MHY440, a Novel Topoisomerase Ι Inhibitor, Induces Cell Cycle Arrest and Apoptosis via a ROS-Dependent DNA Damage Signaling Pathway in AGS Human Gastric Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Effects of MHY440 on Topo I and DNA Damage Signaling Pathway in AGS Cells
2.2. Molecular Docking Simulation of Topo I with MHY440
2.3. Effects of MHY440 on the Growth of AGS Cells
2.4. Effects of MHY440 on the Cell Cycle in AGS Cells
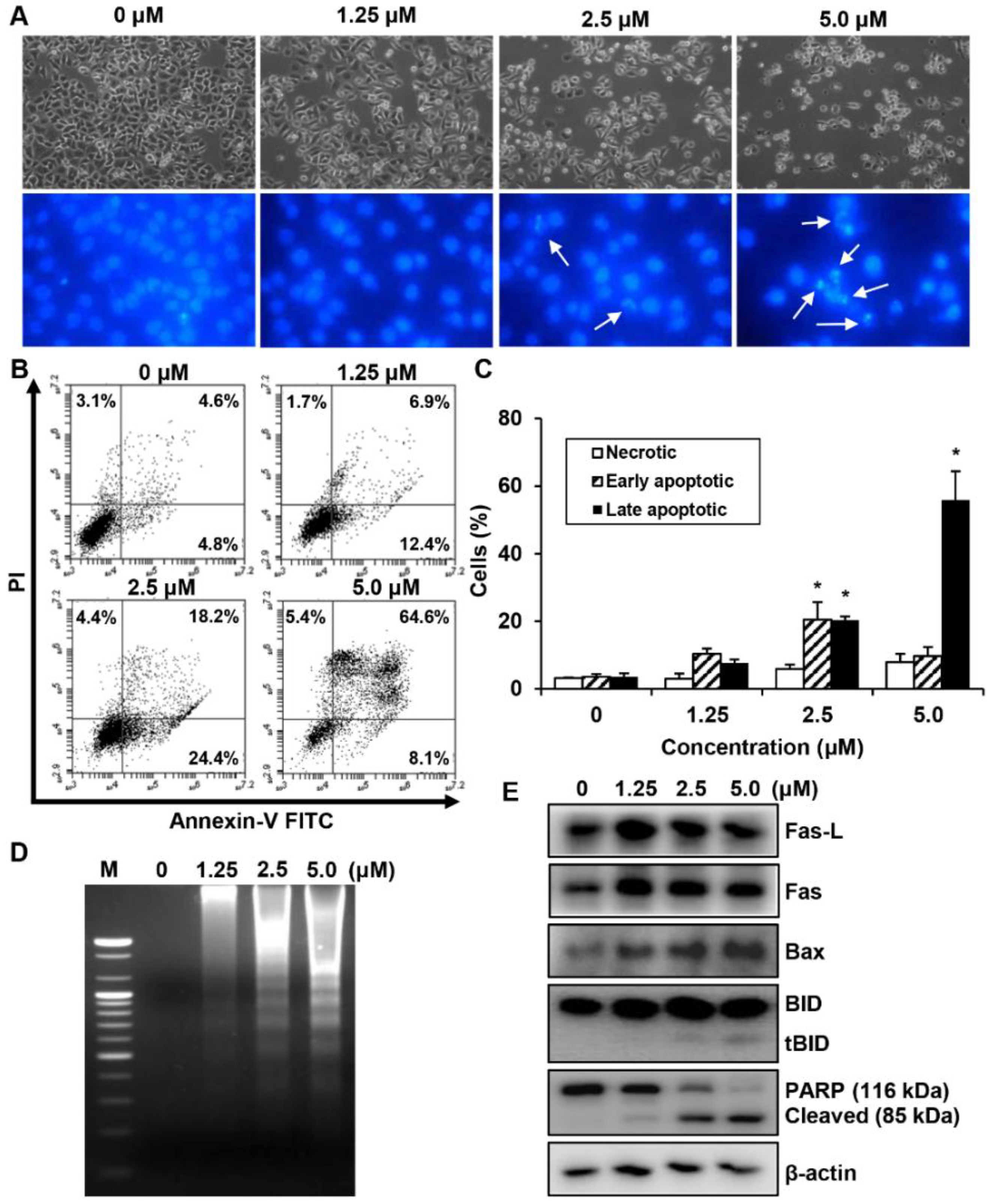
2.5. Effects of MHY440 on the Induction of Apoptosis in AGS Cells
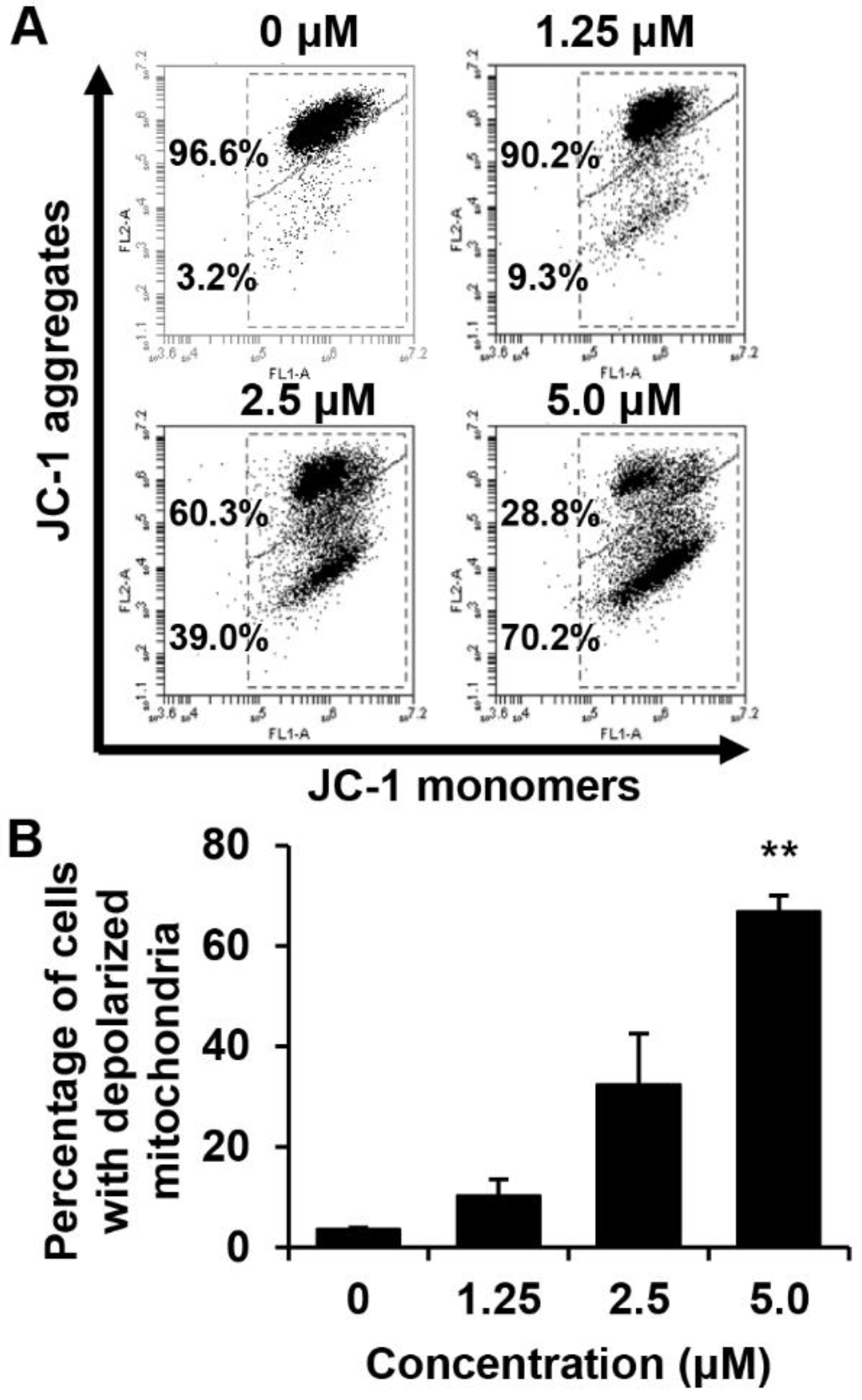
2.6. Effects of MHY440 on the Mitochondrial Membrane Potential (MMP)
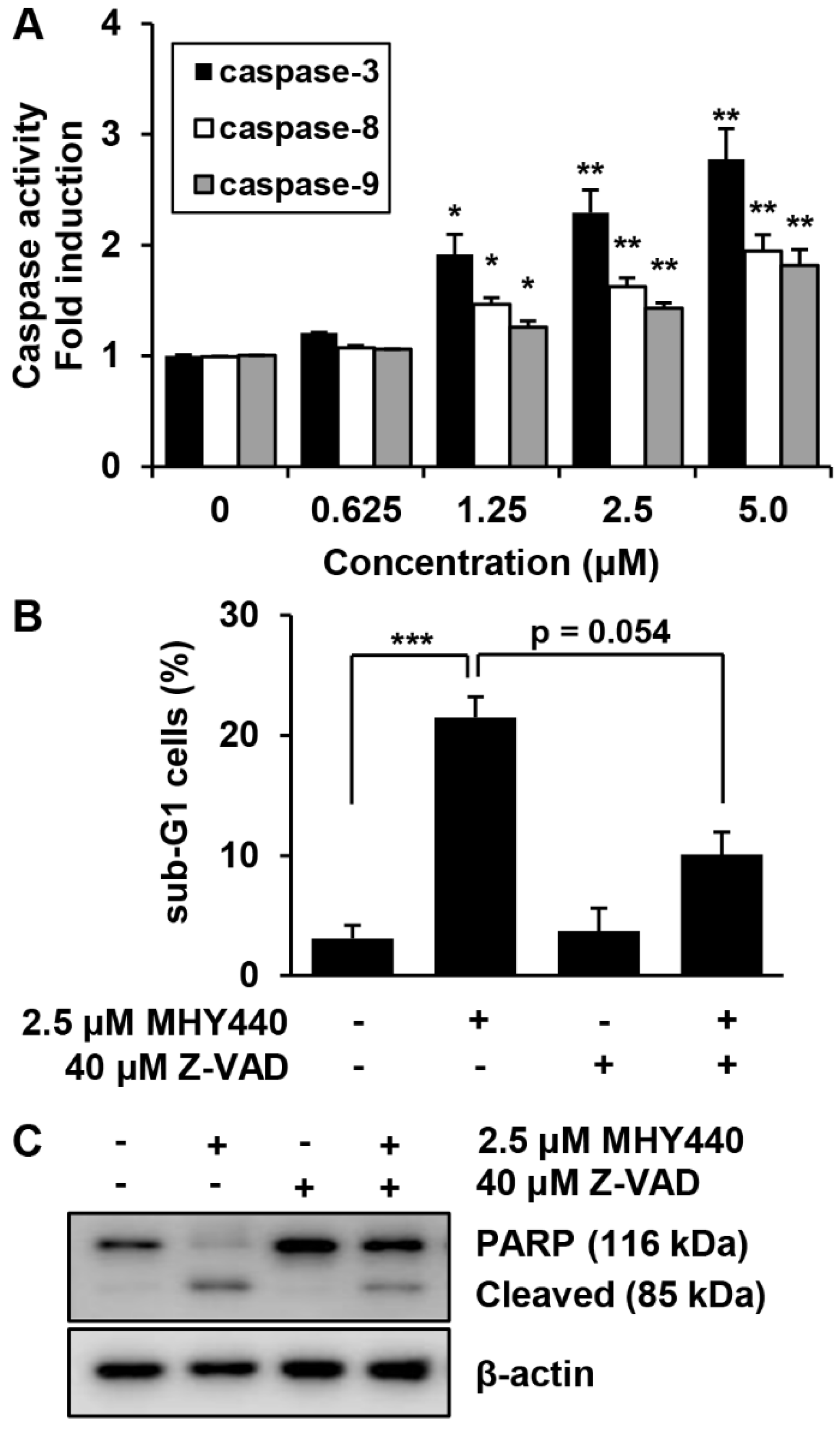
2.7. Effects of MHY440 on the Caspase Activation
2.8. Effects of MHY440 on the ROS Generation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Determination of Topo I Activity
4.3. Docking Simulation of Topo I and MHY440
4.4. Cell Culture and Cell Viability Analysis
4.5. Cell Cycle Analysis
4.6. Western Blot Analysis
4.7. Nuclear Staining with Hoechst 33342
4.8. Annexin V Staining
4.9. DNA Fragmentation Assay
4.10. Measurement of Mitochondrial Membrane Potential (MMP, ΔΨm)
4.11. Measurement of Caspase Activity
4.12. Measurement of Intracellular ROS Accumulation
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Venerito, M.; Vasapolli, R.; Rokkas, T.; Malfertheiner, P. Gastric cancer: Epidemiology, prevention, and therapy. Helicobacter 2018, 23 (Suppl. 1), e12518. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Lee, E.S.; Community of Population-Based Regional Cancer Registries. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2015. Cancer Res. Treat. 2018, 50, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. Cellular roles of DNA topoisomerases: A molecular perspective. Nat. Rev. Mol. Cell. Biol. 2002, 3, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.C. A journey in the world of DNA rings and beyond. Annu. Rev. Biochem. 2009, 78, 31–54. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. DNA topoisomerase II and its growing repertoire of biological functions. Nat. Rev. Cancer 2009, 9, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Champoux, J.J. DNA topoisomerases: Structure, function, and mechanism. Annu. Rev. Biochem. 2001, 70, 369–413. [Google Scholar] [CrossRef]
- Wu, C.C.; Huang, K.F.; Yang, T.Y.; Li, Y.L.; Wen, C.L.; Hsu, S.L.; Chen, T.H. The topoisomerase 1 inhibitor Austrobailignan-1 isolated from Koelreuteria henryi induces a G2/M-phase arrest and cell death independently of p53 in non-small cell lung cancer cells. PLoS ONE 2015, 10, e0132052. [Google Scholar] [CrossRef]
- Fulda, S.; Gorman, A.M.; Hori, O.; Samali, A. Cellular stress responses: Cell survival and cell death. Int. J. Cell. Biol. 2010, 2010, 214074. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.L.; Kang, Y.J.; Sung, B.; Hwang, S.Y.; Jang, J.Y.; Oh, H.J.; Ahn, Y.R.; Kim, D.H.; Kim, S.J.; Ullah, S.; et al. MHY451 induces cell cycle arrest and apoptosis by ROS generation in HCT116 human colorectal cancer cells. Oncol. Rep. 2017, 38, 1783–1789. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.L.; Perego, P.; Zunino, F. Mechanisms of cellular resistance to camptothecins. Curr. Med. Chem. 2006, 13, 3291–3305. [Google Scholar] [CrossRef] [PubMed]
