Structural Modifications of Nature-Inspired Indoloquinolines: A Mini Review of Their Potential Antiproliferative Activity
Abstract
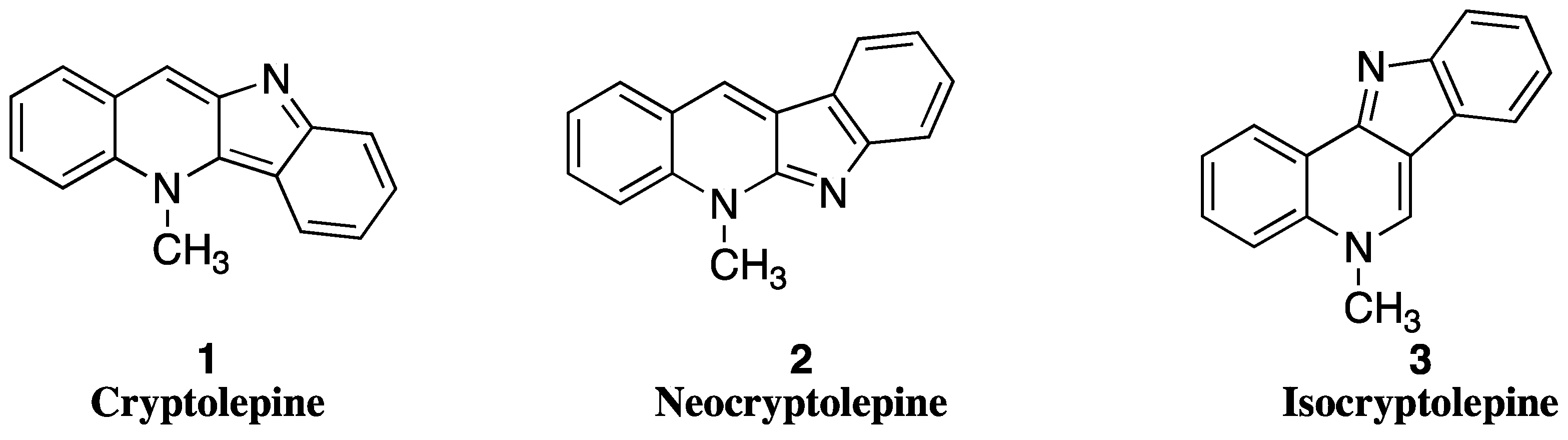
1. Introduction
1.1. Antitumoral Activity of Cryptolepine
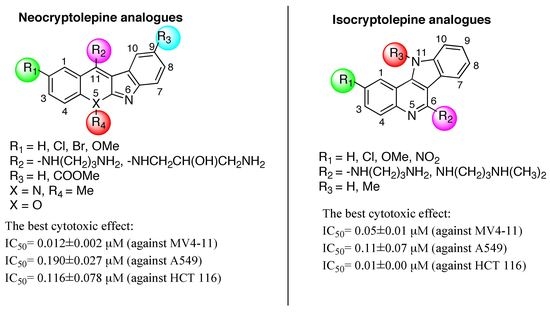
1.2. Antitumoral Activity of Neocryptolepines and Isocryptolepines
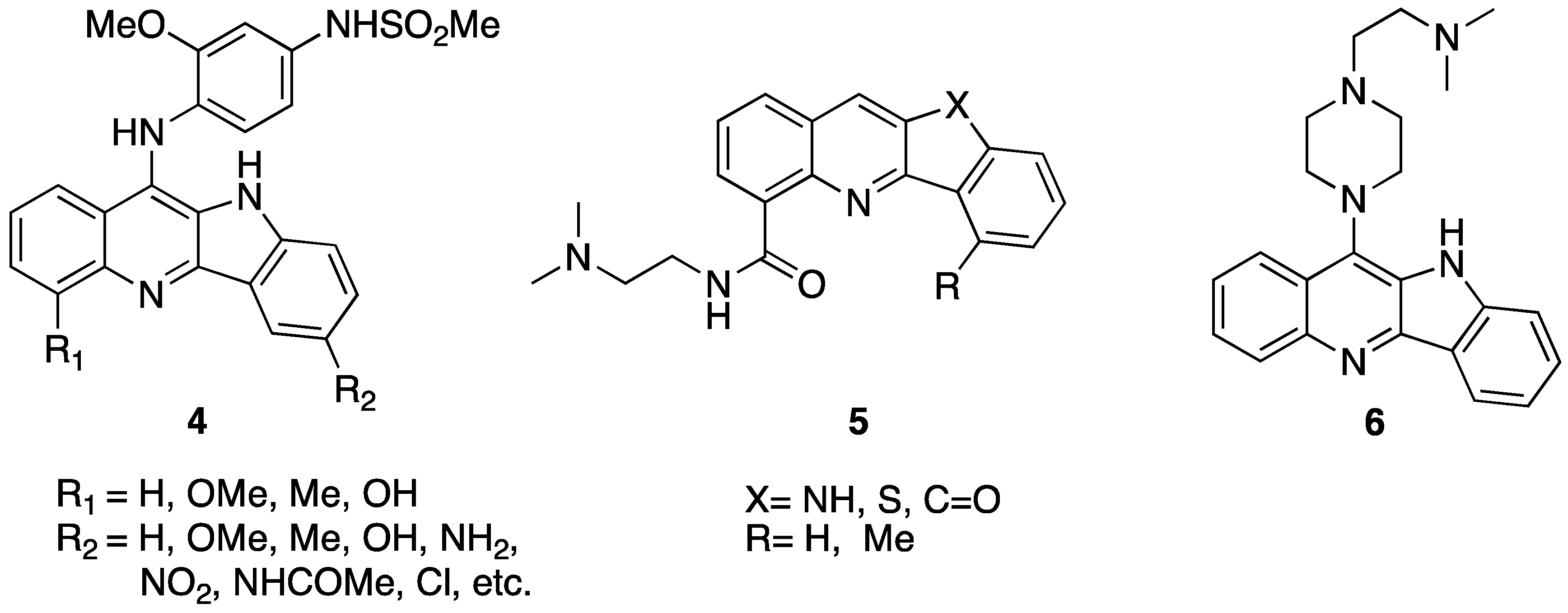
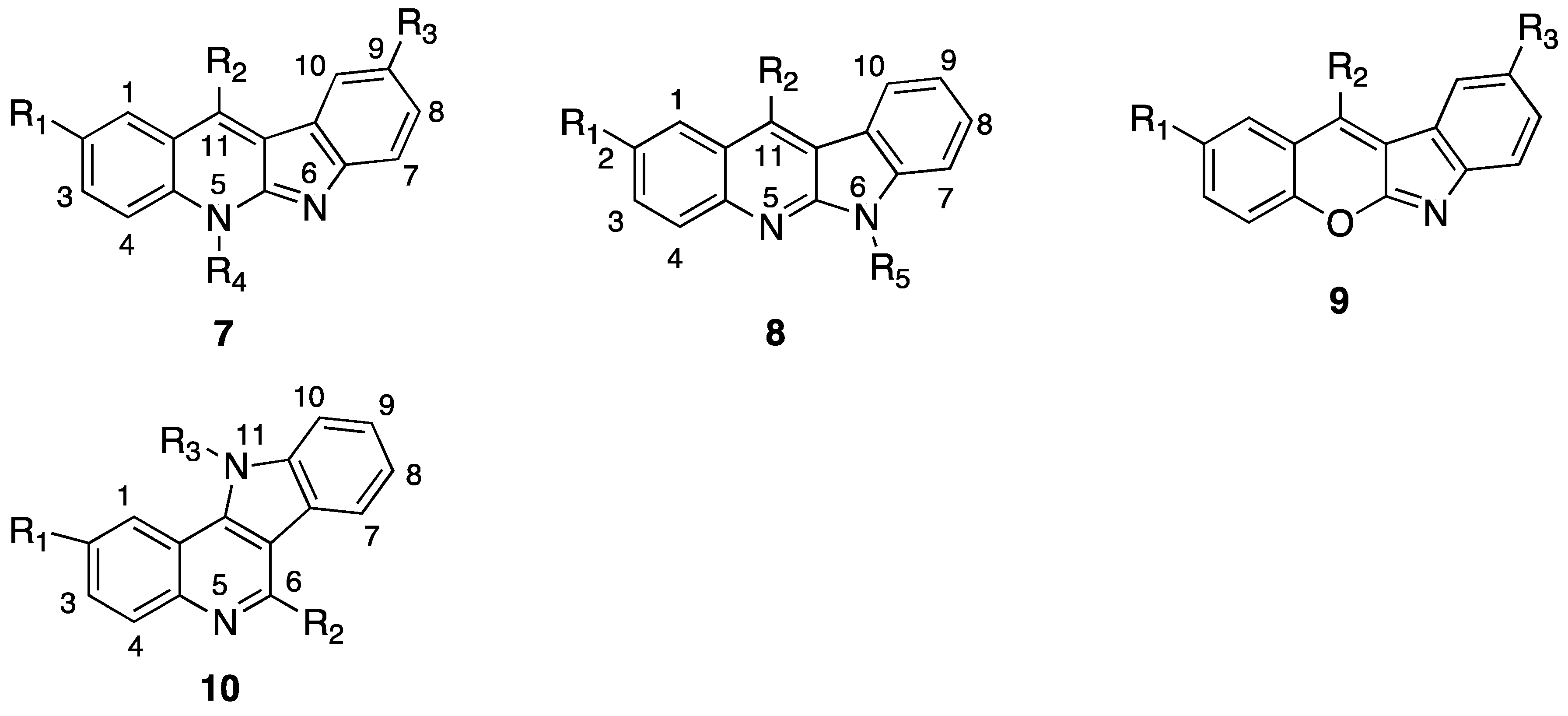
2. Compare Study
3. Conclusions
Funding
Conflicts of Interest
References
- Oliver-Bever, B. Medicinal Plants in Tropical West Africa; Cambridge University Press: Cambridge, UK, 1986; p. 41. [Google Scholar]
- Cimanga, K.; Bruyne, T.; Pieters, L.; Claeys, M.; Vlietinck, A. New alkaloids from Cryptolepis sanguinolenta. Tetrahedron Lett. 1996, 37, 1703–1706. [Google Scholar] [CrossRef]
- Bailly, C.; Laine, W.; Baldeyrou, B.; De Pauw-Gillet, M.C.; Colson, P.; Houssier, C.; Cimanga, K.; Van Miert, S.; Vlietinck, A.J.; Pieters, L. DNA intercalation topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti-Cancer Drug Des. 2000, 15, 191–201. [Google Scholar]
- Hurley, L. DNA and its associated processes as targets for cancer therapy. Nat. Rev. Cancer 2002, 2, 188–200. [Google Scholar] [CrossRef]
- Yamato, M.; Hashigaki, K.; Takeuchi, Y.; Ikeda, Y. Synthesis and antitumor activity of fused tetracyclic quinoline derivatives. 1. J. Med. Chem. 1989, 32, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Deady, L.W.; Kaye, A.J.; Finlay, G.J.; Baguley, B.C.; Denny, W.A. Synthesis and antitumor properties of N-[2-(Dimethylamino)ethyl] carboxamide derivatives of fused tetracyclic quinolines and quinoxalines: A new class of putative Topoisomerase inhibitors. J. Med. Chem. 1997, 40, 2040–2046. [Google Scholar] [CrossRef] [PubMed]
