CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium wilfordii
Abstract
:1. Introduction
2. Results
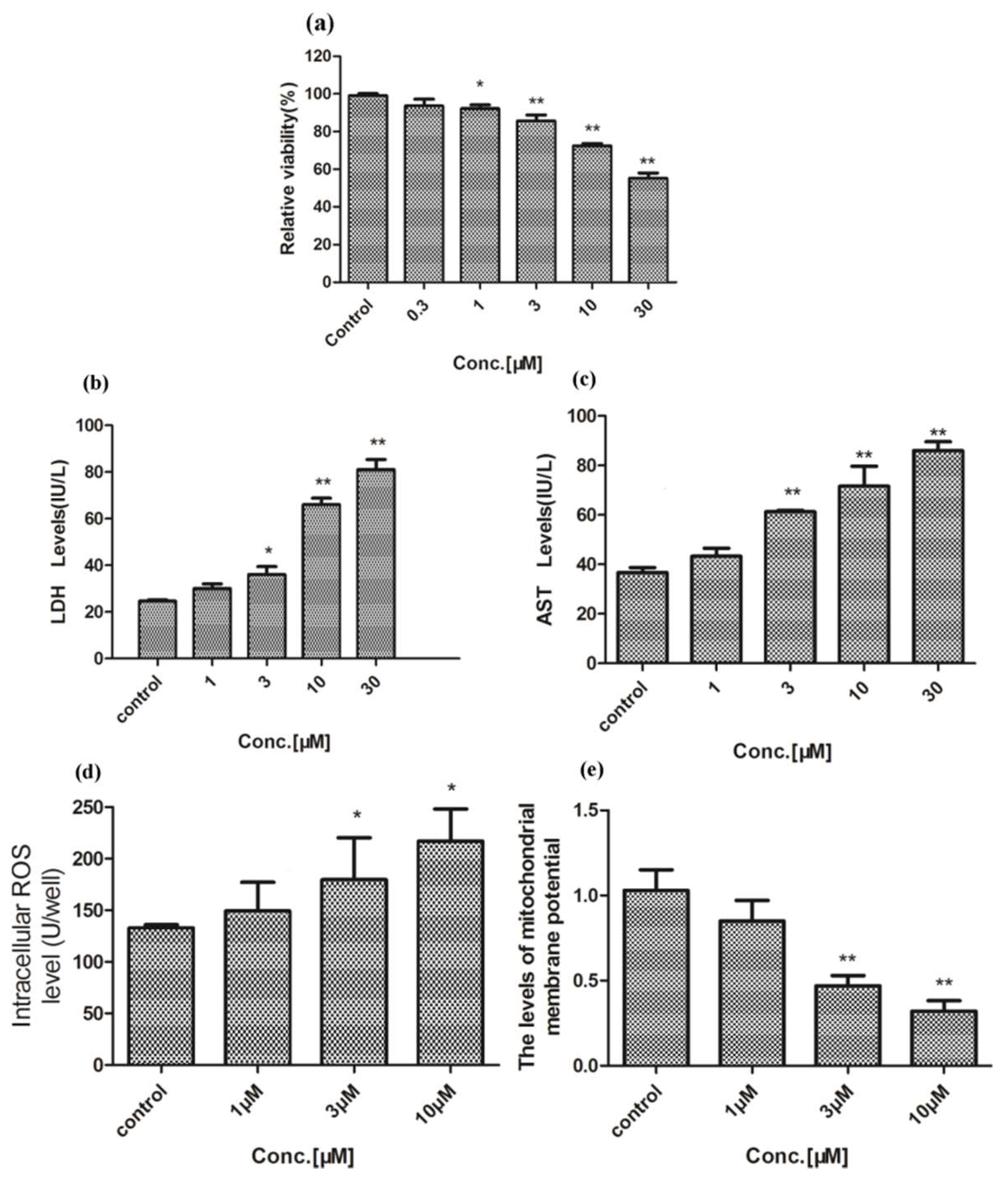
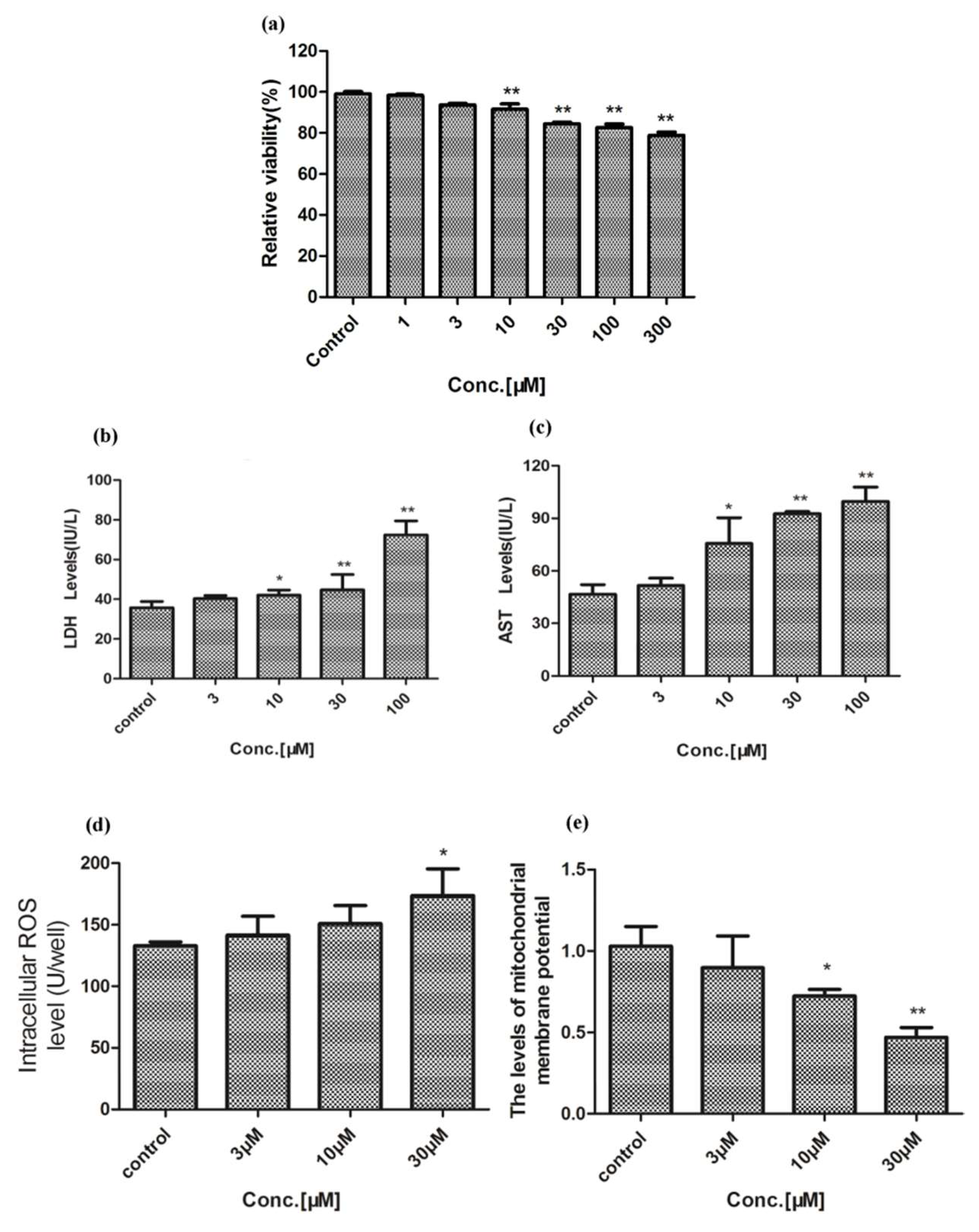
2.1. Hepatotoxicity of Celastrol and Triptolide
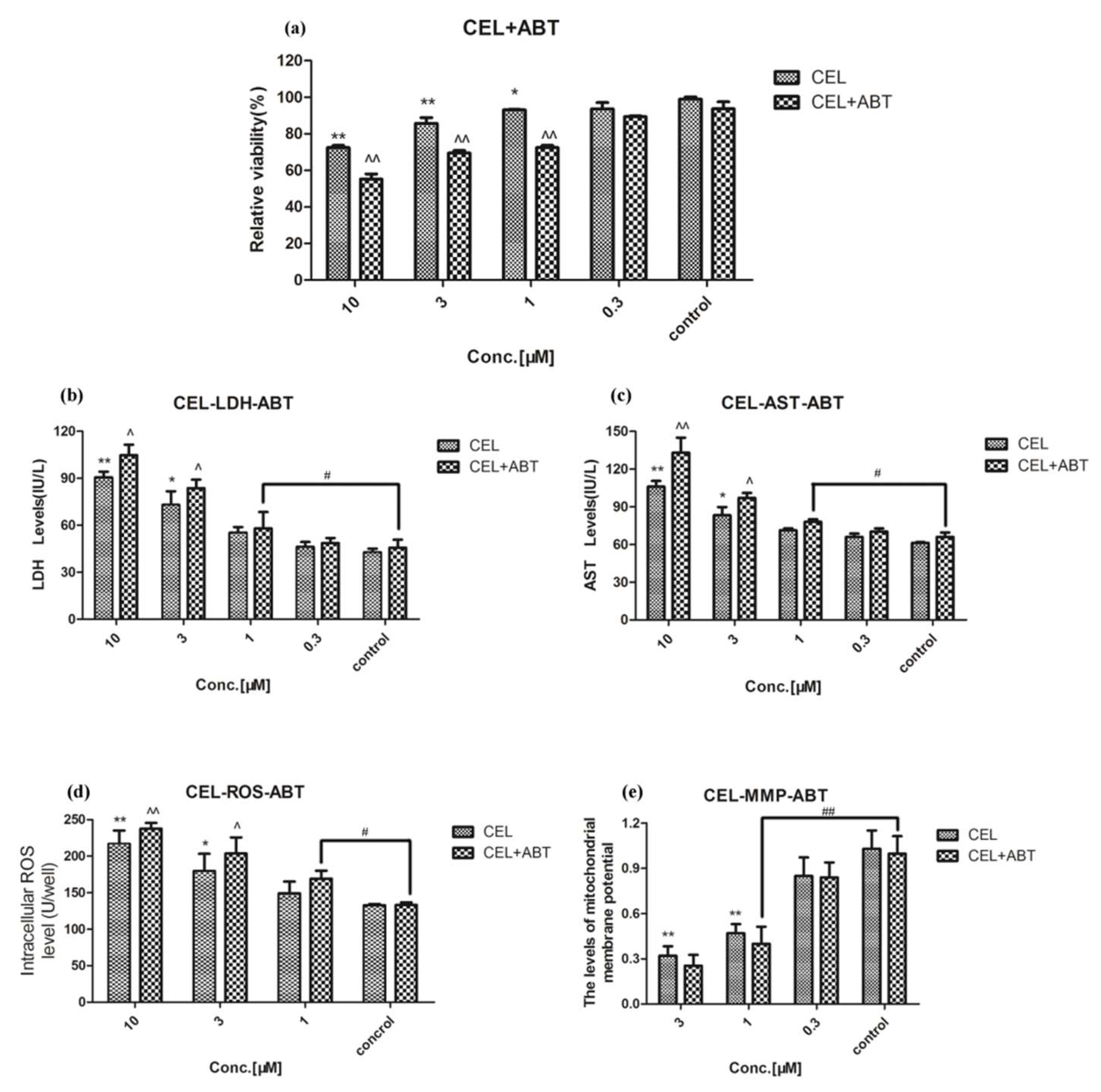
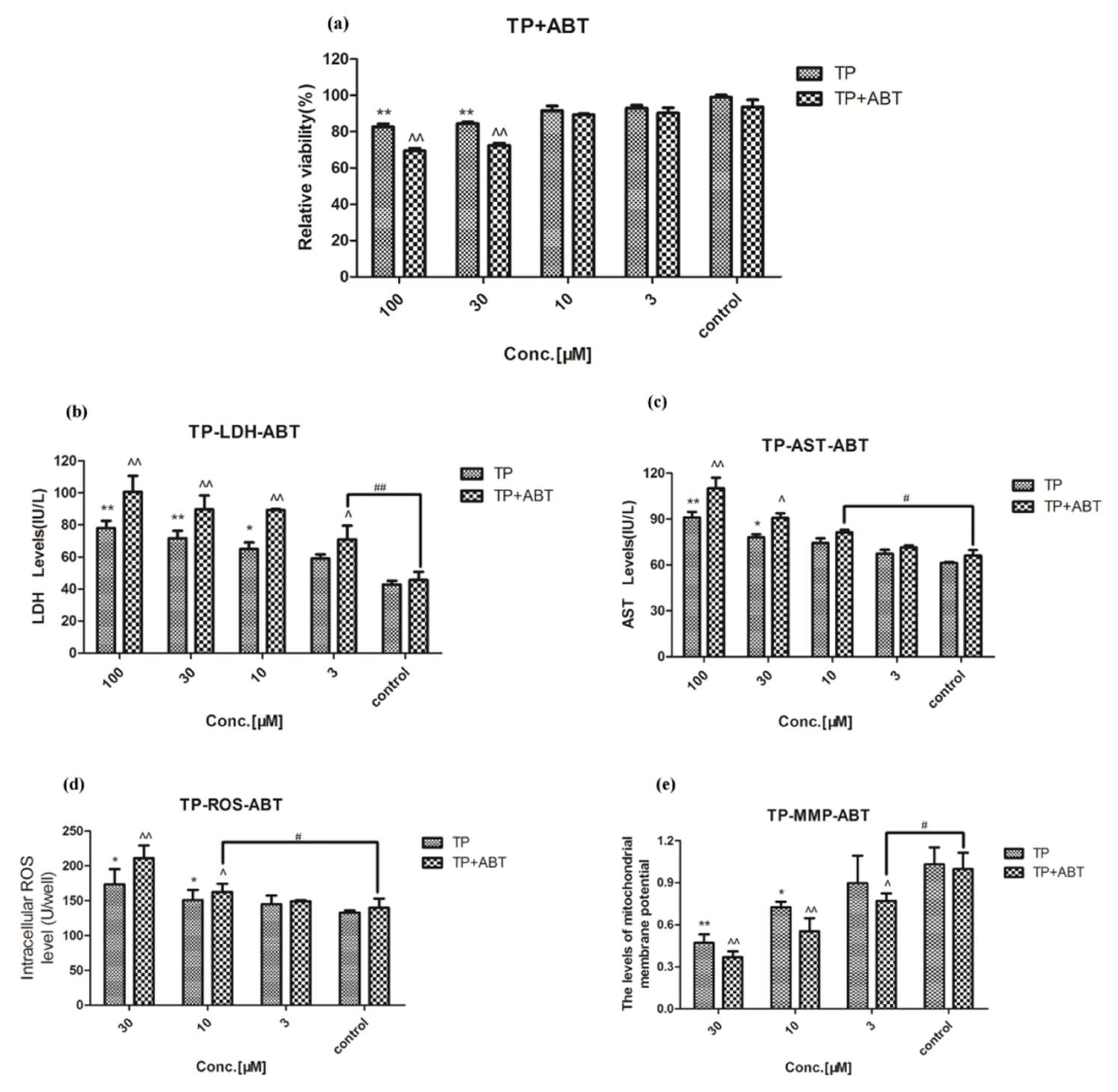
2.2. Effect of Celastrol and Triptolide on Primary Rat Hepatocytes Model with ABT
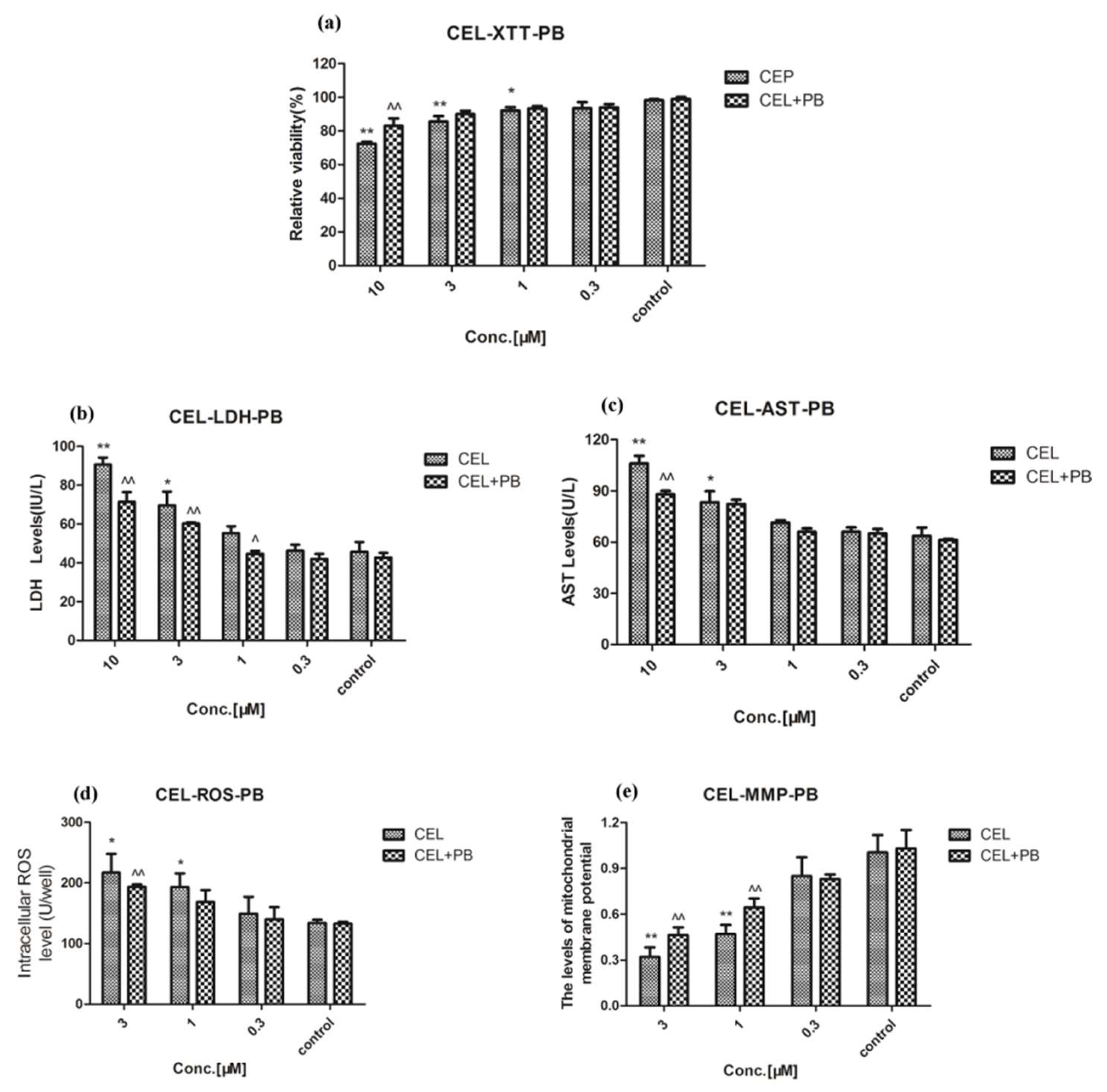
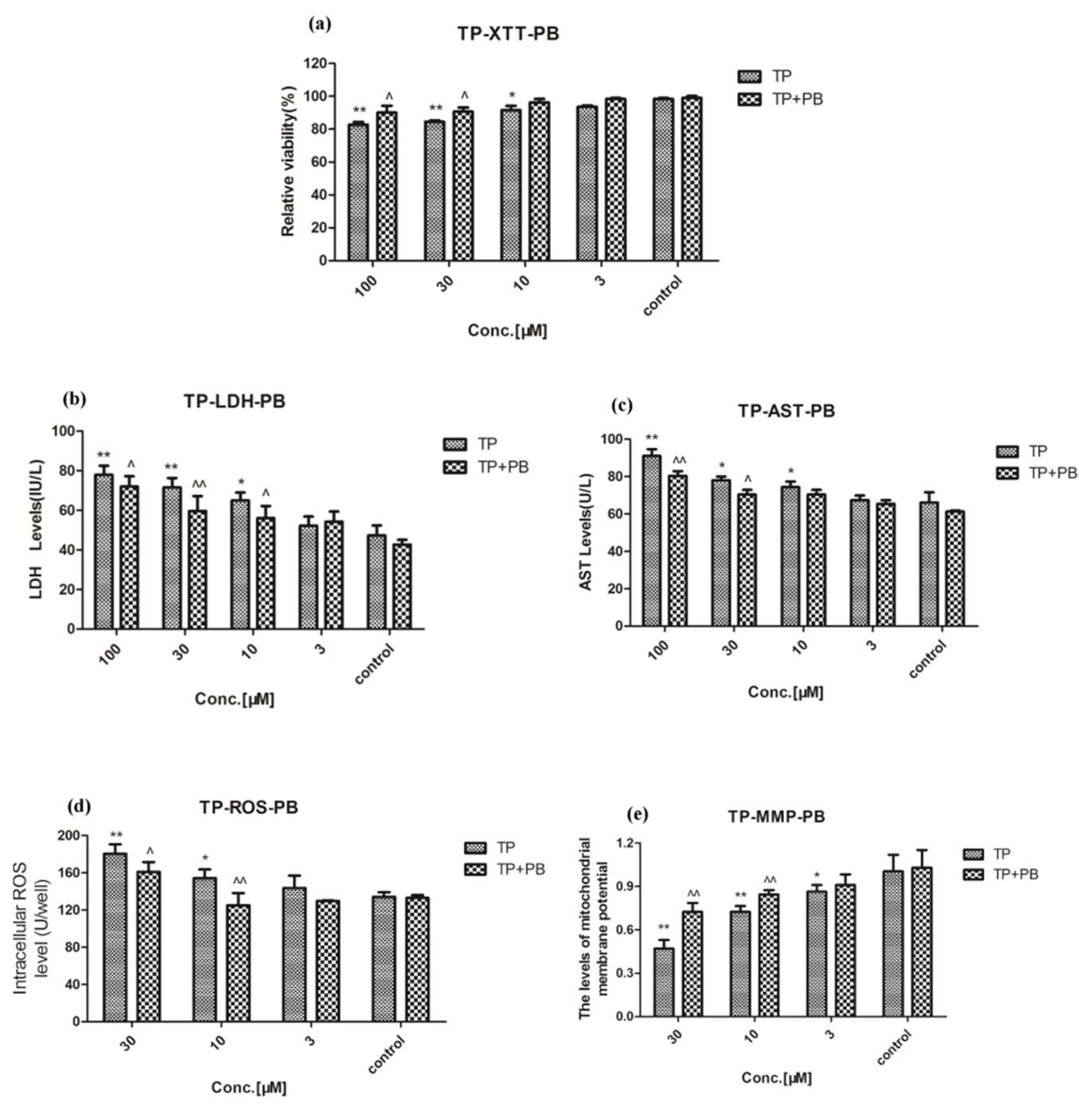
2.3. Effect of Celastrol and Triptolide on Primary Rat Hepatocytes Model with PB
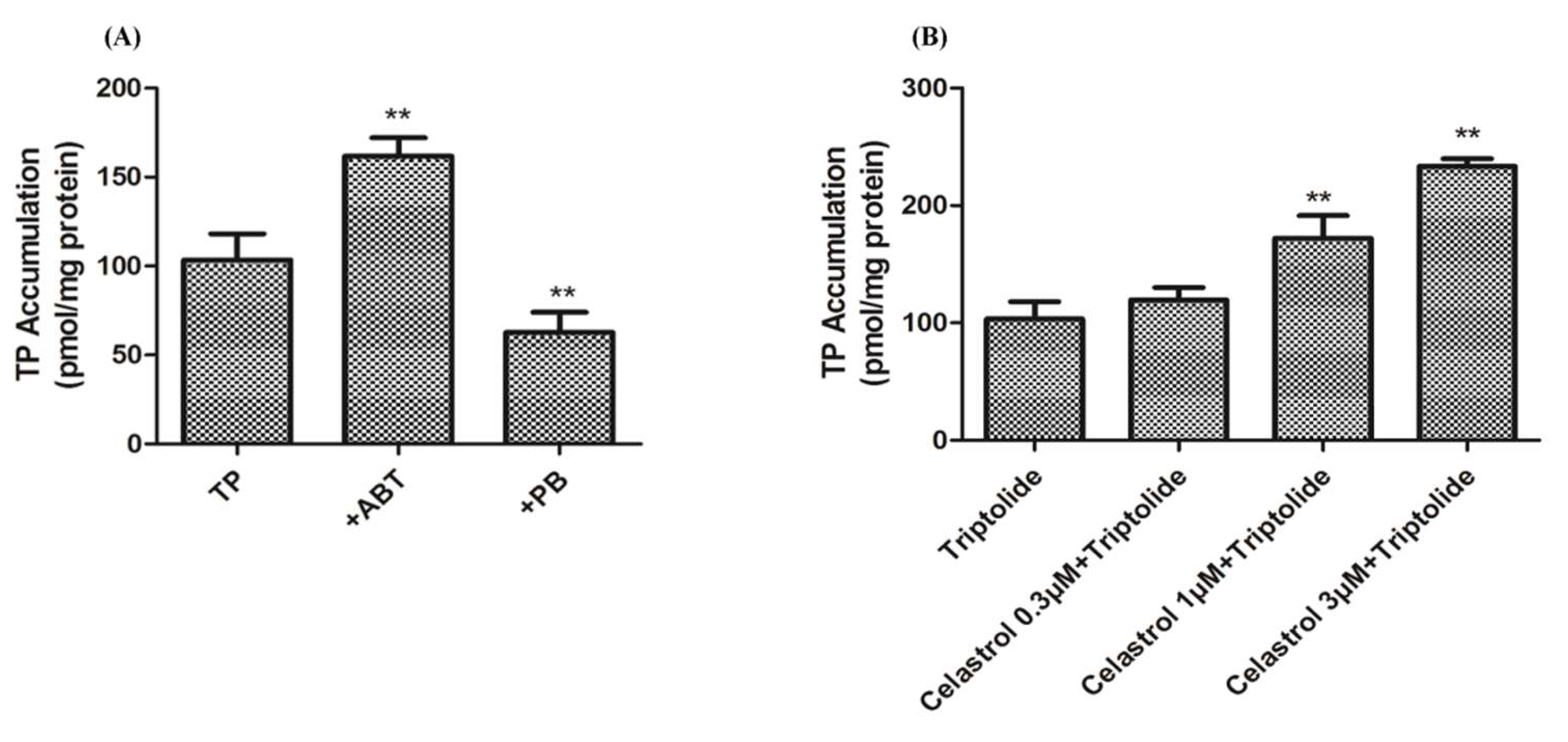
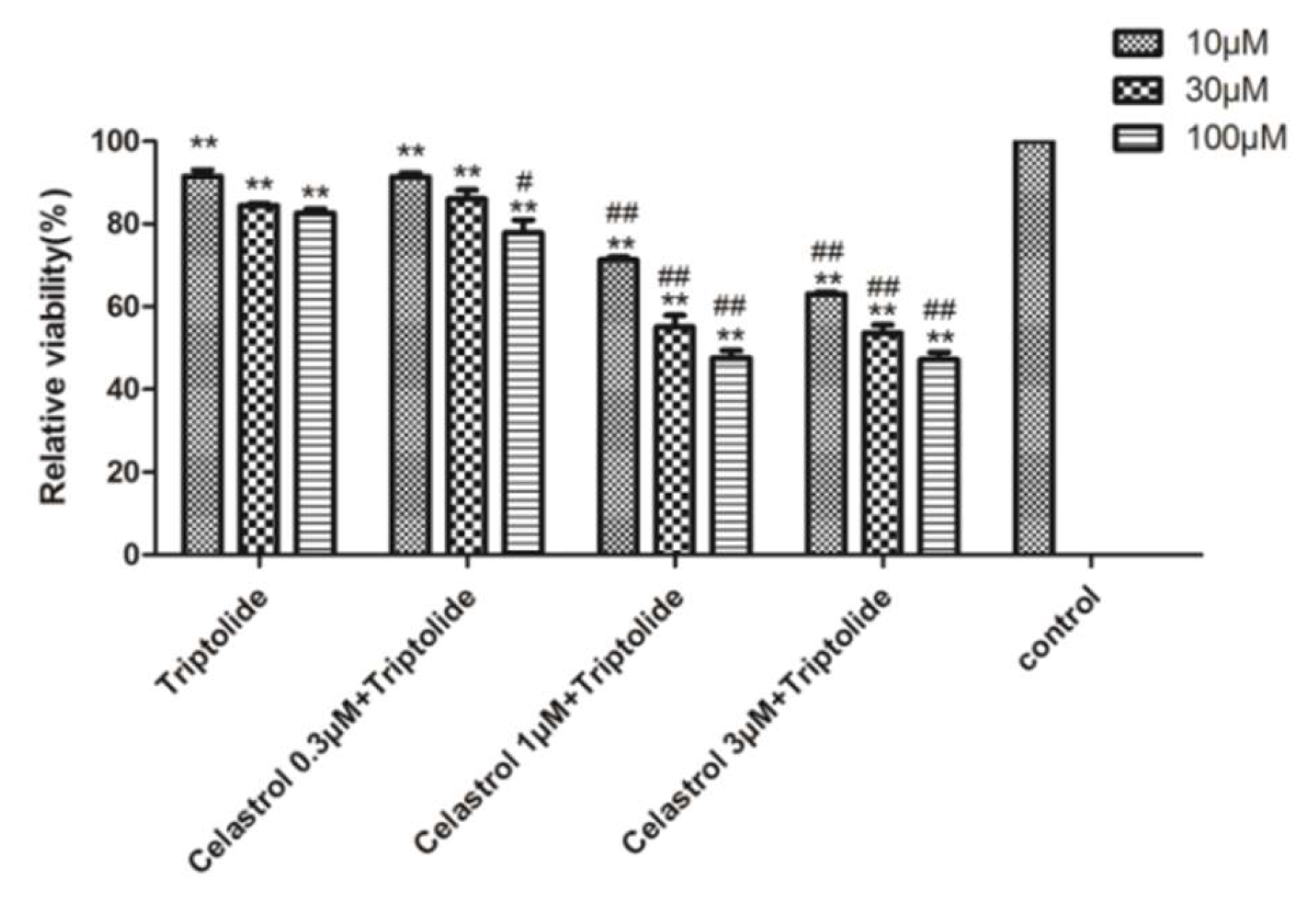
2.4. Drug-Drug Interaction between Celastrol and Triptolide
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals
4.3. Primary Rat Hepatocytes Model for Evaluating the Toxicity of Celastrol and Triptolide
4.3.1. Isolation and Culture of Hepatocyte
4.3.2. XTT Assay
4.3.3. The Determination of LDH and AST Level
4.3.4. Detection of ROS Generation
4.3.5. The Determination of MMP
4.4. Evaluate Toxicity of Celastrol and Triptolide on Primary Rat Hepatocytes with Subdued Enzyme Activity
4.5. Evaluating the Toxicity of Celastrol and Triptolide on Primary Rat Hepatocytes with Enhanced Enzyme Activity
4.6. Cell Amount of Triptolide Assay
4.7. LC-MS/MS Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CYP450 | Cytochrome P450 |
| XTT | 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H- tetrazolium-5-carboxanilide |
| LDH | lactic dehydrogenase |
| AST | aspartate aminotransferase |