- Allen, W.L.; Coyle, V.M.; Johnston, P.G. Predicting the outcome of chemotherapy for colorectal cancer. Curr. Opin. Pharmacol. 2006, 6, 332–336. [Google Scholar] [CrossRef] [PubMed]
- Fellows, I.M.; Schwaebe, M.; Dexheimer, T.S.; Vankayalapati, H.; Gleason-Guzman, M.; Whitten, J.P.; Hurley, L.H. Determination of the importance of the stereochemistry of psorospermin in topoisomerase II-induced alkylation of DNA and in vitro and in vivo biological activity. Mol. Cancer Ther. 2005, 4, 1729–1739. [Google Scholar] [CrossRef] [PubMed]
- Boutefnouchet, S.; Gaboriaud-Kolar, N.; Nguyen, T.M.; Depauw, S.; David-Cordonnier, M.H.; Pfeiffer, B.; Leonce, S.; Pierre, A.; Tillequin, F.; Lallemand, M.C.; et al. Synthesis, cytotoxic activity, and mechanism of action of furo[2,3-c]acridin-6-one and benzo[b]furo[3,2-h]acridin-6-one analogues of psorospermin and acronycine. J. Med. Chem. 2008, 51, 7287–7297. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Tho, L.M.; Xu, N.; Gillespie, D.A. The ATM-Chk2 and ATR-Chk1 pathways in DNA damage signaling and cancer. Adv. Cancer Res. 2010, 108, 73–112. [Google Scholar] [CrossRef]
- Turner, T.; Caspari, T. When heat casts a spell on the DNA damage checkpoints. Open. Biol. 2014, 4, 140008. [Google Scholar] [CrossRef]
- Boohaker, R.J.; Xu, B. The versatile functions of ATM kinase. Biomed. J. 2014, 37, 3–9. [Google Scholar] [CrossRef]