- Bonjean, K.; De Pauw-Gillet, M.C.; Defresne, M.P.; Colson, P.; Houssier, C.; Dassonneville, L.; Bailly, C.; Greimers, R.; Wright, C.; Quetin-Leclercq, J.; et al. The DNA intercalating alkaloid cryptolepine interferes with Topoisomerase II and inhibits primarily DNA synthesis in B 16 melanoma cells. Biochemistry 1998, 37, 5136–5146. [Google Scholar] [CrossRef] [PubMed]
- Lisgartent, J.N.; Coll, M.; Portugal, J.; Wright, C.W.; Aymami, J. The antimalarial and cytotoxic drug cryptolepine intercalates into DNA at cytosine-cytosine sites. Nat. Struct. Biol. 2002, 9, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Boddupally, P.V.L.; Hahn, S.; Beman, C.; De, B.; Brooks, T.A.; Gokhale, V.; Hurley, L.H. Anticancer Activity and Cellular Repression of c-MYC by the GQuadruplex-Stabilizing 11-Piperazinylquindoline is Not Dependent on Direct Targeting of the G-Quadruplex in the c-MYC Promoter. J. Med. Chem. 2012, 55, 6076–6086. [Google Scholar] [CrossRef]
- Zhou, J.L.; Lu, Y.J.; Ou, T.-M.; Zhou, J.-M.; Huang, Z.-S.; Zhu, X.-F.; Du, C.-J.; Bu, X.-Z.; Ma, L.; Gu, L.-Q.; et al. Synthesis and evaluation of quindoline derivatives as G-quadruplex inducing and stabilizing ligands and potential inhibitors of telomerase. J. Med. Chem. 2005, 48, 7315–7321. [Google Scholar] [CrossRef]
- Lu, Y.J.; Ou, T.M.; Tan, J.H.; Hou, J.Q.; Shao, W.Y.; Peng, D.; Sun, N.; Wang, X.D.; Wu, W.B.; Bu, X.Z.; et al. 5-N-methylated quindoline derivatives as telomeric G-quadruplex stabilizing ligands: Effects of 5-N positive charge on quadruplex binding affinity and cell proliferation. J. Med. Chem. 2008, 51, 6381–6392. [Google Scholar] [CrossRef]
- Guittat, L.; Alberti, P.; Rosu, F.; Van Miert, S.; Thetiot, E.; Pieters, L.; Gabelica, V.; De Pauw, E.; Ottaviani, A.; Riou, J.-F.; et al. Interaction of cryptolepine and neocryptolepine with unusual DNA structures. Biochimie 2003, 85, 535–547. [Google Scholar] [CrossRef]
