Abstract
The photocatalytic activities of reduced titanium dioxide (TiO2) materials have been investigated by measuring their ability to produce hydroxyl radicals under UV and visible light irradiation. Degussa P25 TiO2 was doped with nitrogen (N), fluorine (F), and/or phosphorus (P) and then subjected to surface modification employing a thermo-physicochemical process in the presence of reducing agent sodium borohydride (NaBH4). The reduced TiO2 materials were characterized by a number of X-ray, spectroscopic and imaging methods. Surface doping of TiO2 was employed to modulate the band gap energies into the visible wavelength region for better overlap with the solar spectrum. Hydroxyl radical generation, central to TiO2 photocatalytic water purification applications, was quantitated using coumarin as a trap under UV and visible light irradiation of the reduced TiO2 materials. At 350 nm irradiation, the yield of hydroxyl radicals generated by the reduced forms of TiO2 was nearly 90% of hydroxyl radicals generated by the Degussa P25 TiO2. Hydroxyl radical generation by these reduced forms of TiO2 was also observed under visible light irradiation (419 and 450 nm). These results demonstrated that simple surface modification of doped TiO2 can lead to visible light activity, which is important for more economical solar-driven applications of TiO2 photocatalysis.
1. Introduction
While titanium dioxide (TiO2) has attracted remarkable attention as a photocatalyst over the past few decades for the treatment of wastewater and the degradation of hazardous chemicals, significant industrial applications and commercial benefits have yet to be realized [1,2,3]. TiO2 photocatalysis requires absorption of a photon, typically in the UV region (λ < 400 nm), to generate an electron (e−CB)/hole(h+VB) pair, Equation (1), which can initiate degradation through the production of reactive oxygen species (ROS) as outlined below in Equations (1)–(5). The recombination of the electron/hole pair is often a dominant process which eliminates the potential for the formation of ROS. In competition with recombination, the electron/hole pairs can migrate to the surface of TiO2 and form ROS in the presence of water and oxygen [4,5,6,7]. While direct oxidation and reduction of target substrates by electron/hole pair can also be envisioned, such direct transformations are generally not competitive in aqueous solution. The primary processes leading to degradation of target pollutants usually involve the formation of ROS, hydroxyl radicals (•OH), Equations (2) and (5), superoxide anion radicals (O2•−), Equation (3), and hydrogen peroxide (H2O2), Equation (4). Although a number of ROS are generated in a TiO2 photo-oxidation process and can lead to the mineralization of organic pollutants, hydroxyl radical (•OH) is considered the primary species leading to degradation [8,9,10].
TiO2 + hv → h+VB + e−CB
H2O + h+VB → H+ + •OH
O2 + e−CB → O2•−
2H2O + 2O2•− → H2O2 + 2HO• + O2
H2O2 + e−CB → 2HO•
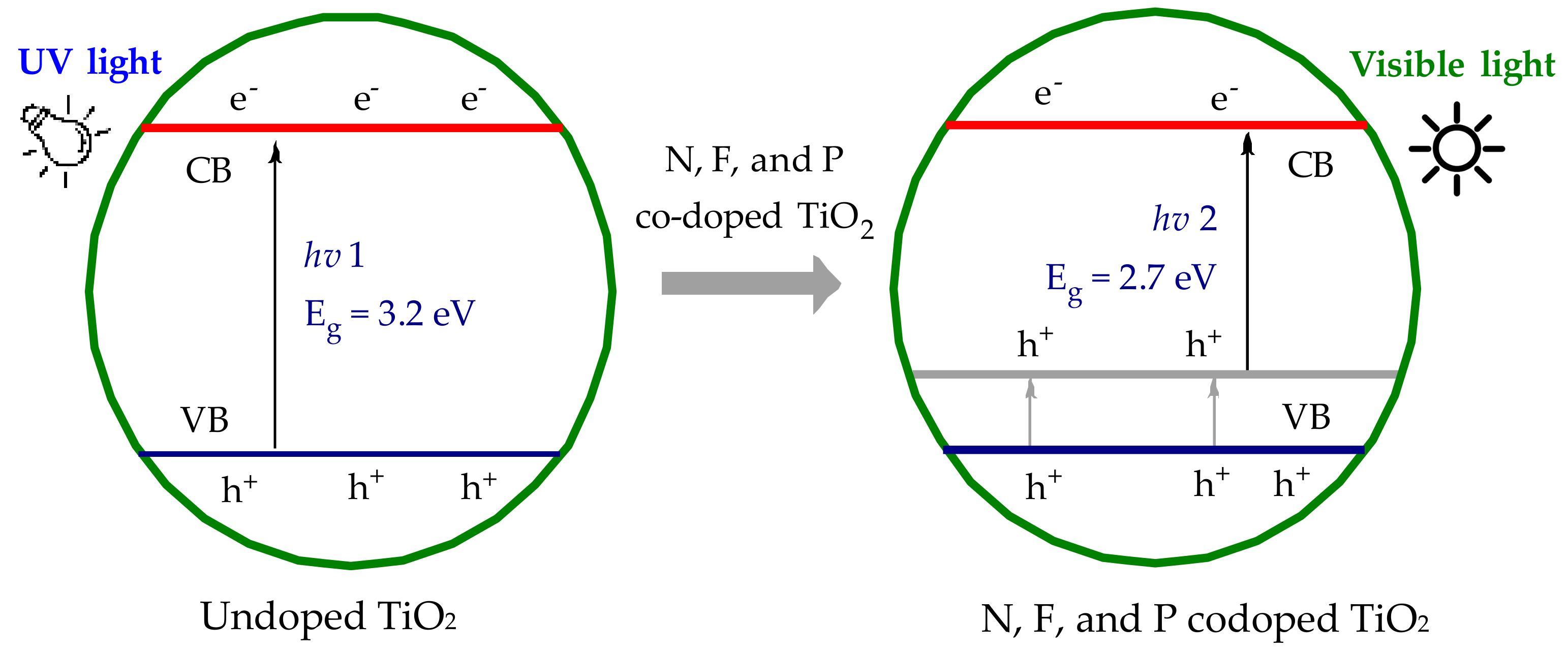
TiO2 is readily available, inexpensive, and chemically stable; however, there are two major drawbacks to TiO2 photocatalysis [3,11]. Firstly, the photo-excitation of unmodified TiO2 requires UV light. Secondly, the quantum yield for photochemical production of ROS is low due to the rapid recombination of electron/hole pairs [7,12]. The solar spectrum is predominately in the visible region, with only ~5% of the total irradiation in the UV region. A major challenge in TiO2 photocatalysis is to utilize the visible region of the solar spectrum. Extension of the TiO2 light absorption profile into the visible light region offers significantly improved economic viability compared to those requiring UV activation. A number of techniques including nonmetal doping with nitrogen (N), sulfur (S), carbon (C), fluorine (F), iodine (I), or boron (B), nonmetal codoping, metal deposition, dye sensitization, semiconductor coupling, and enriching the oxygen content of TiO2 have led to a variety of visible light activated (VLA) TiO2 materials over the last decade [1,3,13,14,15,16,17,18,19,20]. A number of metal doping materials has been previously explored; however, metal doping often produces undesirable metal titanates at high processing temperatures [21,22,23,24]. Nonmetal doping or codoping into the TiO2 modifies the valence band level of TiO2, reducing the effective band gap energy, as shown in Figure 1, and modulating the light absorption into the visible light region [25,26,27,28,29,30]. Although the incorporation of dopant elements can result in the advantage of narrowing band gap energies, dopants can influence charge carrier recombination processes, and the limited solubility of dopants into the bulk materials as well as thermal instability have limited photocatalytic applications of doped TiO2 materials [31,32,33,34,35].
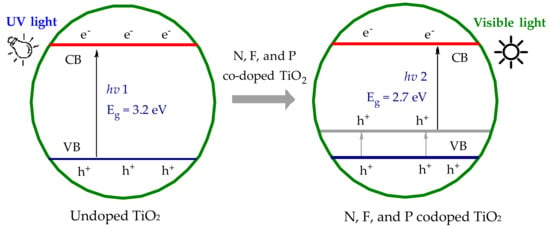
Figure 1.
Schematic band gap energy levels of doped and undoped TiO2; Eg stands for the band energy gap between the valance band (VB) and conduction band (CB).
Simple reduction of Ti within the TiO2 matrix leads to stable colored TiO2 with visible light absorbing properties, and these materials have attracted extensive attention due to their enhanced photocatalytic activity [36,37,38,39]. Notably, black TiO2 prepared from the hydrogenation of TiO2 nanocrystals at high-pressure exhibits relatively low band gap energy and enhanced photocatalytic activity for water splitting and dye degradation [36]. A number of approaches, including high pressure, high temperature, plasma-assisted hydrogenation, and high-temperature aluminum vapor reduction, have been explored for preparation of VLA TiO2 materials [37,38,39,40]. High-pressure and/or high-temperature protocols are often experimentally challenging and/or expensive. Recently, Tan et al. developed a fast and simple chemical reduction method for the production of colored TiO2 through a controllable solid-state reaction of NaBH4 and crystalline TiO2 at moderate temperature (< 300 °C) [41]. The resulting reduced TiO2 material possessed a narrow band gap with a crystalline core/amorphous shell structure (TiO2@TiO2 − x) containing oxygen vacancies at the surface of TiO2 promoting the separation of charge carriers and leading to improved VLA photocatalytic performance. Herein, we synthesized reduced codoped P25 TiO2 through thermal treatment in the presence of NaBH4 in an attempt to extend the TiO2 photocatalytic function into the visible light region.