| ROS | reactive oxygen species |
| MMP | mitochondrial membrane potential |
| ABT | 1-aminobenzotriazole |
| PB | phenobarbital |
| FBS | fetal bovine serum |
| S-P | streptomycin-penicillin |
| EDTA | trypsin-ethylenediaminetetraacetic acid |
| DMSO | dimethyl sulfoxide |
| DMEM | Dulbecco’s modified Eagle’s medium |
| PBS | phosphate buffer saline |
| DCFH-DA | 2′, 7′-dichlorofluorescin diacetate |
| FDA | food and drug administration |
| MI | metabolic intermediate |
| MRM | multiple reaction monitoring |
| ESI-MS | electrospray ionization mass spectrometry |
| SD | standard deviation |
References
- Tao, X.L.; Cush, J.J.; Garret, M.; Lipsky, P.E. A phase I study of ethyl acetate extract of the chinese antirheumatic herb tripterygium wilfordii hook f in rheumatoid arthritis. J. Rheumatol. 2001, 28, 2160–2167. [Google Scholar]
- Chen, Y.F.; Cai, H.D. Investigation of liver damage associated with traditional chinese medicines. Adv. Drug Rea. J. 1999, 24, 27–32. [Google Scholar]
- Gu, S.P.; Fan, Y.W.; Wang, L.F.; Feng, G.; Liu, S.S.; Sheng, Y.; He, X. Analysis on release characteristics of sustained release traditional chinese medicine preparation of leigongteng bilayer tablets based on drug simulating system. Chin. J. Exp. Trad. Med. Formulae 2015, 56, 549–567. [Google Scholar]
- Lipsky, P.E.; Tao, X.L. A potential new treatment for rheumatoid arthritis: Thunder god vine. Semin Arthritis Rheum. 1997, 26, 713–723. [Google Scholar] [CrossRef]