- Wang, L.; Switalska, M.; Mei, Z.-W.; Lu, W.-J.; Takahara, Y.; Feng, X.-W.; El-Sayed, I.E.-T.; Wietrzyk, J.; Inokuchi, T. Synthesis and in vitro antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-indolo[2,3-b]quinolines; structure–activity relationships of neocryptolepines and 6-methyl congeners. Bioorg. Med. Chem. 2012, 20, 4820–4829. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Switalska, M.; Wang, L.; Yonezawa, M.; El-Sayed, I.E.-T.; Wietrzyk, J.; Inokuchi, T. In vitro antiproliferative activity of 11-aminoalkylaminosubstituted 5H-indolo[2,3-b]quinolines; improving activity of neocryptolepines by installation of ester substituent. Med. Chem. Res. 2013, 22, 4492–4504. [Google Scholar] [CrossRef]
- Shaban, E.; Switalska, M.; Wang, L.; Wang, N.; Xiu, F.; Hayashi, I.; Ngoc, T.A.; Nagae, S.; El-Ghlban, S.; Shimoda, S.; et al. Synthesis and In Vitro Antiproliferative Activity of 11-Substituted Neocryptolepines with a Branched ω-Aminoalkylamino Chain. Molecules 2017, 22, 1954. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Świtalska, M.; Wang, L.; Mei, Z.-W.; Edazawa, Y.; Pang, C.-Q.; El-Sayed, I.E.-T.; Wietrzyk, J.; Inokuchi, T. Synthesis and in vitro antiproliferative activity of new 11-aminoalkylaminosubstituted chromeno[2,3-b]indoles. Eur. J. Med. Chem. 2012, 58, 441–451. [Google Scholar] [CrossRef] [PubMed]
- Emam, S.; El Sayed, I.; Ayad, M.; Hathout, H. Synthesis, characterization and anticancer activity of new Schiff bases bearing neocryptolepine. J. Mol. Struct. 2017, 1146, 600–619. [Google Scholar] [CrossRef]
- Emam, S.; El Sayed, I.; Nassar, N. Transition metal complexes of neocryptolepine analogues Part I: Synthesis, spectroscopic characterization, and in vitro anticancer activity of Copper(II) Complexes, Spectrochim. Mol. Biomol. Spectrosc. 2015, 138, 942–953. [Google Scholar] [CrossRef]
- Wang, N.; Świtalska, M.; Wu, M.-Y.; Imai, K.; Ngoc, T.A.; Pang, C.-Q.; Wang, L.; Wietrzyk, J.; Inokuchi, T. Synthesis and in vitro cytotoxic effect of 6-amino-substituted 11H- and 11Me-indolo[3,2-c]quinolines. Eur. J. Med. Chem. 2014, 78, 314–323. [Google Scholar] [CrossRef]