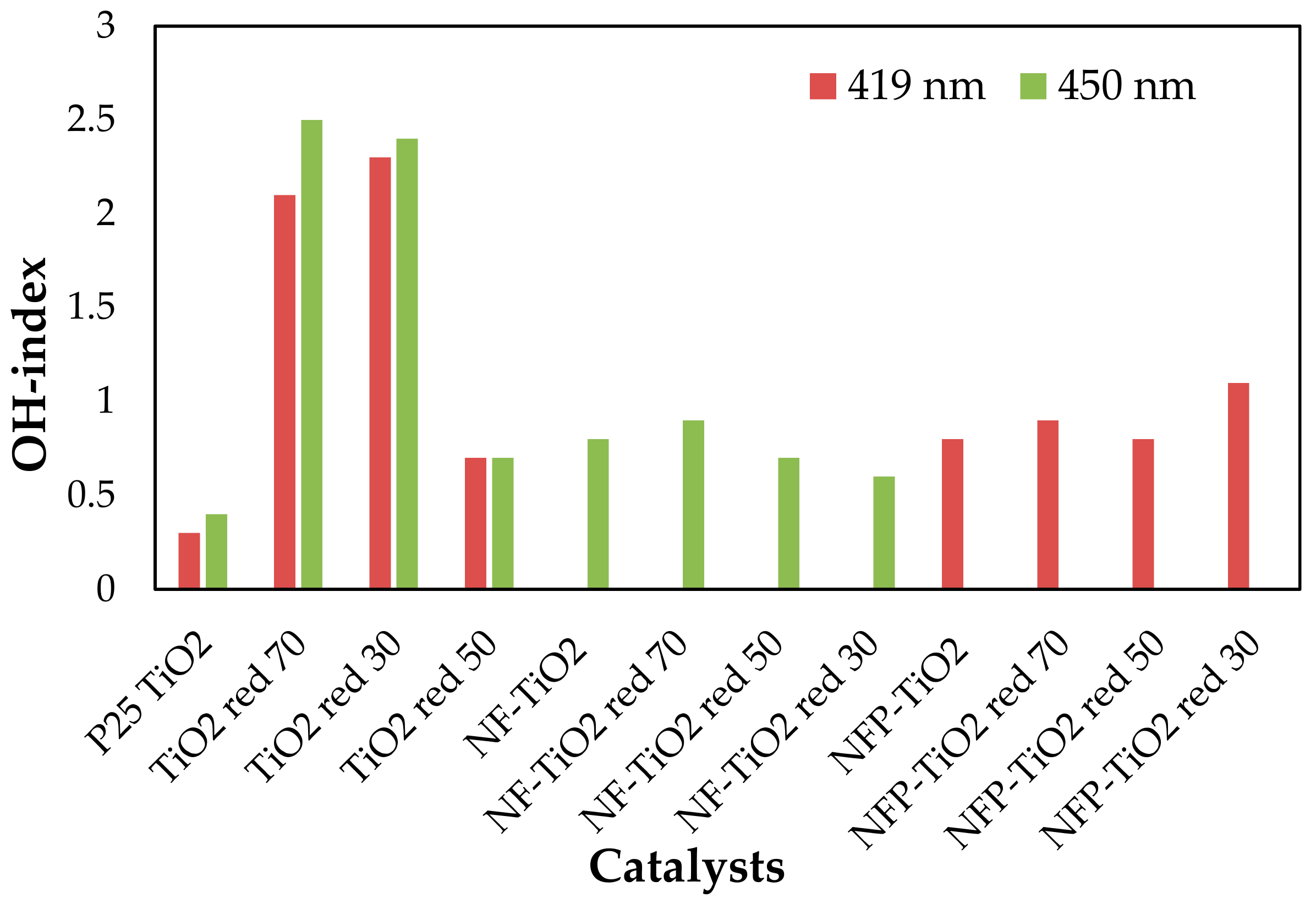
Although reduction and codoping of TiO2 can narrow band gap energies appropriate for the absorption of visible light, enhanced visible light photocatalytic activity is not ensured [3]. The photocatalytic activity is generally associated with a redox reaction initiated by the photogenerated charge carriers. Assessment and comparisons of the photocatalytic activity of different TiO2 materials are challenging due to the variation of experimental conditions, equipment design, flux intensity, loading, band gap, surface area, and crystal lattice and composition. While the application of TiO2 photocatalysis for remediation of pollutants and toxins in aqueous media has been studied extensively, assessing the specific effectiveness of the photocatalyst is still not standardized [42,43,44,45,46,47]. Since •OH is generally the primary species initiating the degradation of target pollutants during TiO2 photocatalysis, monitoring •OH production during TiO2 photocatalysis is an effective way to evaluate TiO2 photocatalytic activity. Noteworthy, Xiang et al. reported an OH-index parameter, as expressed in Equation (6), to assess the activity of a photocatalyst [47].
OH-index = (r/r0) × 100
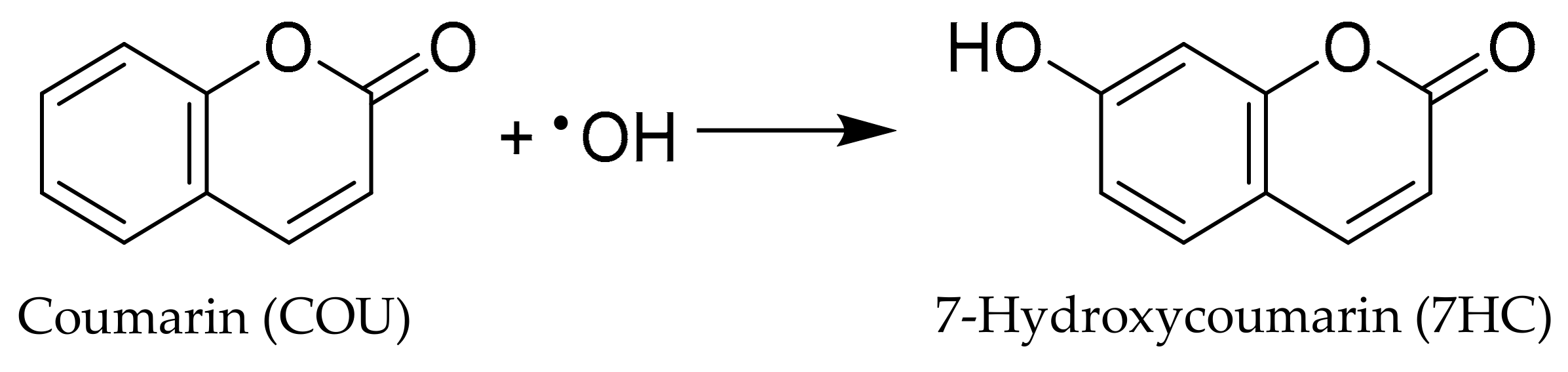
Direct measurement of •OH is difficult because its short wavelength absorbance (<230 nm) makes it difficult to monitor and its high reactivity results in an extremely short lifetime (often 10−9 s) [10]. Given these limitations, indirect methods have been established to trap hydroxyl radicals with the formation of stable hydroxyl radical adducts. Among hydroxyl radical traps, coumarin (COU) forms a hydroxyl radical adduct which can be readily quantitated using fluorescence spectroscopy. We have employed coumarin which yields 7-hydroxycoumarin (7HC) upon trapping of hydroxyl radical, as shown in Scheme 1. The concentration of 7HC measured by fluorescence is proportional to the production of hydroxyl radicals by TiO2 materials under UV and visible light [48].
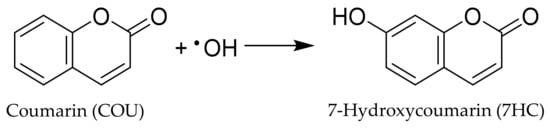
Scheme 1.
The reaction of COU and •OH for highly florescent 7HC production.
One of the purposes of this study was to develop visible light active forms of TiO2 through a simple reduction process. We prepared the VLA TiO2 materials using a controllable solid state thermal physicochemical process. Detailed physical and chemical characterization of the prepared materials, along with their optical properties, were carried out by a number of techniques including X-ray powder diffraction (XRD), high-resolution transmission electron microscope (HRTEM), energy dispersive X-ray fluorescence (EDXRF), ultraviolet-visible (UV-Vis) spectroscopy, and Raman spectroscopy. Hydroxyl radical production is reported as a measure of the viability of these TiO2 materials for the destruction of environmental contaminants in potential water treatment applications. The study also compares the relative photocatalytic efficiency of prepared TiO2 materials using OH-index. Our results provide insight into the development of visible light-activated photocatalysts for the destruction of organic pollutants in water.
2. Results and Discussions
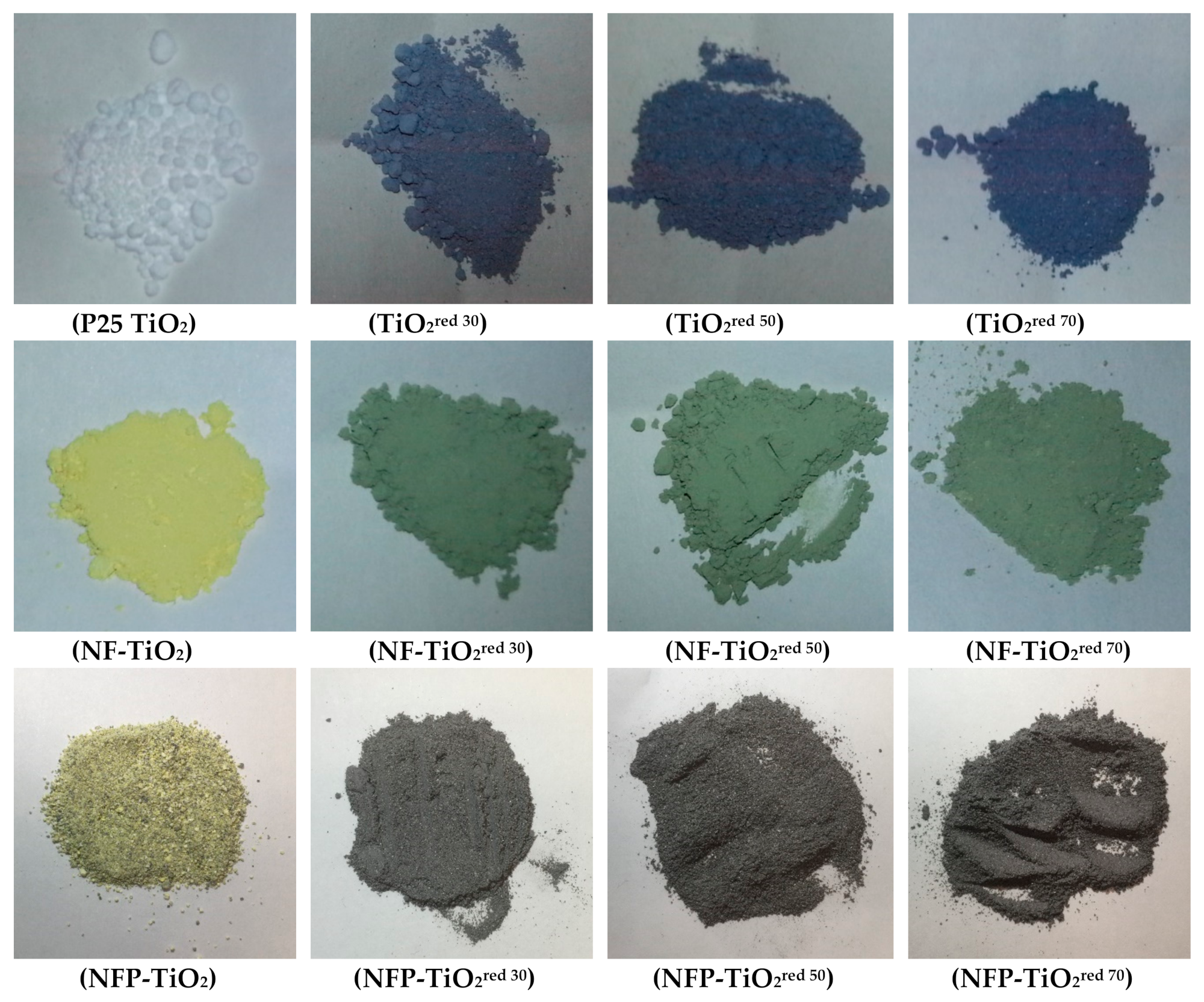
A series of reduced TiO2 materials (identified as TiO2red) for P25 TiO2 and N, F, and/or P codoped TiO2 (NF-TiO2 and NFP-TiO2) were synthesized by thermal treatment with NaBH4 for 30, 50, and 70 min following the reported method [41] as detailed in the experimental section. The specific conditions of thermal treatment used in the preparation of reduced TiO2 materials are tabulated in Table 1, and the optical reflectance of representative samples are shown in Figure 2.