- Bao, J.; Dai, S.M. A chinese herb tripterygium wilfordii hook f. in the treatment of rheumatoid arthritis: Mechanism, efficacy, and safety. Rheumatol. Int. 2011, 31, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Allison, A.C.; Cacabelos, R.; Lombardi, V.R.M.; Xoan, A.A.; Vigo, C. Celastrol, a potent antioxidant and anti-inflammatory drug, as a possible treatment for alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2001, 25, 1341–1357. [Google Scholar] [CrossRef]
- Han, R.; Rostami-Yazdi, M.; Gerdes, S.; Mrowietz, U. Triptolide in the treatment of psoriasis and other immune-mediated inflammatory diseases. Br. J. Clin. Pharmacol. 2012, 74, 424–436. [Google Scholar] [CrossRef]
- Sethi, G.; Ahn, K.S.; Pandey, M.K.; Aggarwal, B.B. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-κB-regulated gene products and tak1-mediated NF-κB activation. Blood 2007, 109, 2727–2735. [Google Scholar]
- Yang, H.; Chen, D.; Cui, Q.C.; Yuan, X.; Dou, Q.P. Celastrol, a triterpene extracted from the chinese “thunder of god vine,” is a potent proteasome inhibitor and suppresses human prostate cancer growth in nude mice. Cancer Res. 2006, 66, 4758–4765. [Google Scholar] [CrossRef]
- Huang, Y.L.; Zhou, Y.X.; Fan, Y.S.; Zhou, D. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing vegfr expression. Cancer Lett. 2008, 264, 101–106. [Google Scholar] [CrossRef]
- Committee of Pharmacopeia. Drug Standard of Ministry of Public Health of the People’s Republic of China; WS3-B-3350-98-2011; Committee of Pharmacopeia: Beijing, China, 2011.