- Okada, M.; Mie, Z.-W.; Hossain, M.I.; Wang, L.; Tominaga, T.; Takebayashi, T.; Murakami, M.; Yasuda, M.; Shigehiro, T.; Kasai, T.; et al. Synthesis and in vitro cancer cell growth inhibition evaluation of 11-amino-modified 5-Me-indolo[2,3-b]quinolines and their COMPARE analyses. Med. Chem. Res. 2016, 25, 879–892. [Google Scholar] [CrossRef]
- Wang, L.; Świtalska, M.; Wang, N.; Du, Z.-J.; Fukumoto, Y.; Diep, N.K.; Kiguchi, R.; Nokami, J.; Wietrzyk, J.; Inokuchi, T.; et al. Design, Synthesis, and Biological Evaluation of Artemisinin-Indoloquinoline Hybrids as Potent Antiproliferative Agents. Molecules 2014, 19, 19021–19033. [Google Scholar] [CrossRef]
- Wang, L.; Moraleda, I.; Iriepa, I.; Romero, A.; López-Muñoz, F.; Chioua, M.; Inokuchi, T.; Bartolini, M.; Marco-Contelles, J. 5-Methyl-N-(8-(5,6,7,8-tetrahydroacridin-9-ylamino)octyl)-5H-indoloij2,3-b]quinolin-11-amine: A highly potent human cholinesterase inhibitor. Med. Chem. Commun. 2017, 8, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.; Yamori, T. JFCR39, a panel of 39 human cancer cell lines, and its application in the discovery and development of anticancer drugs. Bioorg. Med. Chem. 2012, 20, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
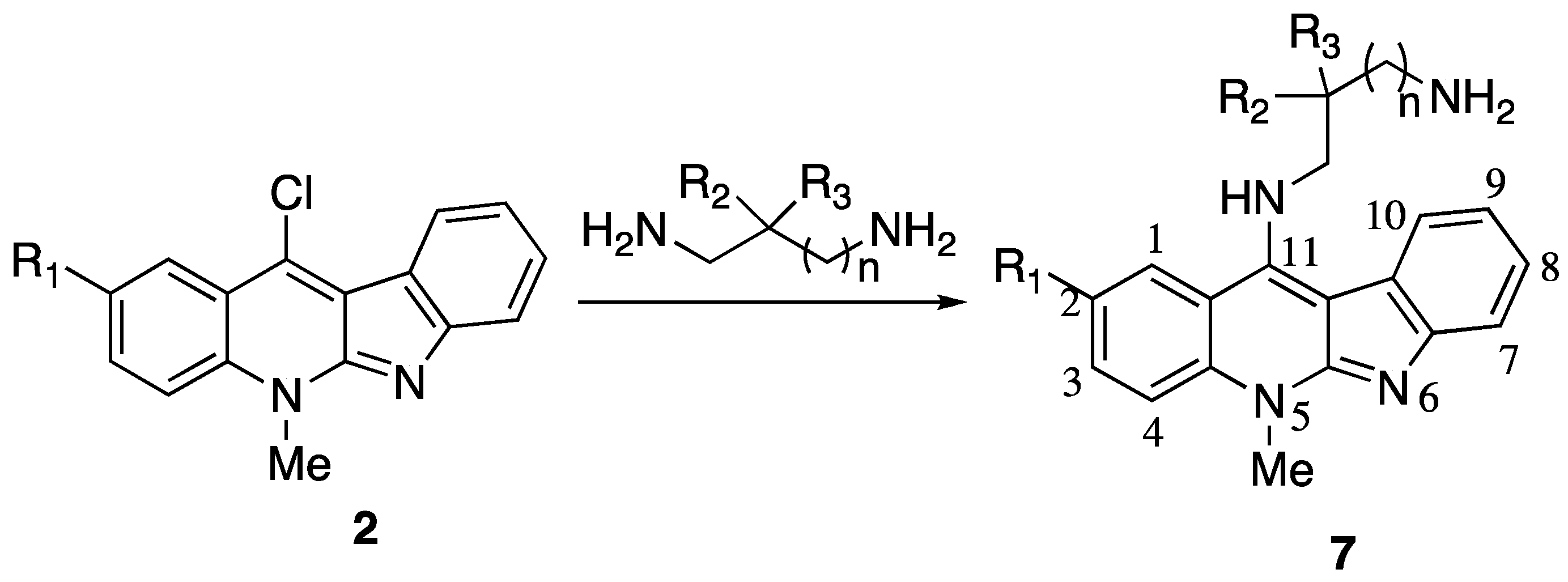
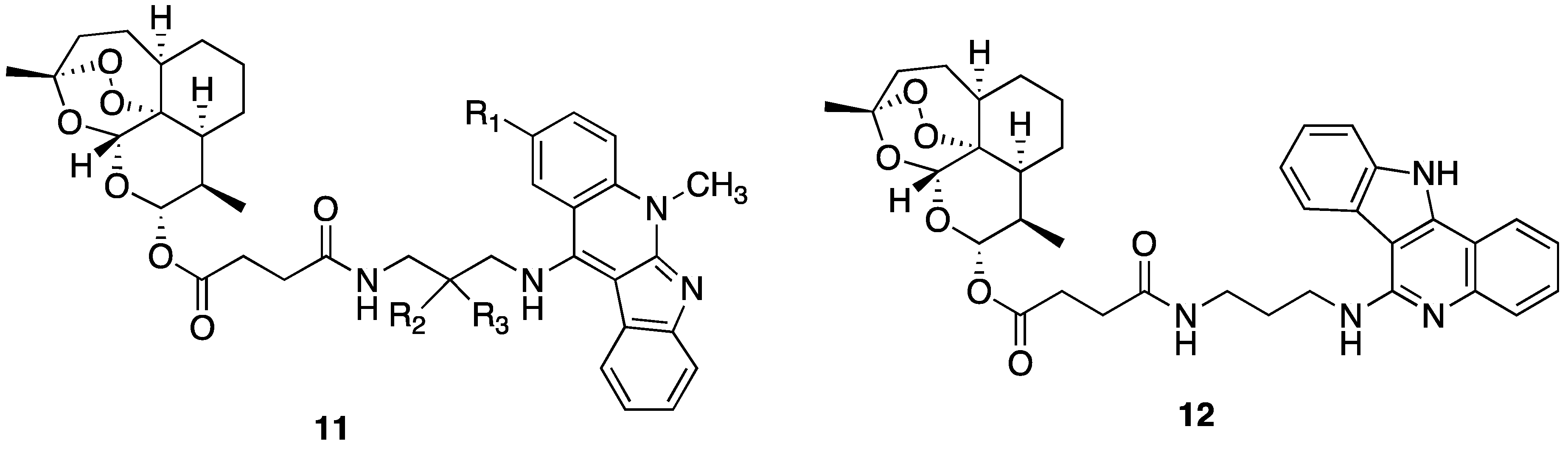
| No. | Substituent | IC50 μM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | R4 | R5 | BALB/3T3 | MV4-11 | A549 | HCT116 | |
| 7a | H | NH(CH2)3NH2 | H | Me | - | 0.884 ± 0.115 | 0.066 ± 0.023 | 0.205 ± 0.079 | 0.302 ± 0.056 |
| 7b | Cl | NH(CH2)3NH2 | H | Me | - | 0.401 ± 0.015 | 0.068 ± 0.018 | 0.761 ± 0.169 | 0.195 ± 0.044 |
| 7c | Br | NH(CH2)3NH2 | H | Me | - | 0.869 ± 0.018 | 0.012 ± 0.002 | 0.543 ± 0.256 | 0.274 ± 0.050 |
| 7d | OMe | NH(CH2)3NH2 | H | Me | - | 0.978 ± 0.021 | 0.102 ± 0.021 | 0.407 ± 0.117 | 0.155 ± 0.042 |
| 7e | Cl | NHCH2CH(OH)CH2NH2 | H | Me | - | 0.896 ± 0.042 | 0.042 ± 0.014 | 0.197 ± 0.028 | 0.138 ± 0.050 |
