Potent Anti-Ovarian Cancer with Inhibitor Activities on Both Topoisomerase II and V600EBRAF of Synthesized Substituted Estrone Candidates
Abstract
1. Introduction
2. Results and Discussion
2.1. Results
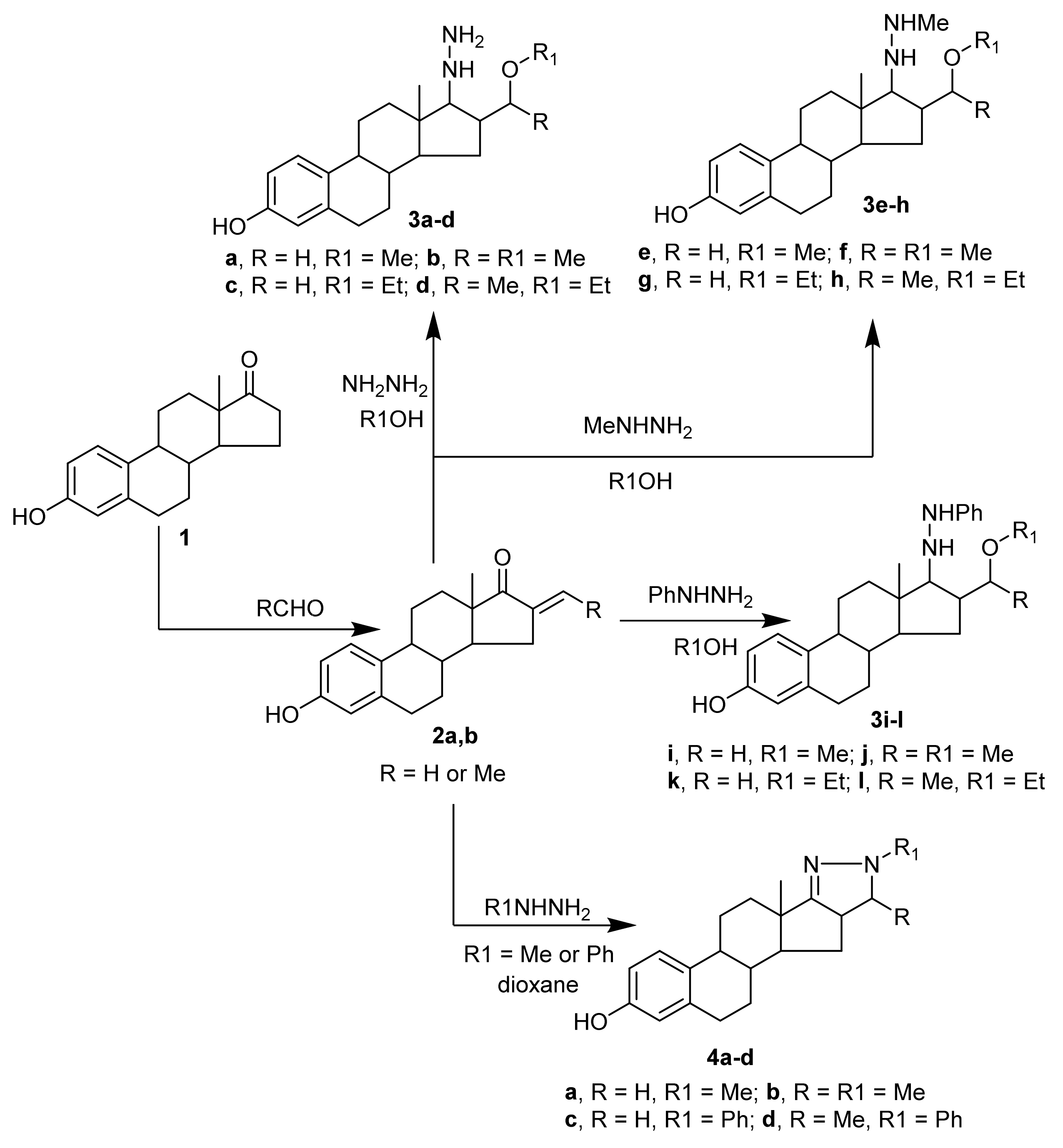
2.1.1. Chenical Synthesis
2.1.2. Biological Screening
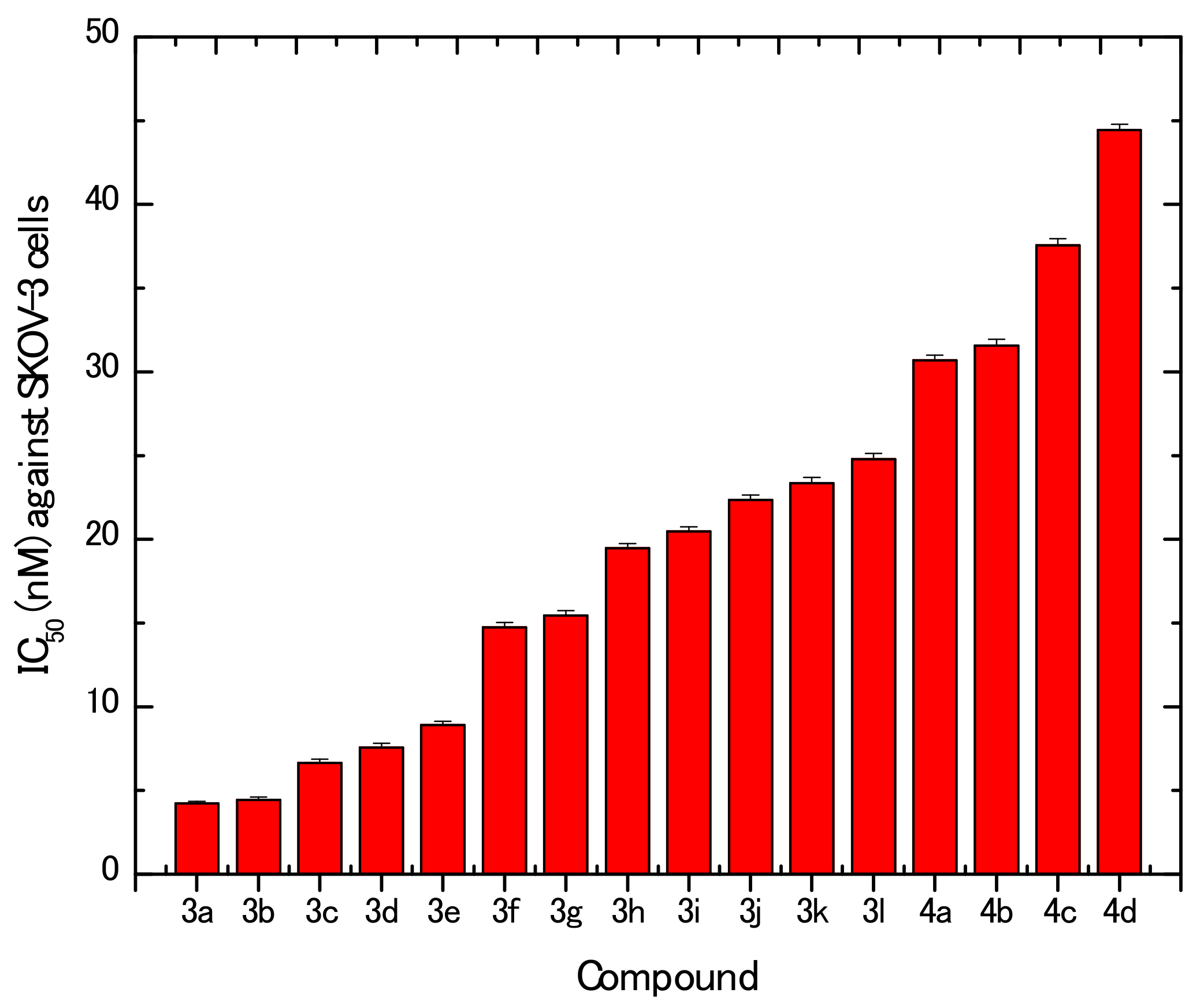
In Vitro Cytotoxic Activities
In Vivo Anti-Ovarian Cancer
2.1.3. Inhibition of Topoisomerase II Activities
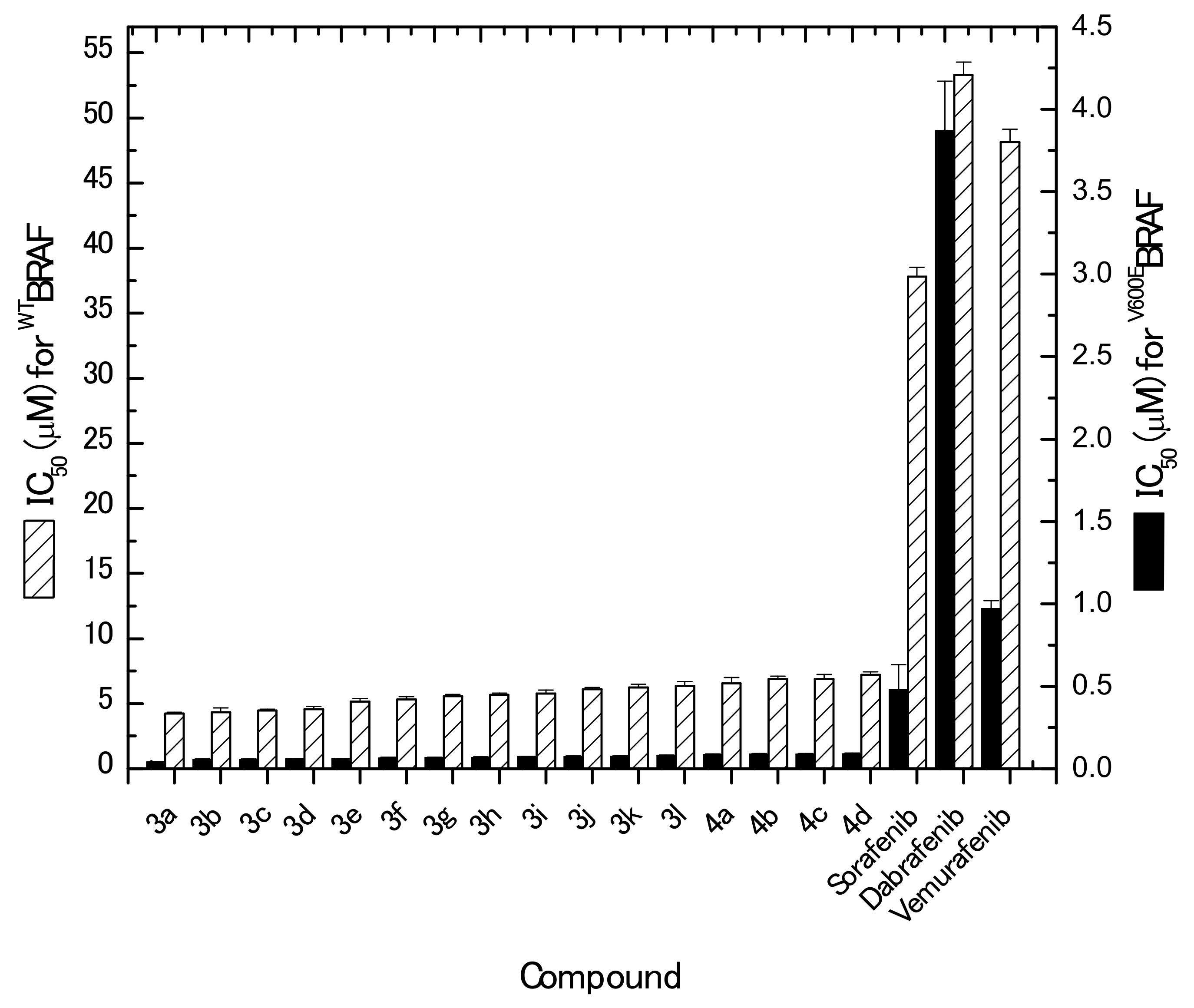
2.1.4. In Vitro Kinase Assay
2.2. Discussion
3. Materials and Methods
3.1. Chemical Synthesis
3.1.1. Synthesis of 3-Hydroxy-16-[substituted]-estra-1(10),2,4-trien-17-ones (2a,b)
3-Hydroxy-16-[ethylene]-estra-1(10),2,4-trien-17-one (2b)
3.1.2. Synthesis of 16-(α-alkoxy-alkane)-17-hydrazino-estra-1(10),2,4-trien[17,16-c]-3-ol and their N-substituted derivatives (3a–l)
16-(α-Methoxy-methane)-17-hydrazino-estra-1(10),2,4-trien-[17,16-c]-3-ol (3a)
16-(α-Methoxy-ethane)-17-hydrazino-estra-1(10),2,4-trien[17,16-c]-3-ol (3b)
16-(α-Ethoxy-methane)-17-hydrazino-estra-1(10),2,4-trien-[17,16-c]-3-ol (3c)
16-(α-Ethoxy-ethane)-17-hydrazino-estra-1(10),2,4-trien-[17,16-c]-3-ol (3d)
16-(α-Methoxy-methane)-17-[N-methyl-hydrazino]estra-1(10),2,4-trien[17,16-c]-3-ol (3e)
16-(α-Methoxy-ethane)-17-[N-methyl-hydrazino]-estra-1(10),2,4-trien-[17,16-c]-3-ol (3f)
16-(α-Ethoxy-methane)-17-[N-methyl-hydrazino]-estra-1(10),2,4-trien-[17,16-c]-3-ol (3g)
16-(α-Ethoxy-ethane)-17-[N-methyl-hydrazino]-estra-1(10),2,4-trien-[17,16-c]-3-ol (3h)
16-(α-Methoxy-methane)-17-[N-phenyl-hydrazino]-estra-1(10),2,4-trien-[17,16-c]-3-ol (3i)
16-(α-Methoxy-ethane)-17-[N-phenyl-hydrazino]-estra-1(10),2,4-trien-[17,16-c]-3-ol (3j)
16-(α-Ethoxy-methane)-17-[N-phenyl-hydrazino]-estra-1(10),2,4-trien-[17,16-c]-3-ol (3k)
16-(α-Ethoxy-ethane)-17-[N-phenyl-hydrazino]-estra-1(10),2,4-trien-[17,16-c]-3-ol (3l)
3.1.3. Synthesis of pyrazoline-3-ol derivatives (4a–d)