- Gu, S.P.; Fu, S.J.; Ally, M.; Xun, M.J.; Sheng, Y.; He, X. Simultaneous determination and quality evaluation of five effective components in tripterygium tablets. Tianjin J. Traditional. Chinese Med. 2015, 32, 38–41. [Google Scholar]
- Bing, Z.; Liu, G.T. Cytotoxic effect of hydrogen peroxide on primary cultured rat hepatocytes and its mecha nisms. Chin. J. Pharmacol. Toxicol. 1996, 10, 260–266. [Google Scholar]
- Seglen, P.O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976, 13, 29–83. [Google Scholar] [PubMed]
- Ismail, T.; Howl, J.; Wheatley, M.; McMaster, P.; Neuberger, J.M.; Strain, A.J. Growth of normal human hepatocytes in primary culture: Effect of hormones and growth factors on dna synthesis. Hepatology 2010, 14, 1076–1082. [Google Scholar] [CrossRef]
- Sarkis, R.; Benoist, S.; Honiger, J.; Baudrimont, M.; Delelo, R.; Balladur, P.; Capeau, J.; Nordinger, B. Transplanted cryopreserved encapsulated porcine hepatocytes are as effective as fresh hepatocytes in preventing death from acute liver failure in rats. Transplantation 2000, 70, 58–64. [Google Scholar] [PubMed]
- US Food and Drug Administration. Guidance for Industry: Drug Interaction Studies-Study Design, Data Analysis, Implications for Dosing, and Labeling Recommendations; US Food and Drug Administration: Silver Spring, MD, USA, 2012.
- Varma, M.V.; Lai, Y.R.; Kimoto, E.; Goosen, T.C.; EL-Kattan, A.F.; Kumar, V. Mechanistic modeling to predict the transporter- and enzyme-mediated drug-drug interactions of repaglinide. Pharm. Res. 2013, 30, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Thummel, K.E.; Wilkinson, G.R. In vitro and in vivo drug interactions involving human CYP3A. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 389–430. [Google Scholar] [CrossRef]