- Vousden, K.H. Activation of the p53 tumor suppressor protein. Biochim. Biophys. Acta 2002, 1602, 47–59. [Google Scholar] [CrossRef]
- Allocati, N.; Di Ilio, C.; De Laurenzi, V. p63/p73 in the control of cell cycle and cell death. Exp. Cell. Res. 2012, 318, 1285–1290. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, S.H.; Lee, S.H.; Meng, X.W.; Loegering, D.A.; Kottke, T.J.; Henzing, A.J.; Ruchaud, S.; Samejima, K.; Earnshaw, W.C. Apoptosis-associated caspase activation assays. Methods 2008, 44, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Khadka, D.B.; Cho, W.J. 3-Arylisoquinolines as novel topoisomerase I inhibitors. Bioorg. Med. Chem. 2011, 19, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y. Topoisomerase I inhibitors: Camptothecins and beyond. Nat. Rev. Cancer 2006, 6, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Pommier, Y.; Leo, E.; Zhang, H.; Marchand, C. DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem. Biol. 2010, 17, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Polo, S.E.; Jackson, S.P. Dynamics of DNA damage response proteins at DNA breaks: A focus on protein modifications. Genes Dev. 2011, 25, 409–433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Chen, S.; Mei, H.; Xuan, J.; Guo, X.; Couch, L.; Dobrovolsky, V.N.; Guo, L.; Mei, N. Ginkgo biloba leaf extract induces DNA damage by inhibiting topoisomerase II activity in human hepatic cells. Sci. Rep. 2015, 5, 14633. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, Z.; Gunasekera, A.H.; Sowin, T.J.; Rosenberg, S.H.; Fesik, S.; Zhang, H. Chk1 mediates S and G2 arrests through Cdc25A degradation in response to DNA-damaging agents. J. Biol. Chem. 2003, 278, 21767–21773. [Google Scholar] [CrossRef]