1`-Methyl-1`H-estra-1(10),2,4-trien-[17,16-c]pyrazoline-3-ol (4a)
1`-Methyl -1`H-5`-methyl-estra-1(10),2,4-trien-[17,16-c]pyrazoline-3-ol (4b)
1`-Phenyl-1`H-estra-1(10),2,4-trien-[17,16-c]pyrazoline-3-ol (4c)
1`-Phenyl-1`H-5`-methyl-estra-1(10),2,4-trien-[17,16-c]pyrazoline-3-ol (4d)
3.2. Biological Screening
3.2.1. In Vitro Cytotoxic Activities
3.2.2. In Vitro Anti-Ovarian Xenograft Model
3.2.3. Topoisomerase II Inhibition
3.2.4. In Vitro Kinase Inhibition
Protein Expression and Purification
In Vitro ELISA-Based Kinase Assay
3.2.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gupta, A.; Kumar, B.S.; Negi, A.S. Current status on development of steroids as anticancer agents. J. Steroid Biochem. Mol. Boil. 2013, 137, 242–270. [Google Scholar] [CrossRef] [PubMed]
- Amr, A.E.-G.E.; El-Naggar, M.; Al-Omar, M.A.; Elsayed, E.A.; Abdalla, M.M. In vitro and in vivo anti-breast cancer activities of some synthesized pyrazolinyl-estran-17-one candidates. Molecules 2018, 23, 1572. [Google Scholar] [CrossRef] [PubMed]
- Afzal, M.; Al-Awadi, S.; Oommen, S. Antioxidant activity of biotransformed sex hormones facilitated by Bacillus stearothermophilus. In Advanced Protocols in Oxidative Stress II, Methods in Molecular Biology; Armstrong, D., Ed.; Springer: Basel, Switzerland, 2010. [Google Scholar]
- Schönecker, B.; Lange, C.; Kötteritzsch, M.; Günther, W.; Weston, J.; Anders, E.; Görls, H. Conformational design for 13α-Steroids. J. Org. Chem. 2000, 65, 5487–5497. [Google Scholar] [CrossRef]
- Jovanovic-Santa, S.; Petrović, J.; Andrić, S.; Kovačević, R.; Ðurendić, E.; Sakač, M.; Lazar, D.; Stanković, S. Synthesis, structure, and screening of estrogenic and antiestrogenic activity of new 3,17-substituted- 16,17-seco-estratriene derivatives. Bioorg. Chem. 2003, 31, 475–484. [Google Scholar] [CrossRef]