- Tredger, J.M.; Stoll, S. Cytochromes P450 - their impact on drug treatment. Hospital Pharmacist. 2002, 9, 167–173. [Google Scholar]
- Wang, D.; Guo, Y.; Wrighton, S.A.; Cooke, G.E.; Sadee, W. Intronic polymorphism in CYP3A4 affects hepatic expression and response to statin drugs. Pharmacogenomics J. 2011, 11, 274–286. [Google Scholar] [CrossRef]
- Ghotbi, R. Genetic, epigenetic and environmental factors of importance for CYP1A2 catalyzed drug metabolism. Institutionen For Laboratoriemed. 2008. [Google Scholar]
- Faber, M.S.; Jetter, A.; Fuhr, U. Assessment of CYP1A2 activity in clinical practice: Why, how, and when? Basic Clin. Pharmacol. Toxicol. 2005, 97, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Choi, H.K.; Jeong, T.C.; Jahng, Y.D.; Kim, D.H.; Lee, S.H.; Lee, S.K. Selective inhibitory effects of mollugin on CYP1A2 in human liver microsomes. Food Chem. Toxicol. 2013, 51, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Hamadeh, H.K. Gene expression analysis reveals chemical-specific profiles. Toxicol Sci. 2002, 67, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Yang, J.; Zhao, X.; Jiao, X.L.; Zhao, W.H.; Ma, Q.; Tang, Z.Y.; Dai, R.K. Induction of P450 3A1/2 and 2A6 by gemfibrozil in sprague-dawley rats. Pharmacol. Rep. 2011, 63, 157–164. [Google Scholar] [CrossRef]