| 7f | Br | NHCH2CH(OH)CH2NH2 | H | Me | - | 0.864 ± 0.015 | 0.057 ± 0.015 | 0.190 ± 0.027 | 0.117 ± 0.055 |
| 7g | H | NH(CH2)3NH2 | COOMe | Me | - | 0.933 ± 0.047 | 0.044 ± 0.011 | 0.820 ± 0.456 | 0.176 ± 0.055 |
| 7h | Br | NH(CH2)3NH2 | COOMe | Me | - | 0.773 ± 0.023 | 0.050 ± 0.016 | 0.302 ± 0.138 | 0.164 ± 0.070 |
| 7i | Cl | NH(CH2)3NH2 | COOMe | Me | - | 0.833 ± 0.030 | 0.056 ± 0.035 | 0.194 ± 0.063 | 0.116 ± 0.078 |
| 7j | Cl | NH(CH2)3NH2 | Br | Me | - | 0.834 ± 0.067 | 0.076 ± 0.034 | 0.464 ± 0.011 | 0.284 ± 0.142 |
| 8 | Cl | NH(CH2)2NH2 | H | - | Me | 0.437 ± 0.400 | 0.456 ± 0.123 | 8.282 ± 0.585 | 6.989 ± 1.416 |
| 9 | OMe | NH(CH2)2NH2 | H | - | - | 6.87 ± 1.74 | 0.12 ± 0.07 | 9.29 ± 1.10 | 1.93 ± 0.24 |
| Cisplatin | 8.53 ± 3.53 | 2.36 ± 0.68 | 7.83 ± 2.60 | 3.47 ± 0.77 | |||||
| Doxorubicin HCl | 1.08 ± 0.03 | 0.006 ± 0.002 | 0.33 ± 0.10 | 0.39 ± 0.10 | |||||
| No. | Substituent | IC50 μM | |||||
|---|---|---|---|---|---|---|---|
| R1 | R2 | R3 | BALB/3T3 | MV4-11 | A549 | HCT116 | |
| 10a | H | NH(CH2)3NH2 | H | 1.05 ± 0.13 | 0.12 ± 0.01 | 0.17 ± 0.05 | 0.26 ± 0.11 |
| 10b | OMe | NH(CH2)3NH2 | H | 0.31 ± 0.03 | 0.12 ± 0.05 | 0.16 ± 0.03 | 0.07 ± 0.03 |
| 10c | NO2 | NH(CH2)3NH2 | H | 0.36 ± 0.15 | 0.15 ± 0.06 | 0.35 ± 0.22 | 0.10 ± 0.02 |
| 10d | H | NH(CH2)3NH2 | Me | 0.95 ± 0.24 | 0.11 ± 0.04 | 0.18 ± 0.03 | 0.06 ± 0.02 |
| 10e | NO2 | NH(CH2)3NH2 | Me | 0.08 ± 0.02 | 0.05 ± 0.01 | 0.11 ± 0.07 | 0.01 ± 0.00 |
| 10f | H | NH(CH2)3NH(CH3)2 | H | 1.18 ± 0.20 | 0.41 ± 0.31 | 0.87 ± 0.27 | 0.55 ± 0.17 |
| 10g | Cl | NH(CH2)3NH(CH3)2 | Me | 0.82 ± 0.08 | 0.08 ± 0.03 | 0.57 ± 0.31 | 0.10 ± 0.02 |
| Compound | MDA-MB-453 IC50(μM) | WiDr IC50(μM) | SKOv3 IC50(μM) |
|---|---|---|---|
| Neocryptolepine | 7.48 ± 4.42 | N.T. | N.T. |
| 7a | 0.50 ± 0.24 | 0.64 ± 0.13 | 2.7 ± 0.42 |
| 7d | 0.20 ± 0.09 | 0.37 ± 0.07 | 1.3 ± 0.18 |
| Cisplatin | 7.6 ± 0.7 a | 8.84 ± 0.52 c | 18.2 ± 0.1 d |