- Minorics, R.; Bózsity, N.; Wölfling, J.; Mernyák, E.; Schneider, G.; Márki, A.; Falkay, G.; Ocsovszki, I.; Zupkó, I. Antiproliferative effect of normal and 13-epi-D-homoestrone and their 3-methyl ethers on human reproductive cancer cell lines. J. Steroid Biochem. Mol. Biol. 2013, 132, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Ayan, D.P.; Maltais, R.; Roy, J.; Poirier, D. Impact of estradiol structural modifications (18-methyl and/or 17-hydroxy inversion of configuration) on the in vitro and in vivo estrogenic activity. In Proceedings of the Endocrine Society’s 93rd Annual Meeting & Expo, Boston, MA, USA, 4–7 June 2011; Volume 127. [Google Scholar]
- Szabó, J.; Bacsa, I.; Wölfling, J.; Schneider, G.; Zupkó, I.; Varga, M.; Herman, B.E.; Kalmár, L.; Szécsi, M.; Mernyák, E. Synthesis and in vitro pharmacological evaluation of N-[(1-benzyl-1,2,3-triazol-4-yl)methyl]- carboxamides on D-secoestrone scaffolds. J. Enzyme Inhib. Med. Chem. 2016, 31, 574–579. [Google Scholar] [CrossRef] [PubMed]
- Szabó, J.; Jerkovics, N.; Schneider, G.; Wölfling, J.; Bózsity, N.; Minorics, R.; Zupkó, I.; Mernyák, E.; De Sousa, M.E. Synthesis and in vitro antiproliferative evaluation of c-13 epimers of triazolyl-d-secoestrone alcohols: the first potent 13α-d-secoestrone derivative. Molecules 2016, 21, 611. [Google Scholar] [CrossRef] [PubMed]
- Szabó, J.; Pataki, Z.; Wölfling, J.; Schneider, G.; Bózsity, N.; Minorics, R.; Zupkó, I.; Mernyák, E. Synthesis and biological evaluation of 13α-estrone derivatives as potential antiproliferative agents. Steroids 2016, 113, 14–21. [Google Scholar] [CrossRef]
- Mernyák, E.; Kovács, I.; Minorics, R.; Sere, P.; Czégány, D.; Sinka, I.; Wölfling, J.; Schneider, G.; Újfaludi, Z.; Boros, I.; et al. Synthesis of trans-16-triazolyl-13α-methyl-17-estradiol diastereomers and the effects of structural modifications on their in vitro antiproliferative activities. J. Steroid Biochem. Mol. Boil. 2015, 150, 123–134. [Google Scholar] [CrossRef]