- Arinç, E.; Arslan, S.; Bozcaarmutlu, A.; Adali, O. Effects of diabetes on rabbit kidney and lung CYP2E1 and CYP2B4 expression and drug metabolism and potentiation of carcinogenic activity of n-nitrosodimethylamine in kidney and lung. Food Chem. Toxicol. 2007, 45, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Zuber, R.; Anzenbacherová, E.; Anzenbacher, P. Cytochromes P450 and experimental models of drug metabolism. J. Cell Mol. Med. 2002, 6, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Matsuki, Y.; Yamaguchi, T.; Oguri, K. Effect of a ligand selective for peripheral benzodiazepine receptors on the expression of rat hepatic p-450 cytochromes: Assessment of the effect in vivo and in a hepatocyte culture system. Drug Metab. Dispos. 1999, 27, 1242–1247. [Google Scholar] [PubMed]
- Donato, M.T.; Viitala, P.; Rodriguezantona, C.; Lindfors, A.; Castell, J.V.; Raunio, H.; Gómez-Lechón, M.J.; Pelkonen, O. CYP2A5/CYP2A6 expression in mouse and human hepatocytes treated with various in vivo inducers. Drug Metab. Dispos. 2000, 28, 1321–1326. [Google Scholar]
- Le marchand, L.; Guo, C.F.; Benhanmon, S.; Bouchardy, C.; Cascorbi, I.; Clapper, M.L.; Garte, S.; Haugen, A.; Ingelman-Sundberg, M.; Kihare, M.; et al. Pooled analysis of the CYP1A1 exon 7 polymorphism and lung cancer (United States). Cancer Causes Control 2003, 14, 339–346. [Google Scholar] [CrossRef]
- Stresser, D.M.; Kupfer, D. Monospecific antipeptide antibody to cytochrome P-450 2B6. Drug Metab. Dispos. 1999, 27, 517–525. [Google Scholar]