Table 1.
The prepared TiO2 materials as a function of reduction time with NaBH4 along with their thicknesses of the amorphous layer.
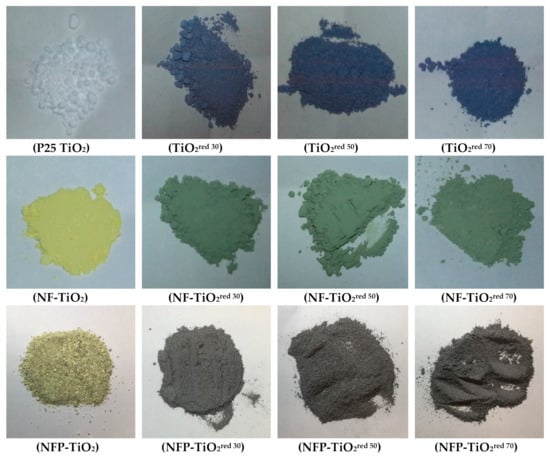
Figure 2.
Color photographs of reduced TiO2 synthesized by thermal treatment with NaBH4.
2.1. Phase Structure Analysis
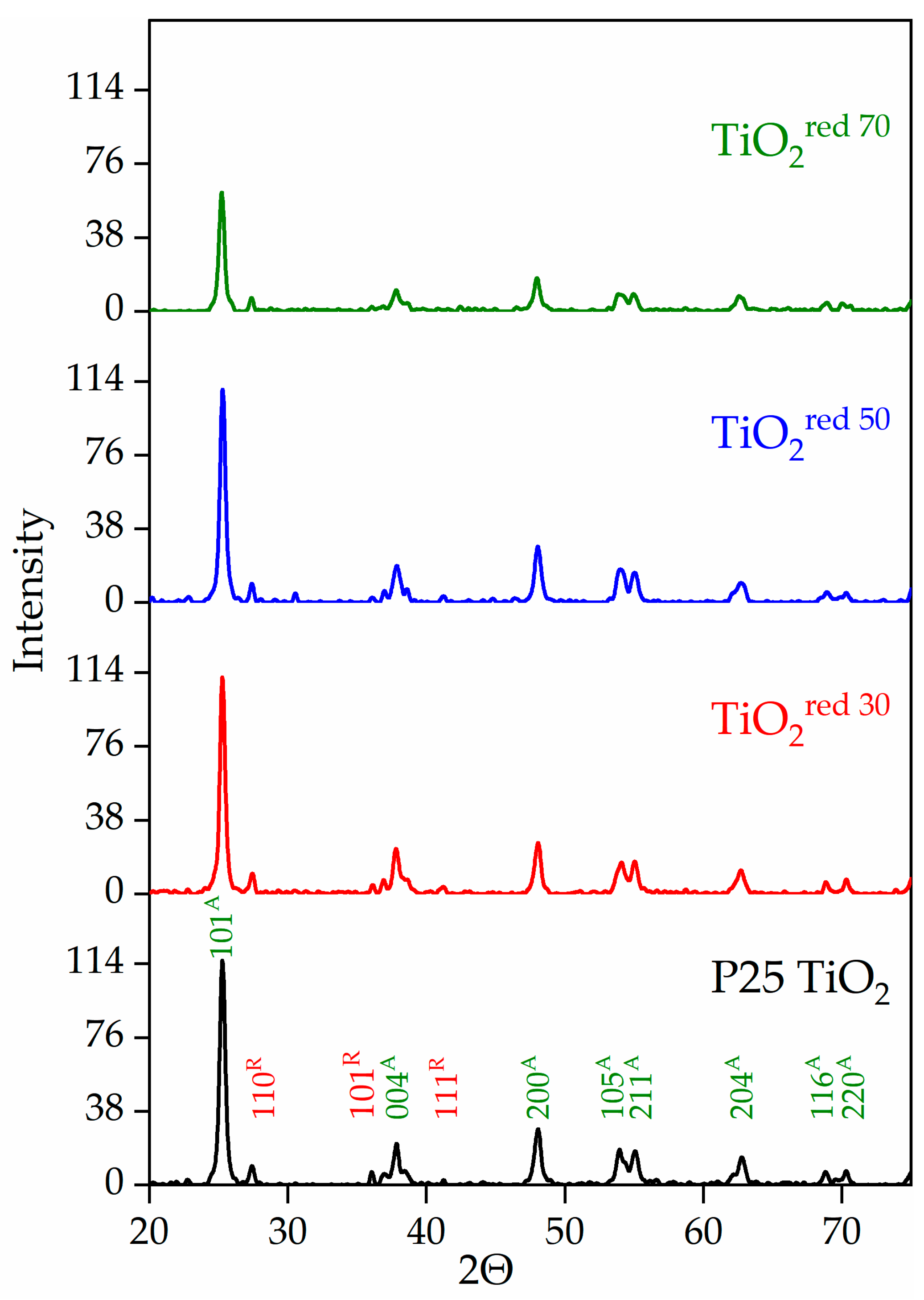
The performance of a photocatalyst is often dependent on the many factors, including the crystal structure, surface area, and composition [18,22,49]. TiO2 can exist as three different forms; anatase, rutile, and brookite [21,49,50,51,52]. Anatase is considered the most photocatalytically active crystal form. The photocatalytic activity of TiO2 can vary dramatically based on its lattice structure, and thus, X-ray powder diffraction (XRD) patterns were used to analyze the crystalline properties of synthesized TiO2 samples. The XRD data of P25 TiO2 and reduced TiO2 are provided in Figure 3. All observed peaks in the XRD patterns of the reference P25 TiO2 and the reduced TiO2red were indexed to anatase and rutile phases. The intensity of XRD peaks for TiO2red 70 associated with the anatase phase, however, decreased, indicating lower crystallization due to surface modification at longer treatment times without a significant change of crystallinity. Anatase (tetragonal structure, a and b) 3.78 Å; c) 9.50 Å) and rutile (tetragonal structure, a and b) 4.58 Å; c) 2.95 Å) are the two major forms of crystalline TiO2. Titanium oxide can also exist in another thermodynamically unstable form called brookite (rhombohedral structure, a) 5.43 Å; b) 9.16 Å; c) 5.13 Å). The brookite phase was not detected in any of the samples in this study. Previous experiments showed that anatase is kinetically more stable than rutile while rutile is reported to be more thermodynamically stable than anatase at normal atmospheric pressure and temperature [11,51]. It has been found that a crystalline mixture of the two different phases of titanium dioxide, such as anatase and rutile is more photoactive than 100% anatase [51,52,53].
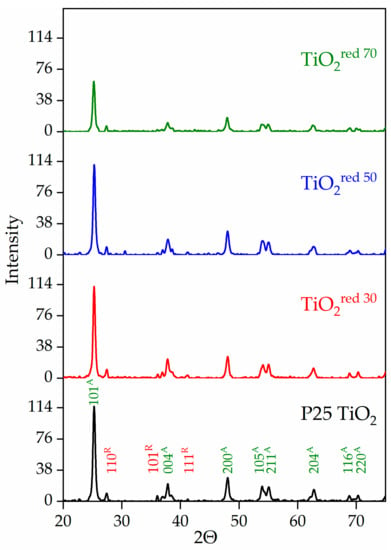
Figure 3.
Comparison of XRD patterns among P25 TiO2 and reduced forms of P25 TiO2 materials (green and red Millar indices represent the anatase and rutile phases, respectively).