- Rao, P.N.; Cessac, J.W.; Tinley, T.L.; Mooberry, S.L. Synthesis and antimitotic activity of novel 2-methoxyestradiol analogs. Steroids 2002, 67, 1079–1089. [Google Scholar] [CrossRef]
- Sundov, D.; Čarić, A.; Mrklić, I.; Gugic, D.; Čapkun, V.; Hofman, I.D.; Mise, B.P.; Tomic, S. P53, MAPK, topoisomerase II alpha and Ki67 immunohistochemical expression and KRAS/BRAF mutation in ovarian serous carcinomas. Diagn. Pathol. 2013, 8, 21. [Google Scholar] [CrossRef]
- Kaur, P.V.; Kaur, S. DNA Topoisomerase II: promising target for anticancer drugs. In Multi-Targeted Approach to Treatment of Cancer; Gandhi, V., Ed.; Springer: Basel, Switzerland, 2015; pp. 323–338. [Google Scholar]
- Nijenhuis, C.; Lucas, L.; Rosing, H.; Huitema, A.; Mergui-Roelvink, M.; Jamieson, G.C.; Fox, J.; Mould, D.; Schellens, J.; Beijnen, J. Metabolism and disposition of the anticancer quinolone derivative vosaroxin, a novel inhibitor of topoisomerase II. Investig. New Drugs 2017, 35, 478–490. [Google Scholar] [CrossRef]
- Rahman, M.A.; Salajegheh, A.; Smith, R.A.; Lam, A.K.-Y.; Ariana, A. Inhibition of BRAF kinase suppresses cellular proliferation, but not enough for complete growth arrest in BRAF V600E mutated papillary and undifferentiated thyroid carcinomas. Endocrine 2016, 54, 129–138. [Google Scholar] [CrossRef]
- Rahman, M.A.; Salajegheh, A.; Smith, R.A.; Lam, A.K.-Y.; Ariana, A. Multiple proliferation-survival signalling pathways are simultaneously active in BRAF V600E mutated thyroid carcinomas. Exp. Mol. Pathol. 2015, 99, 492–497. [Google Scholar] [CrossRef]
- McCubrey, J.A.; Steelman, L.S.; Abrams, S.L.; Lee, J.T.; Chang, F.; Bertrand, F.E.; Navolanic, P.M.; Terrian, D.M.; Franklin, R.A.; D’Assoro, A.B.; et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv. Enzym. Regul. 2006, 46, 249–279. [Google Scholar] [CrossRef]