| Doxorubicin | 0.086 ± 0.006 b | 0.089 e | 0.52 |
| No. | Substituent | IC50 μM | ||||
|---|---|---|---|---|---|---|
| Compounds | R1 | C11-Substituent | MV4-11 | BALB/3T3 | A549 | HCT116 |
| Cisplatin | - | 2.820 ± 0.450 | 8.700 ± 0.097 | 9.870 ± 2.400 | 8.500 ± 0.540 | |
| Doxorubicin HCl | - | 0.006 ± 0.002 | 1.078 ± 0.033 | 0.329 ± 0.097 | 0.390 ± 0.098 | |
| 2a | H | Cl | 1.312 ± 0.262 | - | - | - |
| 2b | Br | Cl | 0.810 ± 0.145 | - | - | - |
| 7l | H | 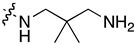 | 0.150 ± 0.060 | 4.789 ± 2.018 | 1.512 ± 0.198 | 1.262 ± 0.361 |
| 7m | Cl |  | 0.119 ± 0.043 | 9.131 ± 0.844 | 1.455 ± 0.168 | 1.373 ± 0.351 |
| 7n | Me | 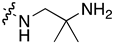 | 0.105 ± 0.027 | 10.558 ± 0.330 | 1.795 ± 0.270 | 2.370 ± 0.481 |
| 7o | Cl | 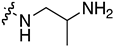 | 0.103 ± 0.014 | 1.018 ± 0.017 | 1.269 ± 0.118 | 1.204 ± 0.283 |
| 7p | Br |  | 0.078 ± 0.020 | 0.939 ± 0.018 | 0.988 ± 0.164 | 0.842 ± 0.367 |
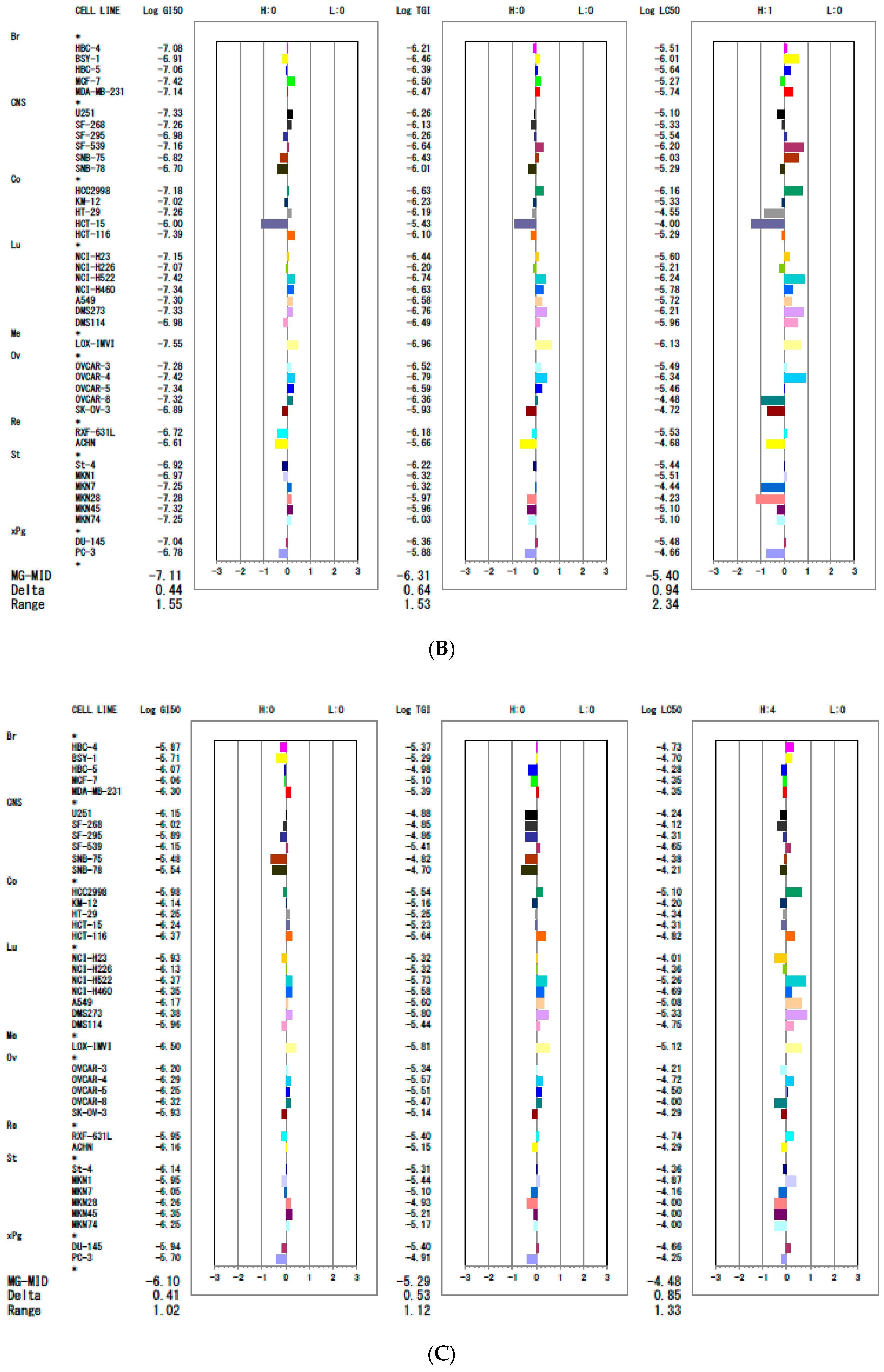
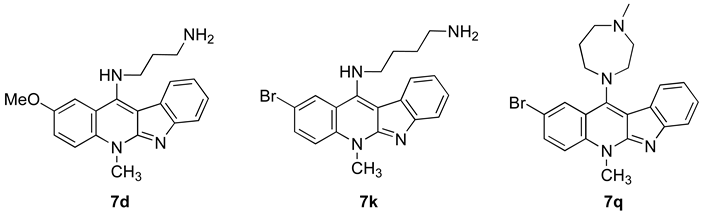 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
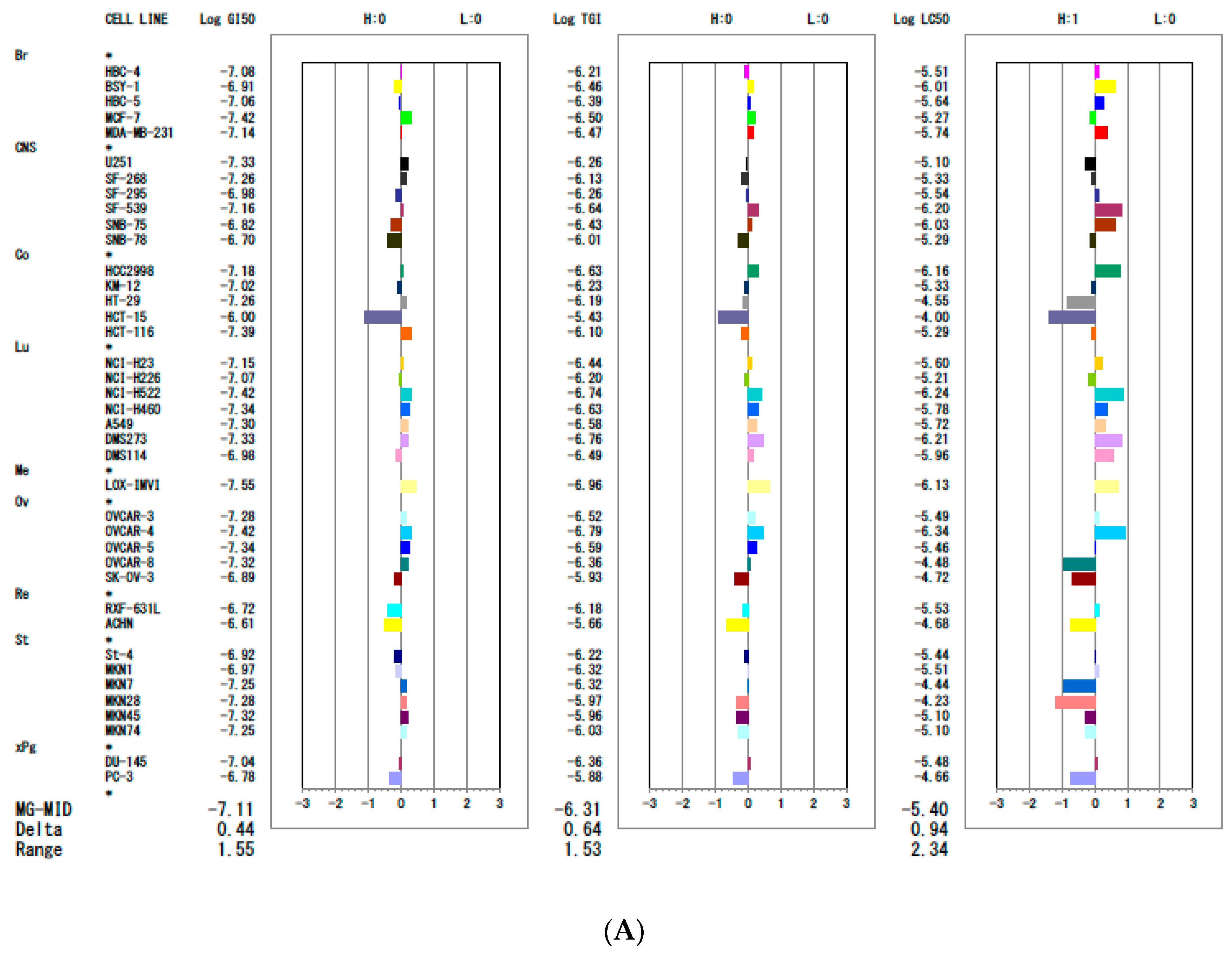
| Tissue of Origin | Cell Line | 7d | 7k | 7q | ||||||
| GI 50 (µM) | TGI (µM) | IC50 (µM) | GI 50 (µM) | TGI (µM) | IC50 (µM) | GI 50 (µM) | TGI (µM) | IC50 (µM) | ||
| Breast | HBC-4 | 0.06 | 0.80 | 9.5 | 0.08 | 0.61 | 6.1 | 1.3 | 4.3 | 19 |
| BSY-1 | 0.11 | 0.30 | 0.82 | 0.12 | 0.35 | 3.5 | 2.0 | 5.2 | 20 | |
| HBC-5 | 0.06 | 0.33 | 3.4 | 0.09 | 0.41 | 4.1 | 0.86 | 10 | 53 | |
| MCF-7 | 0.04 | 0.24 | 17 | 0.03 | 0.31 | 3.1 | 0.87 | 7.9 | 44 | |
| MDA-MB-231 | 0.08 | 0.33 | 6.9 | 0.07 | 0.34 | 3.4 | 0.50 | 4.1 | 44 | |
| CNS | U251 | 0.04 | 0.47 | 17 | 0.05 | 0.55 | 7.9 | 0.7 | 13 | 58 |
| SF-268 | 0.05 | 0.59 | 26 | 0.06 | 0.75 | 4.7 | 0.94 | 14 | 76 | |
| SF-295 | 0.10 | 0.81 | 14 | 0.11 | 0.55 | 2.9 | 1.3 | 14 | 49 | |