2.2. Microstructural and Morphological Analysis
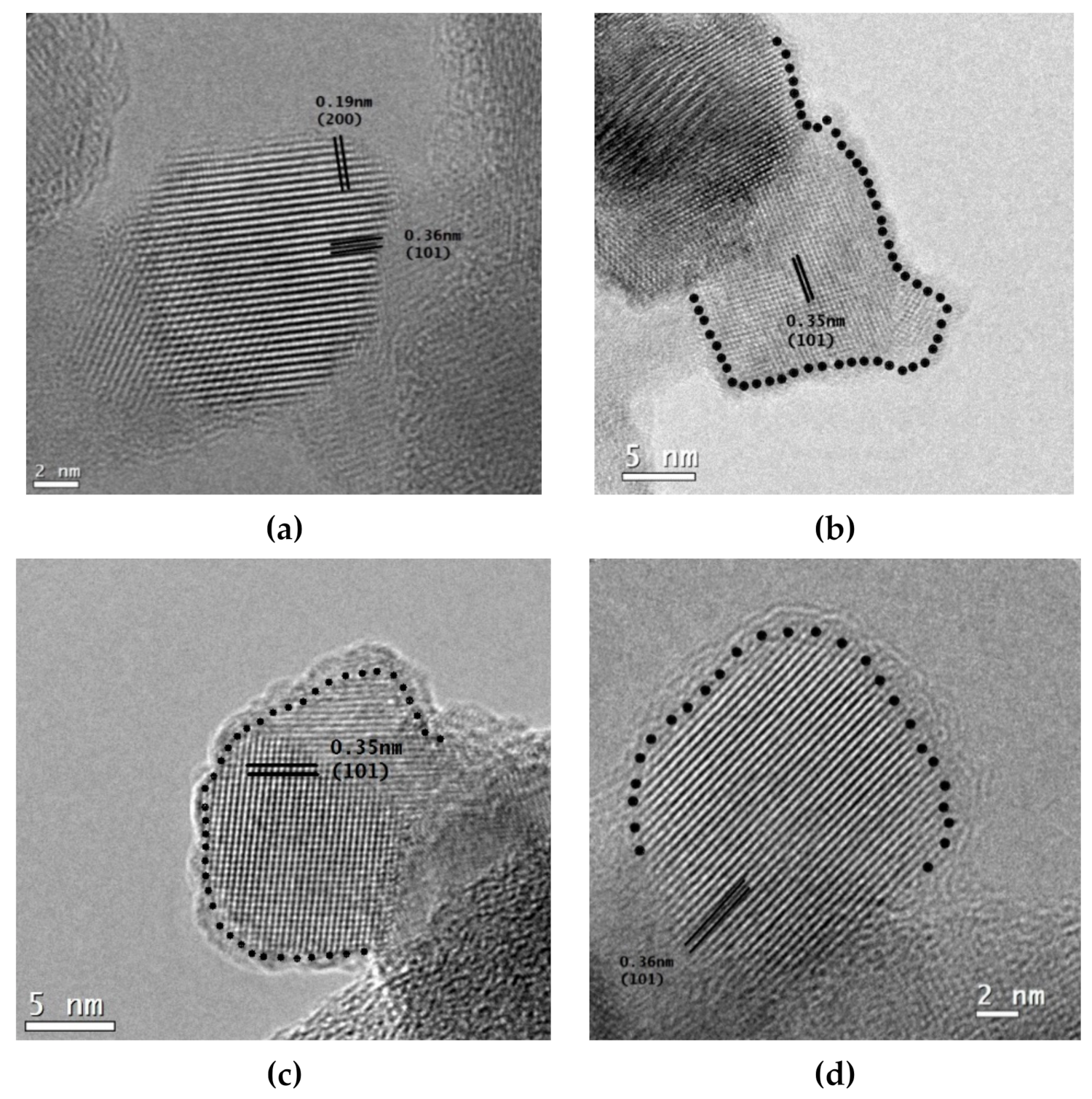
Micrographs of high-resolution transmission electron microscopy (HRTEM) of P25 TiO2 materials are presented in Figure 4, while the reduced forms of NF-TiO2, and NFP-TiO2 are included in the Supplementary Materials (Figure S1). The high crystalline order of the TiO2 nanoparticles with the characteristic lattice of anatase possessing indexed planes of (101) and (200) was observed in all samples. A thin amorphous layer was observed at the surface of the reduced TiO2 nanoparticles, indicative of surface modification. The measured thicknesses of the amorphous layers are tabulated in Table 1. The values of the amorphous layer and the characteristics of the crystalline order of the anatase suggest that we can modulate the amorphous layer by changing the duration of thermal treatment and consequently modulate the band gap energies into the visible light region.
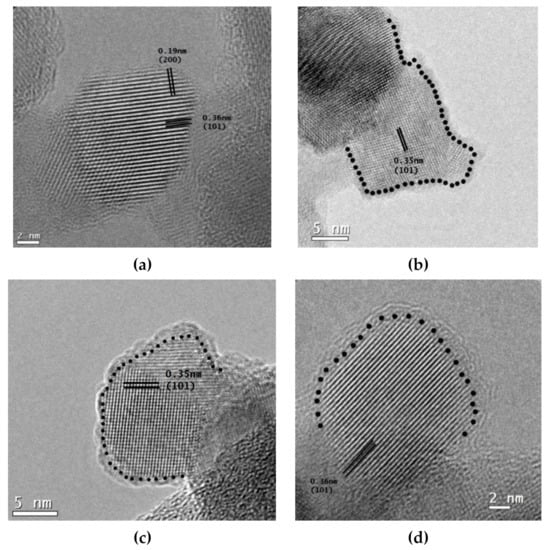
Figure 4.
HRTEM images of TiO2 nanocrystals: (a) P25 TiO2 before reduction, (b) TiO2red 30 (1.2 nm of the amorphous layer), (c) TiO2red 50 (1.5 nm of the amorphous layer), and (d) TiO2red 70 (2.0 nm of the amorphous layer).
2.3. Energy Dispersive X-ray Fluorescence (EDXRF) Analysis
Our results demonstrated effective doping of TiO2 with nonmetals N, F, and P. Chemical composition as weight percentage for the synthesized catalyst NF-TiO2 was N (7.17%), O (42.46%), F (3.40%), and Ti (46.97%) and for the NFP-TiO2 the composition was determined as N (9.17%), O (37.28%), F (12.56%), P (7.35%), and Ti (33.65%). The XRF spectrum of doped samples NF-TiO2 and NFP-TiO2 are presented in Supplementary Materials (Figure S2). Degussa P25 has an average crystalline size of 21 nm with a specific surface area of 50 m2/g [54]. The particle size of 22.3 nm was calculated for the NFP samples processed at 600 °C, with a modest increase to 29.5 nm at 1000 °C and 40.7 nm 1100 °C reported [11].
2.4. Ultraviolet-visible (UV-Vis) and Raman Spectroscopy
The colors of the prepared TiO2, presented in Figure 2, indicate visible light absorption. The UV-visible diffuse reflectance spectra of the reduced TiO2 samples, provided in Supplementary Materials (Figure S3), were used to determine the specific absorption edges and band gap energies. The results demonstrated the band gap energies of doped samples shift to the visible light region compared to the unmodified P25 TiO2. A reduction of the band gap energy from 3.1 eV to 2.23 eV was observed for P25 TiO2 after treatment for approximately one hour. For NF-TiO2 particles, the band gap energy dropped by 0.8 eV to 1.30 eV. The NFP-TiO2 particles possess a band gap of 2.7 eV, decreasing to 2.35 eV upon reduction. The insertion of N atoms into TiO2 lattices is reported to create a new interband state above the valence band of TiO2 leading to the observed absorbance shift into the visible light region [55]. Since the absorbance edges of the prepared samples shifted into the visible light region, we assigned surface modification and production of an amorphous layer on the TiO2 particle results to changes in the band gap energy and visible light absorption properties. The band gap energies calculated by Kubelka–Munk plots are summarized in Table 2.

Table 2.
Band gap of NaBH4 treated P25 TiO2, NF-TiO2, and NFP-TiO2 series and their corresponding photoexcited absorption wavelength.
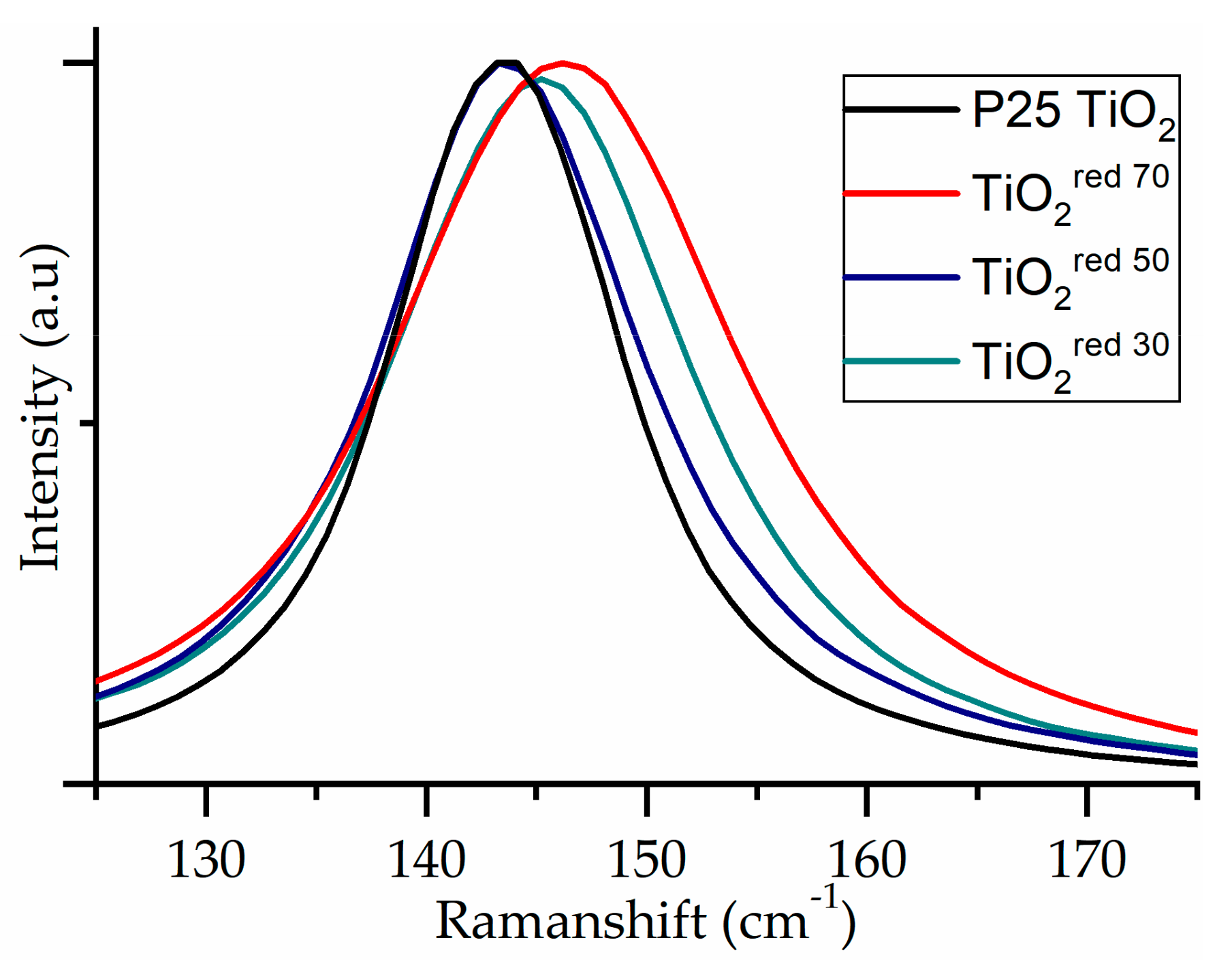
Raman spectra of the TiO2 materials exhibited the characteristic bands corresponding to Eg1, Bg1, Ag1, and Eg3 at 146, 396, 516, and 637 cm-1, respectively (data shown in Supplementary Materials Table S1), for the vibrational modes of the anatase phase of TiO2 [56]. Small shifts of the vibration mode were observed, as shown in Figure 5. The modest shift is assigned to the surface disorder as a result of the amorphous layer over the TiO2 nanoparticle (possible formation of oxygen vacancies or nonstoichiometry defects). Amorphous surface layers can also lead to peak broadening. Oxygen vacancy promotes the charge carrier separation and can potentially enhance the photocatalytic performance [41].