- Ouf, N.H.; Amr, A.E. Synthesis and anti-inflammatory activity of some pyrimidines and thieno- pyrimidines using 1-(2-benzo[d][1,3]dioxol-5-yl)vinyl)-4-mercapto-6-methyl-pyrimidine-5-yl)-ethan-2- one as a starting material. Monatsh. fur Chem. 2008, 139, 579–585. [Google Scholar] [CrossRef]
- Amr, A.E.; Abdulla, M.M. Synthesis and anti-inflammatory activities of new cynopyrane derivatives fused with steroidal nuclei. Archiv. der Pharm. 2008, 339, 88–95. [Google Scholar] [CrossRef]
- Khalifa, N.M.; Al-Omar, M.A.; Amr, A.E.; Haiba, M.E. Antiviral activity of some new polycyclic nucleoside pyrene candidate against HIV-1 and HSV-1 virus. Int. J. Biol. Macromol. 2013, 54, 51. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Mohamed, S.F.; Amr, A.E.-G.E.; Abdalla, M.M. Synthesis and reactions of thiosemicarbazides, triazoles, and Schiff bases as antihypertensive α-blocking agents. Monatsh. Chem. 2008, 139, 1083–1090. [Google Scholar] [CrossRef]
- Amr, A.E.-G.E.; Elsayed, E.A.; Al-Omar, M.A.; Eldin, H.O.B.; Nossier, E.S.; Abdallah, M.M.; Eldin, H.B. Design, synthesis, anticancer evaluation and molecular modeling of novel estrogen derivatives. Molecules 2019, 24, 416. [Google Scholar] [CrossRef]
- Day, J.M.; Foster, P.A.; Tutill, H.J.; Schmidlin, F.; Sharland, C.M.; Hargrave, J.D.; Vicker, N.; Potter, B.V.L.; Reed, M.J.; Purohit, A. STX2171, a 17-hydroxysteroid dehydrogenase type 3 inhibitor, is efficacious in vivo in a novel hormone-dependent prostate cancer model. Endocr. Relat. Cancer 2013, 20, 53–64. [Google Scholar] [CrossRef]
- Donald, P.; Ho-Jin, C.; Arezki, A.; Roch, P.B.; Sheng-Xiang, L. Estrone and estradiol C-16 derivatives as inhibitors of type 1 17β-hydroxysteroid dehydrogenase. Mol. Cell. Endocrinol. 2006, 248, 236–238. [Google Scholar]
- Elsayed, E.A.; Sharaf-Eldin, M.; Wadaan, M. In vitro evaluation of cytotoxic activities of essential oil from moringa oleifera seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines. Asian Pac. J. Cancer Prev. 2015, 16, 4671–4675. [Google Scholar] [CrossRef]
