Separation of Five Iridoid Glycosides from Lonicerae Japonicae Flos Using High-Speed Counter-Current Chromatography and Their Anti-Inflammatory and Antibacterial Activities
Abstract
:1. Introduction
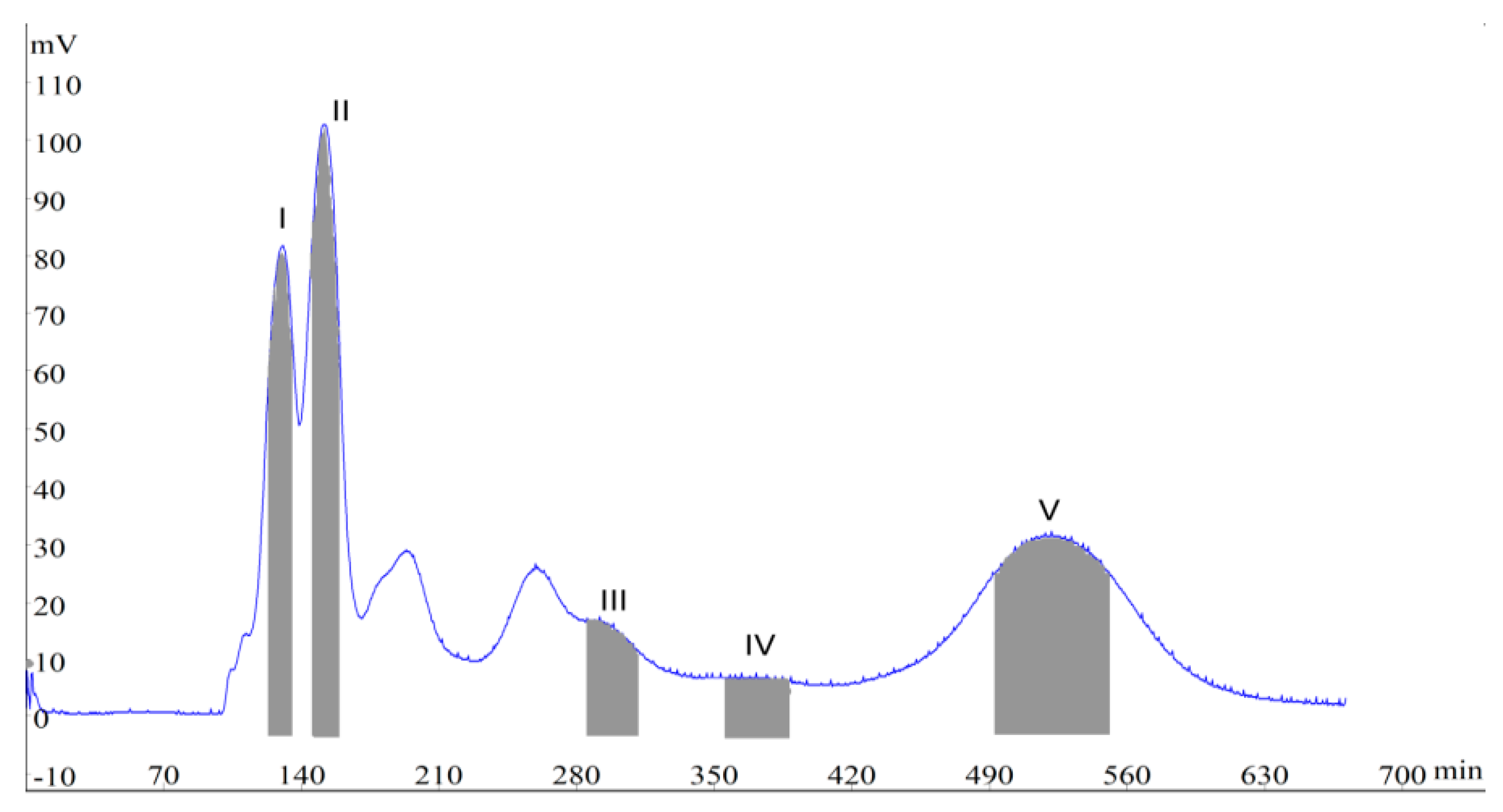
2. Results and Discussion
2.1. Selection of Two-Phase Solvent Systems
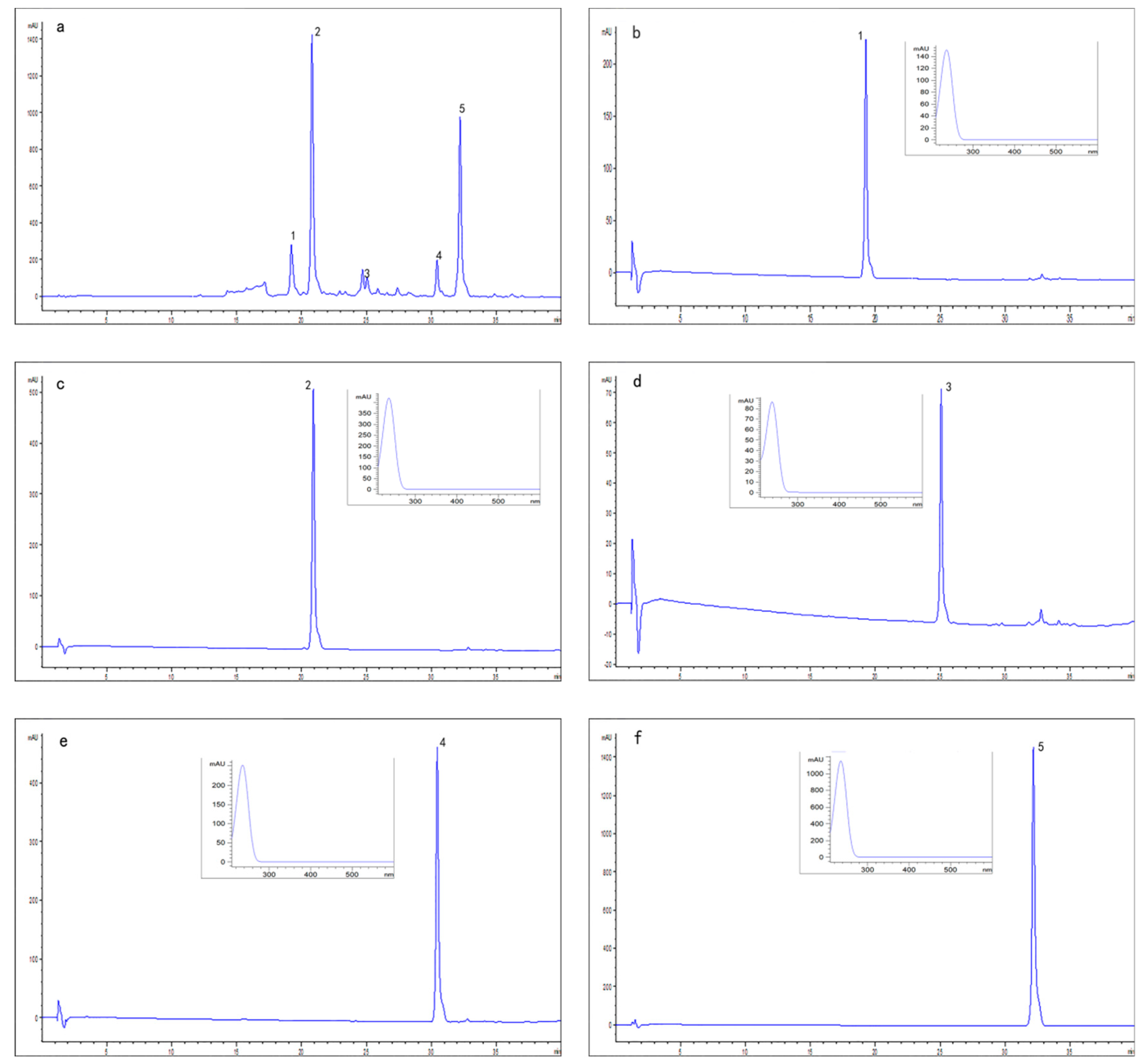
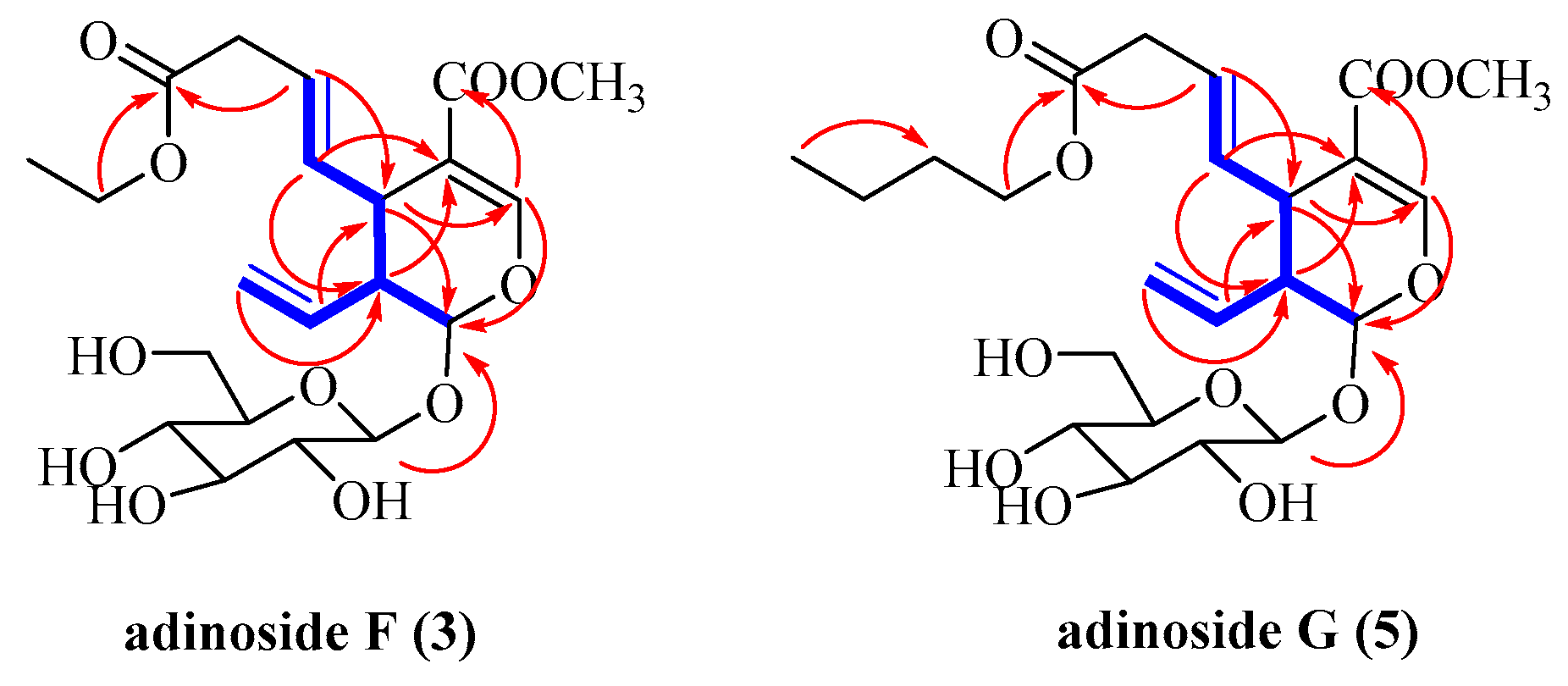
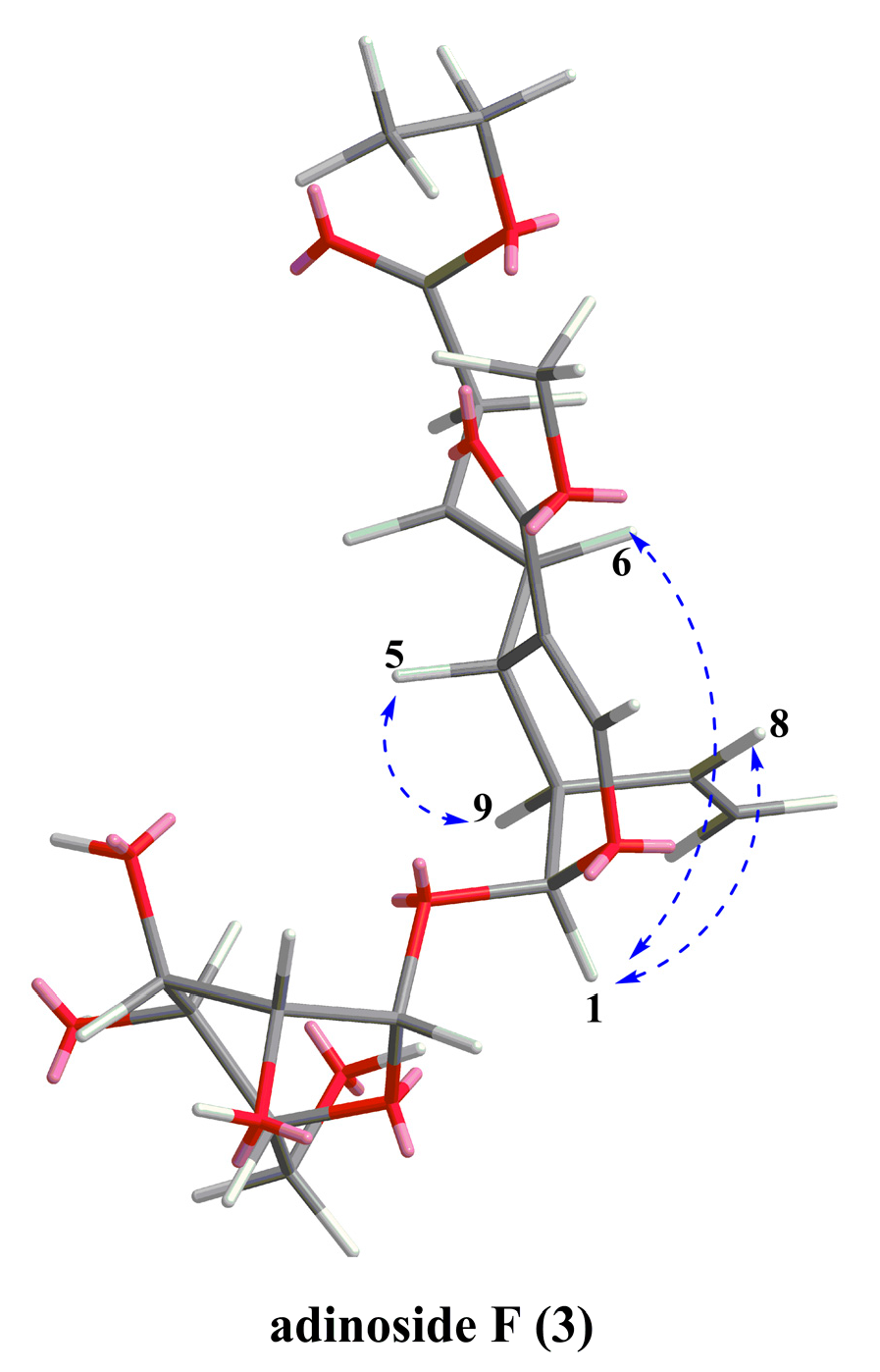
2.2. Identification of Compounds
2.3. Anti-Inflammatory and Antibacterial Activities
3. Materials and Methods
3.1. Plant Materials and Reagents
3.2. Instruments