- Wesierska-Gadek, J.; Maurer, M. Promotion of apoptosis in cancer cells by selective purine-derived pharmacological CDK inhibitors: One outcome, many mechanisms. Curr. Pharm. Des. 2011, 17, 256–271. [Google Scholar] [CrossRef]
- Sun, H.; Hou, H.; Lu, P.; Zhang, L.; Zhao, F.; Ge, C.; Wang, T.; Yao, M.; Li, J. Isocorydine inhibits cell proliferation in hepatocellular carcinoma cell lines by inducing G2/m cell cycle arrest and apoptosis. PLoS ONE 2012, 7, e36808. [Google Scholar] [CrossRef]
- Abraham, R.T. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes Dev. 2001, 15, 2177–2196. [Google Scholar] [CrossRef] [PubMed]
- Norbury, C.J.; Zhivotovsky, B. DNA damage-induced apoptosis. Oncogene 2004, 23, 2797–2808. [Google Scholar] [CrossRef] [PubMed]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef] [PubMed]
- Arbab, I.A.; Looi, C.Y.; Abdul, A.B.; Cheah, F.K.; Wong, W.F.; Sukari, M.A.; Abdullah, R.; Mohan, S.; Syam, S.; Arya, A.; et al. Dentatin induces apoptosis in prostate cancer cells via Bcl-2, Bcl-xL, Survivin downregulation, caspase-9, -3/7 activation, and NF-κB inhibition. Evid. Based. Complementary Alternat. Med.: ECAM 2012, 2012, 856029. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Hairpin-hairpin molecular beacon interactions for detection of survivin mRNA in malignant SW480 Cells. ACS Appl. Mater. Interfaces 2018, 10, 17028–17039. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Krazinski, B.E.; Kowalczyk, A.E.; Dworakowska, B.; Jakiela, S.; Stobiecka, M. Optical biosensing system for the detection of survivin mRNA in colorectal cancer cells using a graphene oxide carrier-bound oligonucleotide molecular beacon. Nanomaterials 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nakamura, H.; Iyer, A.K. Tumor-targeted induction of oxystress for cancer therapy. J. Drug Target. 2007, 15, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T.; Petermann, E.; Lundin, C.; Hodgson, B.; Sharma, R.A. DNA repair pathways as targets for cancer therapy. Nat. Rev. Cancer 2008, 8, 193–204. [Google Scholar] [CrossRef]
- Sahu, R.P.; Batra, S.; Srivastava, S.K. Activation of ATM/Chk1 by curcumin causes cell cycle arrest and apoptosis in human pancreatic cancer cells. Br. J. Cancer 2009, 100, 1425–1433. [Google Scholar] [CrossRef]
- Staker, B.L.; Hjerrild, K.; Feese, M.D.; Behnke, C.A.; Burgin, A.B., Jr.; Stewart, L. The mechanism of topoisomerase I poisoning by a camptothecin analog. Proc. Natl. Acad. Sci. USA 2002, 99, 15387–15392. [Google Scholar] [CrossRef]
Sample Availability: Sample of the compound MHY440 is available from the authors. |

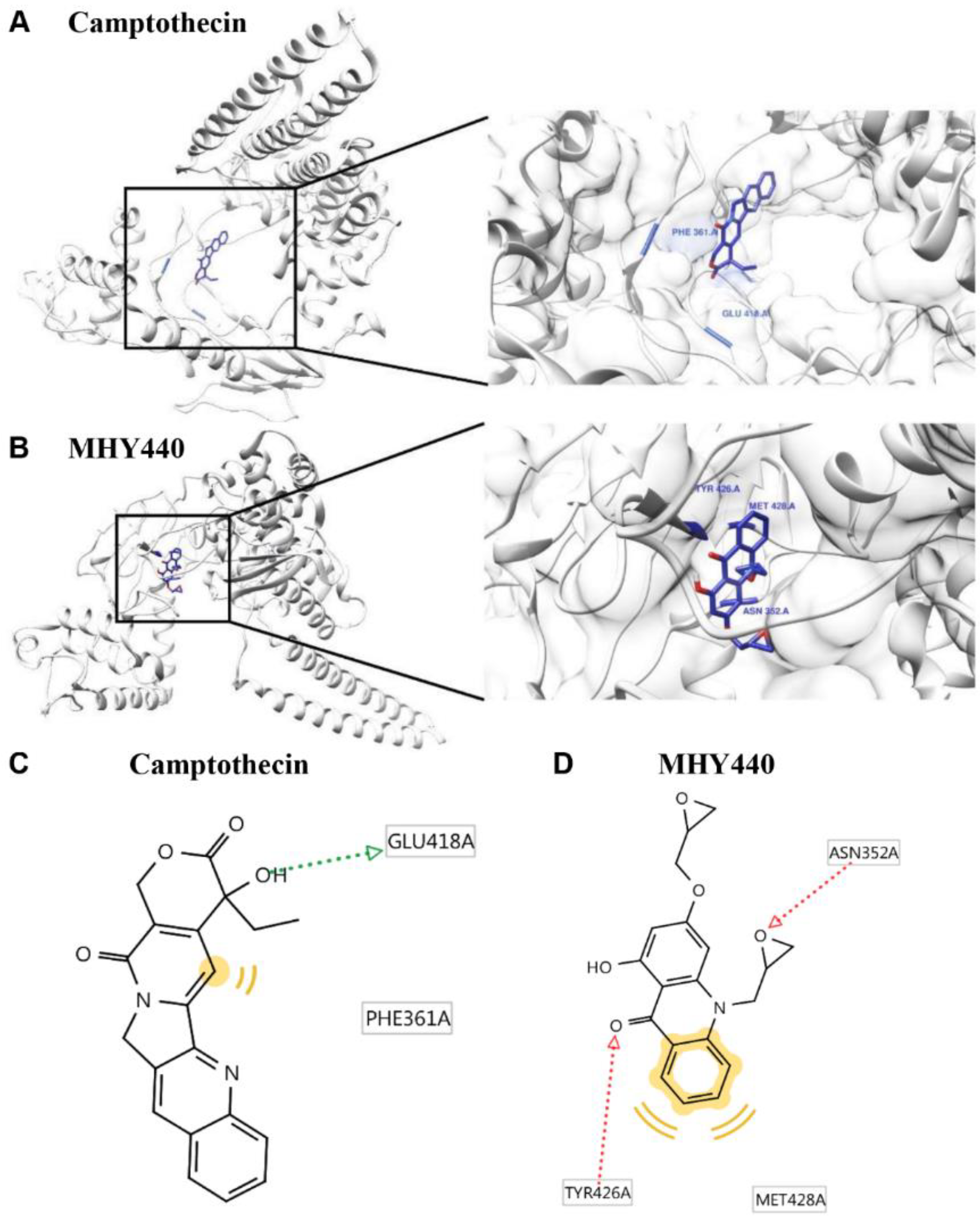



| Compounds | Binding Energy (kcal/mol) a | No. of H-Bond b | H-Bond Interacting Residues b | Van der Waals Bond Interaction Residues b | |
|---|---|---|---|---|---|
| AutoDock Vina | AutoDock 4 | ||||
| Camptothecin | −5.2 | −5.73 | 1 | GLU418 | PHE361 |