- Elsayed, E.A.; Farooq, M.; Dailin, D.; El-Enshasy, H.A.; Othman, N.Z.; Malek, R.; Danial, E.; Wadaan, M. In vitro and in vivo biological screening of kefiran polysaccharide produced by Lactobacillus kefiranofaciens. Biomed. Res. 2017, 28, 594–600. [Google Scholar]
- Al-Salahi, R.; Elsayed, E.A.; El Dib, R.A.; Wadaan, M.; Ezzeldin, E.; Marzouk, M. Synthesis, characterization and cytotoxicity evaluation of 5-hydrazono-[1,2,4]triazolo[1,5-a]quinazolines (Part I). Lat. Am. J. Pharm. 2016, 35, 58–65. [Google Scholar]
- Al-Salahi, R.; Elsayed, E.A.; El Dib, R.A.; Wadaan, M.; Ezzeldin, E.; Marzouk, M. Cytotoxicity of new 5-hydrazono-[1,2,4]triazolo[1,5-a]quinazolines (Part II). Lat. Am. J. Pharm. 2016, 35, 66–73. [Google Scholar]
- Elsayed, E.A.; Sharaf-Eldin, M.A.; El-Enshasy, H.A.; Wadaan, M. In vitro assessment of anticancer properties of Moringa peregrina essential seed oil on different cell lines. Pak. J. Zool. 2016, 48, 853–859. [Google Scholar]
- Farooq, M.; Abu Taha, N.; Butorac, R.R.; Evans, D.A.; Elzatahry, A.A.; Elsayed, E.A.; Wadaan, M.A.M.; Al-Deyab, S.S.; Cowley, A.H.; Li, J. Biological screening of newly synthesized BIAN N-heterocyclic gold carbene complexes in zebrafish embryos. Int. J. Mol. Sci. 2015, 16, 24718–24731. [Google Scholar] [CrossRef]
- McCauley, J.; Zivanovic, A.; Skropeta, D. Bioassays for anticancer activities. Methods Mol. Biol. 2013, 1055, 191–205. [Google Scholar]
- Goyeneche, A.A.; Carón, R.W.; Telleria, C.M. Mifepristone inhibits ovarian cancer cancer cell growth in vitro and in vivo. Clin. Cancer Res. 2007, 13, 3370–3379. [Google Scholar] [CrossRef]
- Nakamura, K.; Sugumi, H.; Yamaguchi, A.; Uenaka, T.; Kotake, Y.; Okada, T.; Kamata, J.; Niijima, J.; Nagasu, T.; Koyanagi, N.; et al. Antitumor activity of ER-37328, a novel carbazole topoisomerase II inhibitor. Mol. Cancer Ther. 2002, 1, 169–75. [Google Scholar]