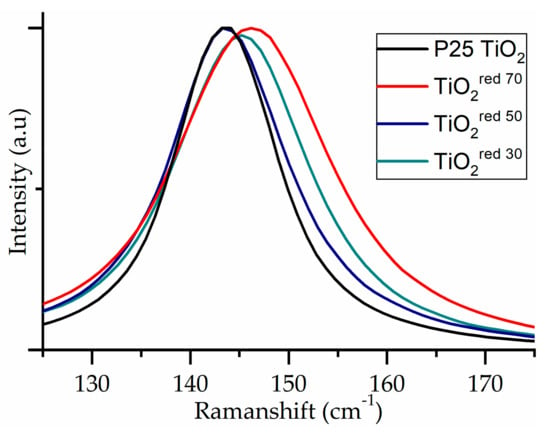
Figure 5.
Raman spectrum of P25 TiO2 series showing shift toward the visible region as a function of reductive treatment time.
2.5. Photocatalytic Hydroxyl Radicals (•OH) Production
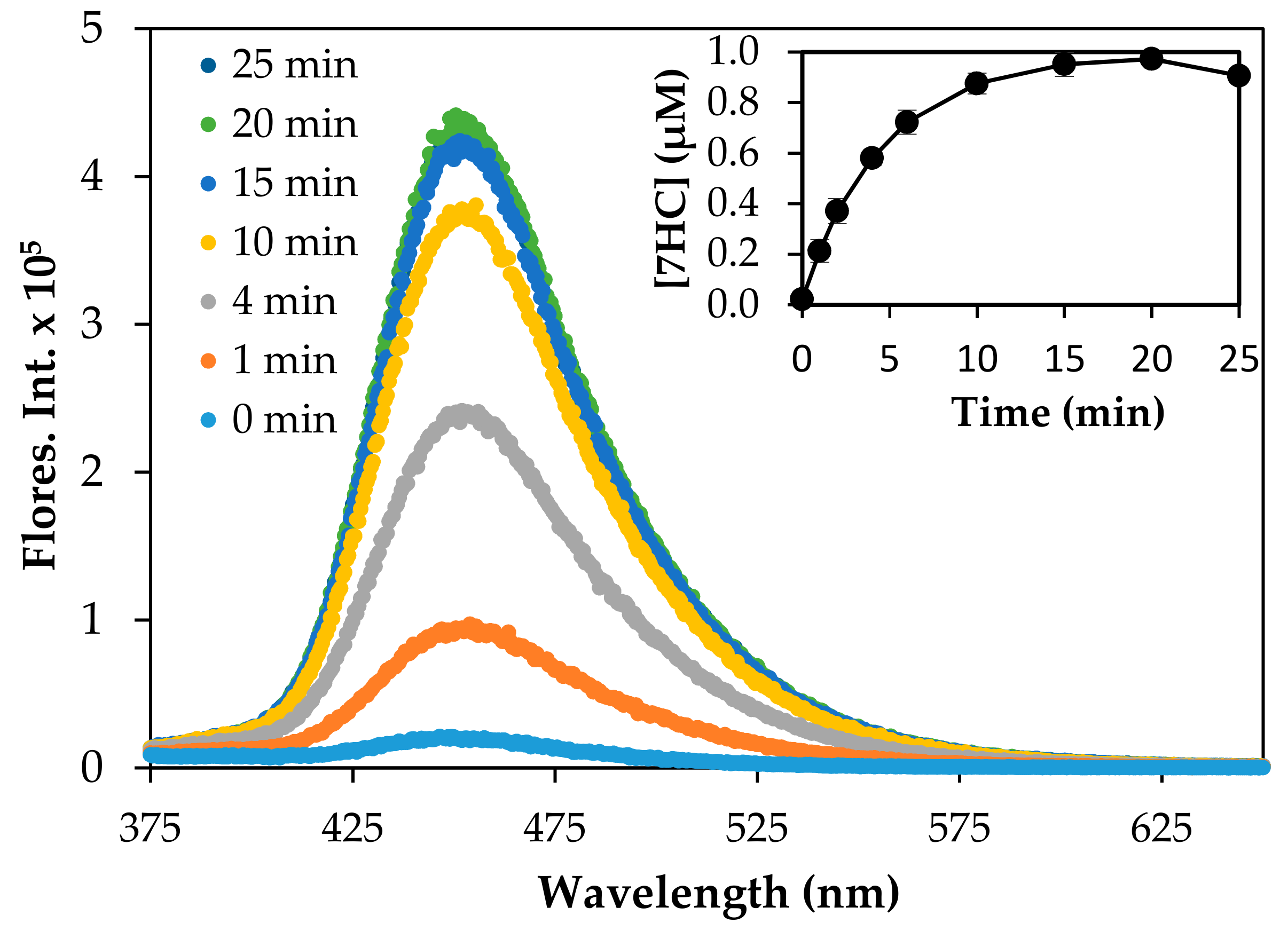
The production of the •OH adduct, 7HC, was monitored to assess the production of •OH by the reduced TiO2 materials at different irradiation wavelengths. Figure 6 shows the growth of fluorescence intensity at 455 nm of the hydroxyl radical trapping agent, 7HC, as a function of irradiation time under 350 UV light in the presence of P25 TiO2, as a reference for comparing different photocatalysts. Control experiments in the dark and in the absence of P25 TiO2 did not yield 7HC. The inset of Figure 6 shows the concentration of 7HC in P25 TiO2 aqueous suspension as a function of irradiation time. Triplicate experiments yielded reproducibility within 95%. The yield of 7HC increased in a linear fashion initially and reached a maximum at approximately 1.0 µM at 20 min of irradiation. Upon continued irradiation, the production of 7HC gradually decreased due to the likely depletion of coumarin, as well as the simultaneous formation and degradation of 7HC at higher concentrations. The formation of 7HC followed pseudo-zero-order kinetics in accordance with previous reports [46,57]. An apparent pseudo-zero-order rate constant (kapp) of 0.16 µM min-1 (R2 = 0.99) was determined under our experimental conditions. The observed rate constant was different from the rate constant of 0.023 µM min-1 reported by Nagarajan et al. [46], likely due to differences in experimental conditions including starting concentration, flux intensity, reactor design, etc. The concentration of 7HC (1.0 µM) produced at 20 min was equivalent to [•OH] = 16.4 µM, assuming trapping efficiency of 6.1% of total •OH [46,57].
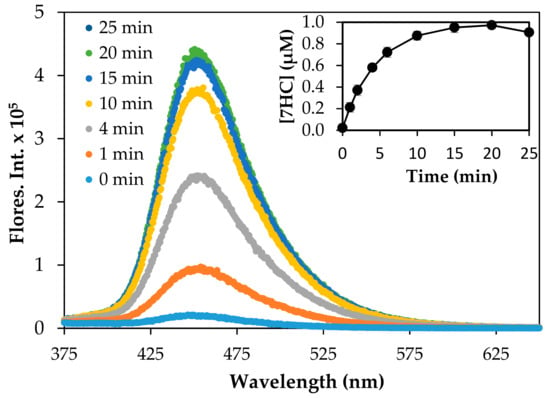
Figure 6.
Change of fluorescence intensity observed at 455 nm during the irradiation of P25 TiO2 (0.10 g/L) suspended in a 125 µM COU aqueous solution. The plot of 7HC vs irradiation time is shown in the inset. The irradiation of P25 TiO2 suspension was carried out at 350 nm.
2.6. Evaluation of the Photocatalytic Performance
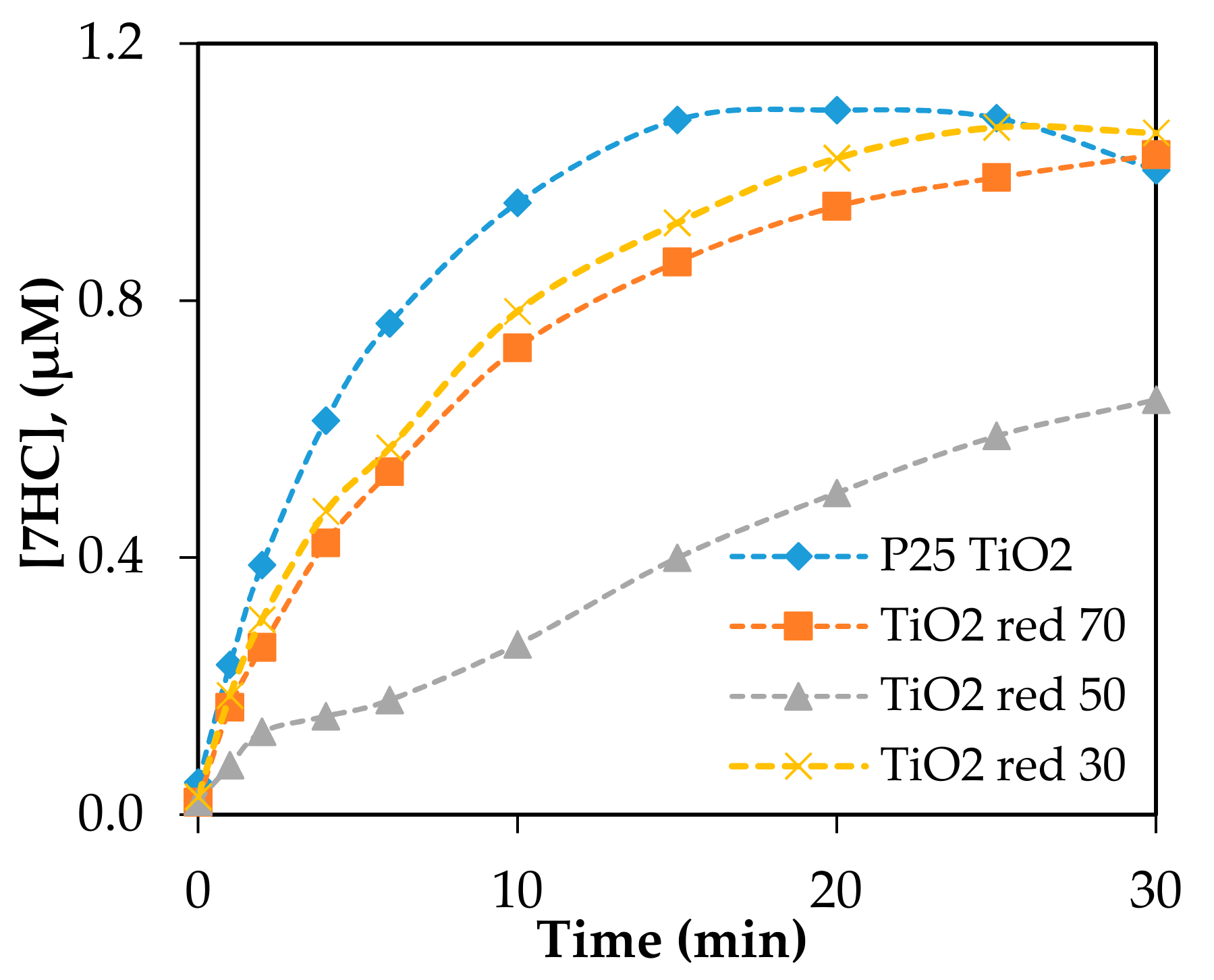
The photocatalytic production of 7HC of the reduced TiO2 materials was carried out under the same conditions employed for the baseline using P25 TiO2 irradiation at λ = 350, 419, and 450 nm. Significant amounts of •OH, quantitated based on the formation of 7HC, were produced by reduced P25 TiO2 under UV (350 nm) irradiation shown in Figure 7. The formation of 7HC by reduced TiO2red was slower than the reference P25 TiO2 and followed the order of TiO2red 30> TiO2red 70> TiO2red 50. Under 350 nm, the productions of hydroxyl radicals by reduced, doped NF-TiO2 and NFP-TiO2 were insignificant (data shown in Supplementary Materials Figure S4). No direct correlation was observed with the treatment time by NaBH4, •OH production, and the band gap energies.