- Ogilvie, B.W.; Zhang, D.; Li, W.; Rodrigues, A.D.; Parkinson, A. Glucuronidation converts gemfibrozil to a potent, metabolism-dependent inhibitor of CYP2C8: Implications for drug-drug interactions. Drug Metab. Dispos. 2006, 34, 191–197. [Google Scholar] [CrossRef]
- Ye, X.C.; Li, W.Y.; Yan, Y.; Mao, C.W.; Cai, R.X.; Xu, H.B.; Yang, X.L. Effects of cytochrome P450 3A inducer dexamethasone on the metabolism and toxicity of triptolide in rat. Toxicol. Lett. 2010, 192, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.H.; He, X.; Zhang, F.L.; He, L.N.; Chen, J.X.; Wang, L.L.; An, L.J.; Fan, Y.W. Inhibitory mechanisms of celastrol on human liver cytochrome P450 1A2, 2C19, 2D6, 2E1 and 3A4. Xenobiotica 2015, 45, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Seglen, P.O. Preparation of rat liver cells. I. effect of Ca2+ on enzymatic dispersion of isolated, perfused liver. Exp. Cell Res. 1972, 74, 450–454. [Google Scholar] [CrossRef]
- Mathews, J.M.; Dostal, L.A.; Bend, J.R. Inactivation of rabbit pulmonary cytochrome P-450 in microsomes and isolated perfused lungs by the suicide substrate 1-aminobenzotriazole. J. Pharmacol. Exp. Ther. 1985, 235, 186–190. [Google Scholar]
- Huijzer, J.C.; Adams, J.D., Jr.; Jaw, J.Y.; Yost, G.S. Inhibition of 3-methylindole bio-activation by the cytochrome P-450 suicide substrates 1-aminobenzotriazole and alpha-methylbenzylaminobenzotriazole. Drug Metab. Dispos. 1989, 17, 37–42. [Google Scholar]