- Qin, J.; Xie, P.; Ventocilla, C.; Zhou, G.; Vultur, A.; Chen, Q.; Liu, Q.; Herlyn, M.; Winkler, J.; Marmorstein, R. Identification of a novel family of BRAF(V600E) inhibitors. J. Med. Chem. 2012, 55, 5220–5230. [Google Scholar] [CrossRef]
- Stones, C.J.; Kim, J.E.; Joseph, W.R.; Leung, E.; Marshall, E.S.; Finlay, G.J.; Shelling, A.N.; Baguley, B.C. Comparison of responses of human melanoma cell lines to MEK and BRAF inhibitors. Front. Genet. 2013, 4, 66. [Google Scholar] [CrossRef][Green Version]
Sample Availability: Samples of the compounds are available from the authors. |


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Naggar, M.; E. Amr, A.E.-G.; Fayed, A.A.; A. Elsayed, E.; A. Al-Omar, M.; Abdalla, M.M. Potent Anti-Ovarian Cancer with Inhibitor Activities on Both Topoisomerase II and V600EBRAF of Synthesized Substituted Estrone Candidates. Molecules 2019, 24, 2054. https://doi.org/10.3390/molecules24112054
El-Naggar M, E. Amr AE-G, Fayed AA, A. Elsayed E, A. Al-Omar M, Abdalla MM. Potent Anti-Ovarian Cancer with Inhibitor Activities on Both Topoisomerase II and V600EBRAF of Synthesized Substituted Estrone Candidates. Molecules. 2019; 24(11):2054. https://doi.org/10.3390/molecules24112054
Chicago/Turabian StyleEl-Naggar, Mohamed, Abd El-Galil E. Amr, Ahmed A. Fayed, Elsayed A. Elsayed, Mohamed A. Al-Omar, and Mohamed M. Abdalla. 2019. "Potent Anti-Ovarian Cancer with Inhibitor Activities on Both Topoisomerase II and V600EBRAF of Synthesized Substituted Estrone Candidates" Molecules 24, no. 11: 2054. https://doi.org/10.3390/molecules24112054
APA StyleEl-Naggar, M., E. Amr, A. E.-G., Fayed, A. A., A. Elsayed, E., A. Al-Omar, M., & Abdalla, M. M. (2019). Potent Anti-Ovarian Cancer with Inhibitor Activities on Both Topoisomerase II and V600EBRAF of Synthesized Substituted Estrone Candidates. Molecules, 24(11), 2054. https://doi.org/10.3390/molecules24112054