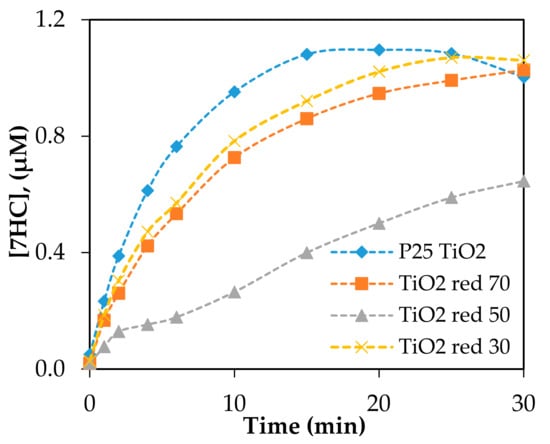
Figure 7.
The production of 7HC by reduced P25 TiO2 photocatalysts irradiated at 350 nm. Catalyst loading was 0.10 g/L; [COU] = 125 µM.
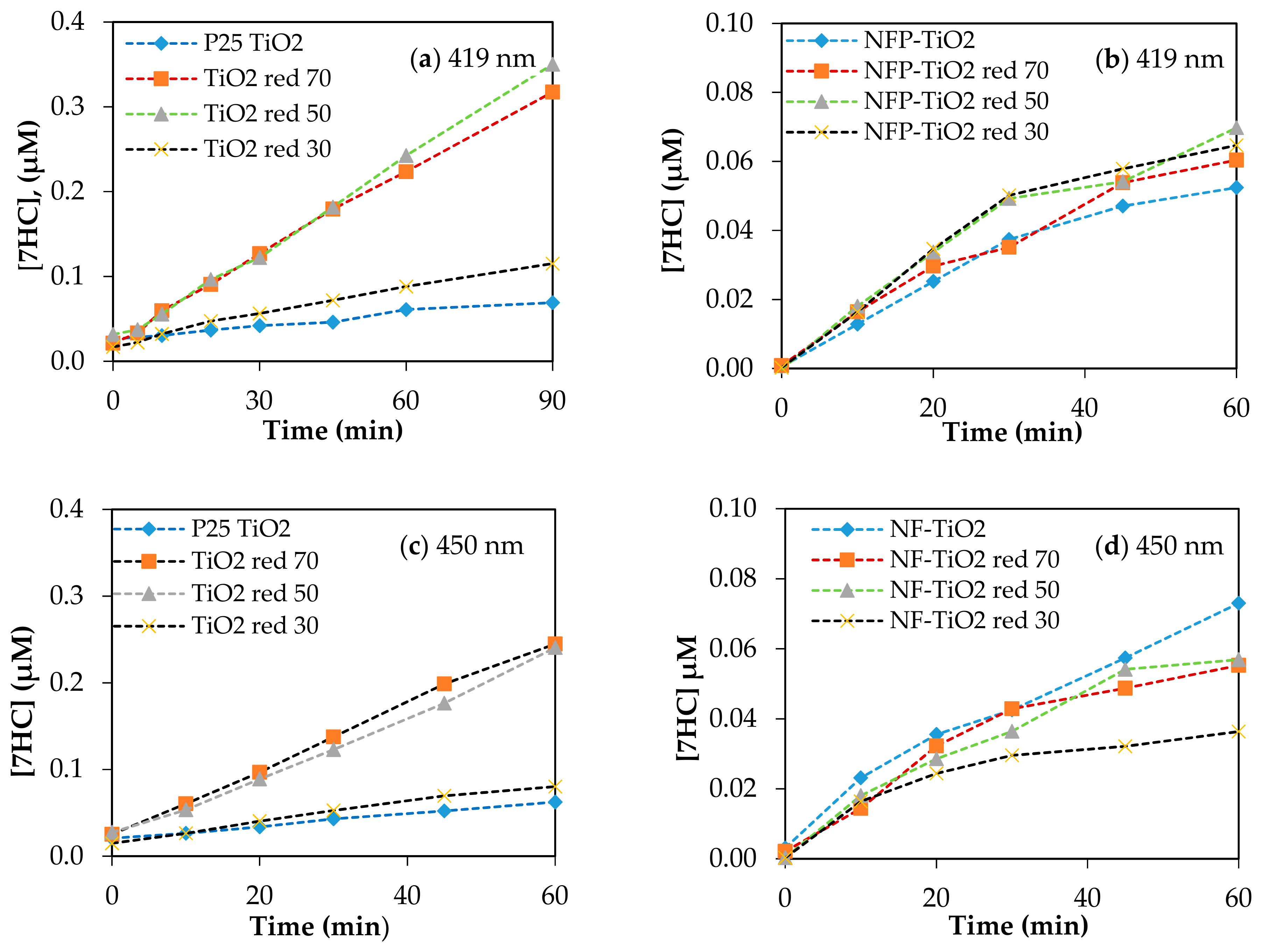
Visible light-activated photocatalysis has been reported to be effective for the degradation of organic pollutants [13,14,23,54]. The band gap of P25 TiO2 is 3.1 eV, and upon reduction with NaBH4, the band gap energy decreases to 2.23 eV, corresponding to light absorption wavelengths ~556 nm. Based on the calculated band gap for NF-TiO2 and NFP-TiO2 series, shown in Table 2, reduced NF-TiO2red and NFP-TiO2red may be active in the near IR region and at ~500 nm, respectively. The photocatalytic production of 7HC in aqueous suspension of the synthesized TiO2 materials under irradiation at 419 and 450 nm is presented in Figure 8. For quantitative estimation of their relative photocatalytic activity, we calculated OH-index for each of the prepared samples using Equation (6) based on the formation rate of 7HC correlated to the production of •OH for only the initial 10 min of reaction to avoid the possible degradation of 7HC. The calculated OH-indexes are summarized in Table 3. Though the formation rate of •OH depends on various factors, including phase structures, particle size, crystallinity, and surface defects, the composition is an important factor in the photolytic activity of TiO2 materials. A graphical comparison production of •OH at visible light is presented in Figure 9.
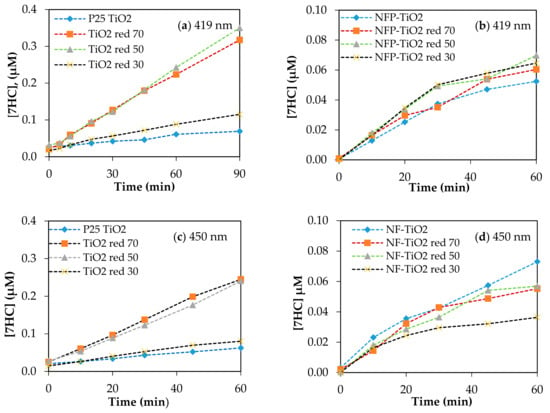
Figure 8.
Production of 7HC using various TiO2 upon irradiation at visible light (a,b) 419 nm; (c,d) 450 nm. Catalyst loading was 0.10g/L; [COU] = 125 µM.

Table 3.
Calculated OH-index of prepared TiO2 materials.
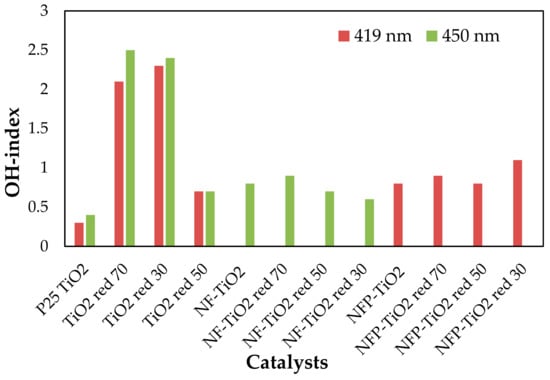
Figure 9.
Relative comparison of OH-index of various TiO2-based photocatalysts at visible light (the data are reproducible with 5% of error based on some triplicate runs).
In general, the larger the OH-index, the higher the photocatalytic activity. P25 TiO2 showed the highest OH-index for UV activation compared to the reduced TiO2 materials. P25 TiO2 is among the most active photocatalysts for the degradation of organic compounds initiated by •OH radicals under UV irradiation. However, under the visible light region, P25 TiO2 did not show appreciable photo activity. In the visible range, NaBH4 treated P25 TiO2, TiO2red 70, TiO2red 50, and TiO2red 30, showed enhanced photocatalytic activity compared to reference P25 TiO2. Though NFP codoped TiO2 exhibited limited photocatalytic activity under visible light, NaBH4 treatment did not make a difference in the performance.