| SF-539 | 0.05 | 0.20 | 0.60 | 0.07 | 0.23 | 0.63 | 0.7 | 3.9 | 22 | |
| SNB-75 | 0.13 | 0.34 | 0.91 | 0.15 | 0.38 | 0.93 | 3.3 | 15 | 42 | |
| SNB-78 | 0.12 | 0.78 | 25 | 0.20 | 0.98 | 5.2 | 2.9 | 20 | 62 | |
| Colon | HCC2998 | 0.05 | 0.22 | 0.74 | 0.07 | 0.23 | 0.69 | 1.0 | 2.9 | 8.0 |
| KM-12 | 0.06 | 0.64 | 23 | 0.09 | 0.59 | 4.6 | 0.72 | 7.0 | 64 | |
| HT-29 | 0.04 | 0.54 | 24 | 0.06 | 0.64 | 28 | 0.56 | 5.6 | 46 | |
| HCT-15 | 0.72 | 6.9 | 50 | 1.0 | 3.7 | 100 | 0.58 | 5.8 | 49 | |
| HCT-116 | 0.04 | 0.57 | 22 | 0.04 | 0.80 | 5.1 | 0.43 | 2.3 | 15 | |
| Lung | NCI-H23 | 0.06 | 0.28 | 1.8 | 0.07 | 0.36 | 2.5 | 1.2 | 4.8 | 98 |
| NCI-H226 | 0.08 | 0.49 | 25 | 0.09 | 0.63 | 6.2 | 0.74 | 4.8 | 44 | |
| NCI-H522 | 0.04 | 0.20 | 0.71 | 0.04 | 0.18 | 0.57 | 0.42 | 1.8 | 5.5 | |
| NCI-H460 | 0.05 | 0.27 | 3.4 | 0.05 | 0.23 | 1.7 | 0.45 | 2.6 | 20 | |
| A549 | 0.06 | 0.23 | 0.85 | 0.05 | 0.26 | 1.9 | 0.68 | 2.5 | 8.3 | |
| DMS273 | 0.04 | 0.18 | 0.70 | 0.05 | 0.18 | 0.61 | 0.42 | 1.6 | 4.7 | |
| DMS114 | 0.08 | 0.26 | 0.72 | 0.10 | 0.33 | 1.10 | 1.1 | 3.7 | 18 | |
| Melanoma | LOX-IMVI | 0.03 | 0.11 | 1.1 | 0.03 | 0.11 | 0.75 | 0.31 | 1.5 | 7.6 |
| Ovarian | OVCAR-3 | 0.06 | 0.28 | 8.0 | 0.05 | 0.30 | 42 | 0.64 | 4.6 | 82 |
| OVCAR-4 | 0.04 | 0.15 | 0.48 | 0.04 | 0.16 | 59 | 0.52 | 2.7 | 19 | |
| OVCAR-5 | 0.05 | 0.35 | 9.5 | 0.05 | 0.26 | 42 | 0.56 | 3.1 | 32 | |
| OVCAR-8 | 0.05 | 0.33 | 23 | 0.05 | 0.43 | 100 | 0.48 | 3.4 | 100 | |
| SK-OV-3 | 0.13 | 1.0 | 20 | 0.13 | 1.2 | 54 | 1.2 | 7.4 | 51 | |
| Renal | RXF-631L | 0.27 | 1.4 | 7.4 | 0.19 | 0.67 | 3.0 | 1.1 | 3.0 | 18 |
| ACHN | 0.27 | 2.1 | 20 | 0.25 | 2.2 | 10 | 0.7 | 7.1 | 51 | |
| Stomach | St-4 | 0.06 | 0.43 | 3.8 | 0.12 | 0.60 | 3.7 | 0.72 | 4.9 | 44 |
| MKN1 | 0.09 | 0.38 | 12 | 0.11 | 0.47 | 3.1 | 1.1 | 3.6 | 14 | |
| MKN7 | 0.04 | 0.34 | 23 | 0.06 | 0.48 | 36 | 0.9 | 8.0 | 69 | |
| MKN28 | 0.05 | 0.9 | 37 | 0.05 | 1.1 | 58 | 0.55 | 12 | 100 | |