| MHY440 | −4.4 | −4.88 | 2 | ASN352, TYR426 | TYR426, MET428 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, J.Y.; Kang, Y.J.; Sung, B.; Kim, M.J.; Park, C.; Kang, D.; Moon, H.R.; Chung, H.Y.; Kim, N.D. MHY440, a Novel Topoisomerase Ι Inhibitor, Induces Cell Cycle Arrest and Apoptosis via a ROS-Dependent DNA Damage Signaling Pathway in AGS Human Gastric Cancer Cells. Molecules 2019, 24, 96. https://doi.org/10.3390/molecules24010096
Jang JY, Kang YJ, Sung B, Kim MJ, Park C, Kang D, Moon HR, Chung HY, Kim ND. MHY440, a Novel Topoisomerase Ι Inhibitor, Induces Cell Cycle Arrest and Apoptosis via a ROS-Dependent DNA Damage Signaling Pathway in AGS Human Gastric Cancer Cells. Molecules. 2019; 24(1):96. https://doi.org/10.3390/molecules24010096
Chicago/Turabian StyleJang, Jung Yoon, Yong Jung Kang, Bokyung Sung, Min Jeong Kim, Chaeun Park, Dongwan Kang, Hyung Ryong Moon, Hae Young Chung, and Nam Deuk Kim. 2019. "MHY440, a Novel Topoisomerase Ι Inhibitor, Induces Cell Cycle Arrest and Apoptosis via a ROS-Dependent DNA Damage Signaling Pathway in AGS Human Gastric Cancer Cells" Molecules 24, no. 1: 96. https://doi.org/10.3390/molecules24010096
APA StyleJang, J. Y., Kang, Y. J., Sung, B., Kim, M. J., Park, C., Kang, D., Moon, H. R., Chung, H. Y., & Kim, N. D. (2019). MHY440, a Novel Topoisomerase Ι Inhibitor, Induces Cell Cycle Arrest and Apoptosis via a ROS-Dependent DNA Damage Signaling Pathway in AGS Human Gastric Cancer Cells. Molecules, 24(1), 96. https://doi.org/10.3390/molecules24010096