3. Materials and Methods
3.1. Materials
P25 TiO2 donated by Degussa was employed as starting material. N and F codoped TiO2 (NF-TiO2) and N, F, and P codoped TiO2 (NFP-TiO2) were prepared by using titanium tetraisopropoxide (97.0%), isopropanol (99.0%), ammonium hexafluorophosphate (NFP) (≥95.0%), urea (N) (≥98.0%), trifluoroacetic acid (TFA) (99.0%), and phosphoric acid (PA) (85 wt.%) A detailed experimental procedure to prepare these samples is given in Ref [11]. NaBH4 obtained from Acros Organics was 98% pure and used without further purification. Coumarin was purchased from MP Biomedicals, LLC. 7-Hydroxycoumarin (7HC) was 99% pure and purchased from Acros Organics. The air used to purge samples was from Trigas of the highest available purity. Millipore water (18 MΩ∙cm) was used for the preparation of all aqueous solutions.
3.2. Preparation of Reduced TiO2 Materials
In a typical procedure, 4.0 g of P25 TiO2 was mixed with 1.5 g of NaBH4 in an agate mortar and thoroughly ground with a pestle for 30 min at room temperature. The resulting mixture was transferred to a porcelain boat and placed in a tubular furnace. Gradient heat was applied at a rate of 10 °C min-1 from room temperature to 300 °C under an argon atmosphere and baked for an additional 30–70 min at 300 °C. The sample was removed from the furnace and allowed to naturally cool to room temperature. The resulting colored TiO2 was washed with deionized water and ethanol several times to eliminate unreacted NaBH4 and undesired byproducts. The washed sample was stored for characterization after drying at 70 °C for one hour. A series of reduced forms of NF-TiO2 (NF-TiO2red) and reduced forms of NFP-TiO2 (NFP-TiO2red) were prepared as a function of reduction time.
3.3. Characterization of Reduced TiO2 Materials
X-ray diffraction patterns were taken for the reduced TiO2 materials employing a Bruker Advance D8 X-ray diffractometer, with Cu Kα (λ = 1.54056) radiation with continuous scanning in the range of 10°< 2Θ <90°. Structure, surface morphology, and microstructure of prepared materials were characterized by high-resolution transmission electron microscope (HRTEM) imaging studies using FEI TITAN G2 80–300 operated at 300 kV. Samples were sonicated and placed onto a carbon-coated copper grid for scanning by microscope. The concentration of N, F, P, and Ti in doped TiO2 samples was determined using the energy dispersive X-ray fluorescence (EDXRF) analyses. The vibrational spectroscopy of hybrid nanostructure was identified using Thermo Scientific XDR Raman microscope employing 532 nm laser at 5 mW power. The UV-Visible absorption spectra of the various TiO2 materials were recorded at room temperature on a Varian Cary 100 Bio UV-Visible spectrophotometer in the wavelength range of 220–800 nm. The band gap of the prepared samples was calculated from Kubelka–Munk plots.
3.4. Photocatalytic Experiment
The illumination of the TiO2 suspension was carried out in a Rayonet Photochemical Reactor equipped with a cooling fan and up to 16 lamps in a merry-go-round arrangement (details of the reactor, model RPR-100, are available at The Southern New England Ultra Violet Company [58]. Three sets of lamps provide specific irradiation centered around 350, 419, or 450 nm. Eight lamps equally distributed within the reactor were employed in each experiment. A phosphor-coated black light of nearly 350 nm was used for UV irradiation and white tungsten bulbs of 419 and 450 nm for visible light irradiations. The intensity of light fluxes for 350 nm, 419 nm, and 450 nm was in 10% deviation according to manufacturer specifications. Pyrex glass reaction vessels with Teflon stoppers (L = 30 cm, D = 2.5 cm) were employed for all photocatalysis experiments. In a typical experiment, 100 mL of 125 µM COU aqueous solution was loaded with 10 mg of catalyst and sonicated in an ultrasonic cleaning bath for 10 min to produce a uniform of suspension. The mixture was gently purged with air for 10 min prior to irradiation. The aqueous suspension was magnetically stirred during irradiation in a photochemical reactor. The reaction vessel was removed from the reactor at desired time intervals, vigorously shaken for 30 s, and a 5.0 mL aliquot withdrawn from the reaction solution and immediately passed through a 0.45 µm PTFE filter. The filter was rinsed with another 5.0 ml of deionized water combined with filtrate to make the total volume of 10 ml for future analyses.
3.5. Measurement of the Formation of Hydroxyl Radicals (•OH) for Assessing the Photocatalytic Activities
The concentrations of •OH during irradiation of the aqueous TiO2 suspensions were measured from the concentration of highly fluorescent hydroxyl radical adduct (7HC) produced from the reaction of •OH with COU. Based on the established yield of 7HC from the COU + •OH reaction, the concentration of •OH production can be determined as a function of irradiation time. The optimal COU concentration ranges for •OH production are reported between 100–1000 µM [12]. Czili et al. observed maximum initial yields when the COU concentration was 100 µM [48]. While •OH generated during TiO2 photocatalysis is primarily formed at the surface, •OHads can diffuse from the TiO2 surface, leading to free •OH. Previous studies have shown free •OH, and •OHads occur at or near the surface and have similar reactivities. The trapping efficiency of •OH in TiO2 suspension reported by Zhang et al. is ~6.1% using 100 µM COU [57]. Newton and Milligan reported similar trapping efficiency of 4.7% [59]. The detection and quantification of 7HC were carried out by a Horiba FluoroMax Spectrofluorometer setting with excitation and emission wavelengths at 332 and 455 nm, respectively. The equipment was calibrated against a series of standard 7HC concentrations ranging from 0.1 to 3.0 µM (0.1, 0.5, 1.0, 1.5, 2.0, and 3.0 µM). The calibration plot, shown in Supplementary Materials (Figure S5) was obtained by plotting the fluorescence intensity measured at 455 nm as a function of 7HC concentration. The correlation coefficient of the calibration plot was ≥ 0.99.
4. Conclusions
The visible light-activated reduced forms of TiO2 materials were prepared by a simple solid state thermo-physicochemical treatment using NaBH4. The materials were subsequently characterized by XRD, HRTEM, EDXRF, UV-Visible, and Raman spectrometry. Optical measurement suggested that physicochemical treatment can modulate the band gap energy-suitable properties for efficient photocatalytic activity. Coumarin was used as a fluorescent probe to trap and measure the •OH generated in catalyst suspension under UV and visible light irradiation. The photocatalytic performances of the synthesized reduced TiO2 materials were assessed by quantitative measurement of hydroxyl radical production under the irradiation of UV and visible light. Finally, OH-index of a photocatalyst using the formation rate of hydroxyl radicals was calculated to compare the relative photocatalytic activity of a photocatalyst. Though P25 TiO2 was found as the most active photocatalyst in the UV region, NaBH4-reduced forms of TiO2 were identified as significantly active photocatalysts under visible irradiation.
Supplementary Materials
The following are available online at https://www.mdpi.com/1420-3049/24/11/2147/s1, Figure S1a: HRTEM images of NF-TiO2. Figure S1b: HRTEM images of NFP-TiO2 nanocrystals, Figure S2: EDXRF spectrum of (a) NF-TiO2, and (b) NFP-TiO2, Figure S3: UV-Visible diffuse reflectance spectra of (a) P25 TiO2 series, (c) NF-TiO2 series, and (e) NFP-TiO2 series. The Kubelka-Munk plot for band energy calculation for (b) P25 TiO2 series, (d) NF-TiO2 series, and (f) NFP-TiO2 series, Figure S4: The production of 7HC at 350 nm by (a) reduced NF-TiO2 and (b) NFP-TiO2 photocatalyst, Figure S5: Spectrofluorometric calibration curve for measuring of 7HC. Table S1a: Raman band position of P25 TiO2 and reduced P25 TiO2 (TiO2red), Table S1b: Raman band position of NF-TiO2 and reduced NF-TiO2red, Table S1c: Raman band position of NFP- TiO2 and reduced NFP-TiO2red.
Author Contributions
K.O. made the concept of the project, design of experiments, oversight of data analysis. A.M.A. did the most of experimental work and wrote the report. M.Á.G.-P. did partial experimental work, and S.C.P. was the provider of doped N, F, and/or P codoped TiO2. All authors discussed the results and commented on the manuscript.