- Linder, C.D.; Renaud, N.A.; Hutzler, J.M. Is 1-aminobenzotria zole an appropriate in vitro tool as a nonspecific cytochrome P450 inactivator? Drug Metab. Dispos. 2009, 37, 10–13. [Google Scholar] [CrossRef]
- Waxman, D.J.; Azaroff, L. Phenobarbital induction of cytochrome p-450 gene expression. Biochem. J. 1992, 281, 577–592. [Google Scholar] [CrossRef]
- Zhai, Y.R.; Wang, L.L.; Chen, J.X.; He, X. Establishment of primary rat hepatocytes model with high cyps activity and its application for evaluating drug-induced liver injury. Drug Eval. Res. 2015, 38, 368–374. [Google Scholar]
Sample Availability: Sample Availability: Samples of the compounds are not available from the authors. |

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, C.; Wu, Z.; Wang, L.; Kanai, Y.; He, X. CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium wilfordii. Molecules 2019, 24, 2162. https://doi.org/10.3390/molecules24112162
Jin C, Wu Z, Wang L, Kanai Y, He X. CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium wilfordii. Molecules. 2019; 24(11):2162. https://doi.org/10.3390/molecules24112162
Chicago/Turabian StyleJin, Chunhuan, Zijun Wu, Lili Wang, Yoshikatsu Kanai, and Xin He. 2019. "CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium wilfordii" Molecules 24, no. 11: 2162. https://doi.org/10.3390/molecules24112162
APA StyleJin, C., Wu, Z., Wang, L., Kanai, Y., & He, X. (2019). CYP450s-Activity Relations of Celastrol to Interact with Triptolide Reveal the Reasons of Hepatotoxicity of Tripterygium wilfordii. Molecules, 24(11), 2162. https://doi.org/10.3390/molecules24112162




