APA StyleWang, Y., Hou, Y., Nie, P., Wang, Y., Ren, X., Wei, Q., & Wang, Q. (2019). A Novel Cold-Adapted and Salt-Tolerant RNase R from Antarctic Sea-Ice Bacterium Psychrobacter sp. ANT206. Molecules, 24(12), 2229. https://doi.org/10.3390/molecules24122229