Acknowledgments
Kevin O’Shea acknowledges the National Science Foundation for the partial support of the project (Grant# CHE-1710111). The authors would like to thank Rachel Fagan for helping with sample preparation.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 for environmental photocatalytic applications: A review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.; Choi, W.; Bahnemann, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Mechanism of the OH radical generation in photocatalysis with TiO2 of different crystalline types. J. Phys. Chem. C 2014, 118, 10824–10832. [Google Scholar] [CrossRef]
- Dvoranová, D.; Barbieriková, Z.; Brezová, V. Radical intermediates in photoinduced reactions on TiO2 (An EPR spin trapping study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Ollis, D.F.; Pelizzetti, E.; Serpone, N. Photocatalyzed destruction of water contaminants. Environ. Sci. Technol. 1991, 25, 1522–1529. [Google Scholar] [CrossRef]
- Abdullah, A.M.; O’Shea, K.E. TiO2 photocatalytic degradation of the flame retardant tris (2-chloroethyl) phosphate (TCEP) in aqueous solution: A detailed kinetic and mechanistic study. J. Photochem. Photobiol. A Chem. 2019, 377, 130–137. [Google Scholar] [CrossRef]
- Ishibashi, K.I.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Detection of active oxidative species in TiO2 photocatalysis using the fluorescence technique. Electrochem. Commun. 2000, 2, 207–210. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Hinder, S.; Pillai, S.C. Improved high temperature stability of anatase TiO2 photocatalysts by N, F, P co-doping. Mater. Des. 2016, 96, 44–53. [Google Scholar] [CrossRef]
- Ishibashi, K.; Fujishima, A.; Watanabe, T.; Hashimoto, K. Quantum yields of active oxidative species formed on TiO2 photocatalyst. J. Photochem. Photobiol. A Chem. 2000, 134, 139–142. [Google Scholar] [CrossRef]
- Zhao, C.; Pelaez, M.; Dionysiou, D.D.; Pillai, S.C.; Byrne, J.A.; O’Shea, K.E. UV and visible light activated TiO2 photocatalysis of 6-hydroxymethyl uracil, a model compound for the potent cyanotoxin cylindrospermopsin. Catal. Today 2014, 224, 70–76. [Google Scholar] [CrossRef]
- Han, C.; Pelaez, M.; Likodimos, V.; Kontos, A.G.; Falaras, P.; O’Shea, K.; Dionysiou, D.D. Innovative visible light-activated sulfur doped TiO2 films for water treatment. Appl. Catal. B Environ. 2011, 107, 77–87. [Google Scholar] [CrossRef]
- Pelaez, M.; Falaras, P.; Kontos, A.G.; De la Cruz, A.A.; O’shea, K.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. A comparative study on the removal of cylindrospermopsin and microcystins from water with NF-TiO2-P25 composite films with visible and UV-vis light photocatalytic activity. Appl. Catal. B Environ. 2012, 121–122, 30–39. [Google Scholar] [CrossRef]
- Byrne, J.A.; Dunlop, P.S.M.; Hamilton, J.W.J.; Fernández-Ibáñez, P.; Polo-López, I.; Sharma, P.K.; Vennard, A.S.M. A review of heterogeneous photocatalysis for water and surface disinfection. Molecules 2015, 20, 5574–5615. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Liu, J.; Chuang, S.S.C.; Jana, S.C. Fabrication of hierarchical V2O5 nanorods on TiO2 nanofibers and their enhanced photocatalytic activity under visible light. ChemCatChem 2018, 10, 3305–3318. [Google Scholar] [CrossRef]
- Ghosh, M.; Lohrasbi, M.; Chuang, S.S.C.; Jana, S.C. Mesoporous titanium dioxide nanofibers with a significantly enhanced photocatalytic activity. ChemCatChem 2016, 8, 2525–2535. [Google Scholar] [CrossRef]
- Yu, W.; Liu, X.; Pan, L.; Li, J.; Liu, J.; Zhang, J.; Li, P.; Chen, C.; Sun, Z. Enhanced visible light photocatalytic degradation of methylene blue by F-doped TiO2. Appl. Surf. Sci. 2014, 319, 107–112. [Google Scholar] [CrossRef]
- Adyani, S.M.; Ghorbani, M. A comparative study of physicochemical and photocatalytic properties of visible light responsive Fe, Gd and P single and tri-doped TiO2 nanomaterials. J. Rare Earths 2018, 36, 72–85. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Hinder, S.J.; Healy, L.F.; Pillai, S.C. A systematic study of the effect of silver on the chelation of formic acid to a titanium precursor and the resulting effect on the anatase to rutile transformation of TiO2. J. Phys. Chem. C 2010, 114, 13026–13034. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Pillai, S.C.; Hareesh, U.S.; Mukundan, P.; Warrier, K.G.K. Synthesis of thermally stable, high surface area anatase-alumina mixed oxides. Mater. Lett. 2000, 43, 286–290. [Google Scholar] [CrossRef]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A Chem. 2007, 189, 258–263. [Google Scholar] [CrossRef]
- Shakil, M.R.; El-Sawy, A.M.; Tasnim, H.; Meguerdichian, A.G.; Jin, J.; Dubrosky, J.P.; Suib, S.L. Single-doped and multidoped transition-metal (Mn, Fe, Co, and Ni) ZnO and their electrocatalytic activities for oxygen reduction reaction. Inorg. Chem. 2018, 57, 9977–9987. [Google Scholar] [CrossRef] [PubMed]
- McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J.D.; Morris, M.A. A facile route to synthesis of S-doped TiO2 nanoparticles for photocatalytic activity. J. Mol. Catal. A Chem. 2015, 406, 51–57. [Google Scholar] [CrossRef]
- Hong, X.; Luo, Z.; Batteas, J.D. Enhanced visible-light absorption and dopant distribution of iodine-TiO2 nanoparticles synthesized by a new facile two-step hydrothermal method. J. Solid State Chem. 2011, 184, 2244–2249. [Google Scholar] [CrossRef]
- Giannakas, A.E.; Antonopoulou, M.; Deligiannakis, Y.; Konstantinou, I. Preparation, characterization of N–I co-doped TiO2 and catalytic performance toward simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl. Catal. B Environ. 2013, 140–141, 636–645. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Y.C.; Nadagouda, M.; Han, C.; O’Shea, K.; El-Sheikh, S.M.; Ismail, A.A.; Dionysiou, D.D. Visible light-sensitized S, N, and C co-doped polymorphic TiO2 for photocatalytic destruction of microcystin-LR. Appl. Catal. B Environ. 2014, 144, 614–621. [Google Scholar] [CrossRef]
- Wei, F.; Ni, L.; Cui, P. Preparation and characterization of N–S-codoped TiO2 photocatalyst and its photocatalytic activity. J. Hazard. Mater. 2008, 156, 135–140. [Google Scholar] [CrossRef]
- Yu, J.; Zhou, M.; Cheng, B.; Zhao, X. Preparation, characterization and photocatalytic activity of in situ N,S-codoped TiO2 powders. J. Mol. Catal. A Chem. 2006, 246, 176–184. [Google Scholar] [CrossRef]
- Dozzi, M.V.; Selli, E. Doping TiO2 with p-block elements: Effects on photocatalytic activity. J. Photochem. Photobiol. C Photochem. Rev. 2013, 14, 13–28. [Google Scholar] [CrossRef]
- Abdullah, A.M.; Debnath, T.; Rüscher, C.H.; Hussain, A. Synthesis and characterization of vanadium substituted potassium tungsten bronzes, KxVyW1-yO3. J. Sci. Res. 2012, 4, 507–514. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.; Bard, A.J. Novel carbon-doped TiO2 nanotube arrays with high aspect ratios for efficient solar water splitting. Nano Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, S.; Paganini, M.C.; Giamello, E.; Selloni, A.; Di Valentin, C.; Pacchioni, G. Origin of photoactivity of nitrogen-doped titanium dioxide under visible light. J. Am. Chem. Soc. 2006, 128, 15666–15671. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science (80-) 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Lu, J.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Hydrogenated titania: synergy of surface modification and morphology improvement for enhanced photocatalytic activity. Chem. Commun. 2012, 48, 5733–5735. [Google Scholar] [CrossRef]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Sinhamahapatra, A.; Jeon, J.-P.; Yu, J.-S. A new approach to prepare highly active and stable black titania for visible light-assisted hydrogen production. Energy Environ. Sci 2015, 8, 3539–3544. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Lin, T.; Yin, H.; Chen, P.; Wan, D.; Xu, F.; Huang, F.; Lin, J.; Xie, X.; et al. Visible-light photocatalytic, solar thermal and photoelectrochemical properties of aluminium-reduced black titania. Energy Environ. Sci. 2013, 6, 3007–3014. [Google Scholar] [CrossRef]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–23. [Google Scholar] [CrossRef] [PubMed]
- Serpone, N.; Sauvé, G.; Koch, R.; Tahiri, H.; Pichat, P.; Piccinini, P.; Pelizzetti, E.; Hidaka, H. Standardization protocol of process efficiencies and activation parameters in heterogeneous photocatalysis: relative photonic efficiencies. J. Photochem. Photobiol. A Chem. 1996, 94, 191–203. [Google Scholar] [CrossRef]
- Serpone, N. Relative photonic efficiencies and quantum yields in heterogeneous photocatalysis. J. Photochem. Photobiol. A Chem. 1997, 104, 1–12. [Google Scholar] [CrossRef]
- Bolton, J.R.; Bircher, K.G.; Tumas, W.; Tolman, C.A. Figures-of-merit for the technical development and application of advanced oxidation technologies for both electric- and solar-driven system (IUPAC technical report). Pure Appl. Chem 2001, 73, 1998–1999. [Google Scholar] [CrossRef]
- Mills, A.; Morris, S. Photomineralization of 4-chlorophenol sensitized by titanium dioxide: a study of the initial kinetics of carbon dioxide photogeneration. J. Photochem. Photobiol. A Chem. 1993, 71, 75–83. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.C.; Fina, F.; Zhang, G.; Randorn, C.; Lawton, L.A.; Irvine, J.T.S.; Robertson, P.K.J. Comparative assessment of visible light and UV active photocatalysts by hydroxyl radical quantification. J. Photochem. Photobiol. A Chem. 2017, 334, 13–19. [Google Scholar] [CrossRef]
- Xiang, Q.; Yu, J.; Wong, P.K. Quantitative characterization of hydroxyl radicals produced by various photocatalysts. J. Colloid Interface Sci. 2011, 357, 163–167. [Google Scholar] [CrossRef]
- Czili, H.; Horváth, A. Applicability of coumarin for detecting and measuring hydroxyl radicals generated by photoexcitation of TiO2 nanoparticles. Appl. Catal. B Environ. 2008, 81, 295–302. [Google Scholar] [CrossRef]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Nolan, N.T.; Seery, M.K.; Pillai, S.C. Spectroscopic investigation of the anatase-to-rutile transformation of sol-gel-synthesized TiO2 photocatalysts. J. Phys. Chem. C 2009, 113, 16151–16157. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of high-temperature stable anatase TiO2 photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef]
- Periyat, P.; McCormack, D.E.; Hinder, S.J.; Pillai, S.C. One-pot synthesis of anionic (nitrogen) and cationic (sulfur) codoped high-temperature stable, visible light active, anatase photocatalysts. J. Phys. Chem. C 2009, 113, 3246–3253. [Google Scholar] [CrossRef]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed]
- Nolan, N.T.; Synnott, D.W.; Seery, M.K.; Hinder, S.J.; Van Wassenhoven, A.; Pillai, S.C. Effect of N-doping on the photocatalytic activity of sol-gel TiO2. J. Hazard. Mater. 2012, 211–212, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Giannakas, A.E.; Seristatidou, E.; Deligiannakis, Y.; Konstantinou, I. Photocatalytic activity of N-doped and N–F co-doped TiO2 and reduction of chromium(VI) in aqueous solution: An EPR study. Appl. Catal. B Environ. 2013, 132–133, 460–468. [Google Scholar] [CrossRef]
- Cai, J.; Huang, Z.; Lv, K.; Sun, J.; Deng, K. Ti powder-assisted synthesis of Ti3+ self-doped TiO2 nanosheets with enhanced visible light photoactivity. RSC Adv. 2014, 4, 19588–19593. [Google Scholar] [CrossRef]
- Zhang, J.; Nosaka, Y. Quantitative detection of OH radicals for investigating the reaction mechanism of various visible-light TiO2 photocatalysts in aqueous suspension. J. Phys. Chem. C 2013, 117, 1383–1391. [Google Scholar] [CrossRef]
- The Southern New England Ultraviolet Company Rayonet—Reactors. Available online: https://rayonet.org/reactors.php?part=RPR-100 (accessed on 5 June 2019).
- Newton, G.L.; Milligan, J.R. Fluorescence detection of hydroxyl radicals. Radiat. Phys. Chem. 2006, 75, 473–478. [Google Scholar] [CrossRef]
Sample Availability: Not available. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).