| MKN45 | 0.05 | 1.0 | 28 | 0.05 | 1.1 | 8.0 | 0.44 | 6.2 | 100 | |
| MKN74 | 0.05 | 1.0 | 52 | 0.06 | 0.94 | 8.0 | 0.56 | 6.8 | 100 | |
| Prostate | DU145 | 0.1 | 0.34 | 17 | 0.09 | 0.43 | 3.3 | 1.2 | 4.0 | 22 |
| PC-3 | 0.16 | 1.3 | 70 | 0.16 | 1.3 | 22 | 2.0 | 12 | 56 | |
| Compound | R1 | R2 | R3 | MV4-11 | A549 | HCT116 | BALB/3T3 |
|---|---|---|---|---|---|---|---|
| 11a | Cl | CH3 | CH3 | 0.072 ± 0.022 | 4.555 ± 2.086 | 0.893 ± 0.397 | 6.423 ± 0.996 |
| 11b | CO2Me | CH3 | CH3 | 0.075 ± 0.001 | 5.060 ± 0.911 | 2.206 ± 0.687 | 5.945 ± 1.163 |
| 12 | CO2Me | H | H | 0.086 ± 0.020 | 0.649 ± 0.080 | 0.130 ± 0.014 | 0.768 ± 0.155 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, N.; Świtalska, M.; Wang, L.; Shaban, E.; Hossain, M.I.; El Sayed, I.E.T.; Wietrzyk, J.; Inokuchi, T. Structural Modifications of Nature-Inspired Indoloquinolines: A Mini Review of Their Potential Antiproliferative Activity. Molecules 2019, 24, 2121. https://doi.org/10.3390/molecules24112121
Wang N, Świtalska M, Wang L, Shaban E, Hossain MI, El Sayed IET, Wietrzyk J, Inokuchi T. Structural Modifications of Nature-Inspired Indoloquinolines: A Mini Review of Their Potential Antiproliferative Activity. Molecules. 2019; 24(11):2121. https://doi.org/10.3390/molecules24112121
Chicago/Turabian StyleWang, Ning, Marta Świtalska, Li Wang, Elkhabiry Shaban, Md Imran Hossain, Ibrahim El Tantawy El Sayed, Joanna Wietrzyk, and Tsutomu Inokuchi. 2019. "Structural Modifications of Nature-Inspired Indoloquinolines: A Mini Review of Their Potential Antiproliferative Activity" Molecules 24, no. 11: 2121. https://doi.org/10.3390/molecules24112121
APA StyleWang, N., Świtalska, M., Wang, L., Shaban, E., Hossain, M. I., El Sayed, I. E. T., Wietrzyk, J., & Inokuchi, T. (2019). Structural Modifications of Nature-Inspired Indoloquinolines: A Mini Review of Their Potential Antiproliferative Activity. Molecules, 24(11), 2121. https://doi.org/10.3390/molecules24112121