3.3. Preparation of Crude Sample
3.4. Selection of Two-Phase Solvent Systems and Preparation of the Sample Solution
3.5. Procedure of HSCCC Separation
3.6. Analysis and Identification of Peak Fractions
3.7. Anti-Inflammatory Assay
3.8. Antibacterial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pharmacopoeia. Chinese Pharmacopoeia; China Medical Science Press: Beijing, China, 2015; p. 221. [Google Scholar]
- Deng, M.; Feng, J.; Song, F.; Pan, Y. The Preparation of Honeysuckle and Astragalus Anti-aging Mask. Guangdong Chem. Industry 2018, 45, 96–97. [Google Scholar]
- Wang, L.; Jiang, Q.; Hu, J.; Zhang, Y.; Li, J. Research Progress on Chemical Constituents of Lonicerae japonicae flos. Biomed. Res. Int. 2016, 2016, 8968940. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Shan, J.; Wang, S.; Cai, B.; Di, L. Transepithelial transport of phenolic acids in Flos Lonicerae Japonicae in intestinal Caco-2 cell monolayers. Food Funct. 2015, 6, 3072–3080. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, T.; Pei, Q.; Liu, S.; Yuan, H. Pharmacokinetics and tissue distribution study of chlorogenic Acid from lonicerae japonicae flos following oral administrations in rats. Evid. Based Complement. Alternat. Med. 2014, 2014, 979414. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, W.S.; Nagappan, A.; Hong, S.H.; Jung, J.H.; Park, C.; Kim, H.J.; Kim, G.Y.; Kim, G.; Jung, J.M.; et al. Flavonoids Isolated from Flowers of Lonicera japonica Thunb. Inhibit Inflammatory Responses in BV2 Microglial Cells by Suppressing TNF-alpha and IL-beta Through PI3K/Akt/NF-kb Signaling Pathways. Phytother. Res. 2016, 30, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Obied, H.K.; Karuso, P.; Prenzler, P.D.; Robards, K. Novel Secoiridoids with Antioxidant Activity from Australian Olive Mill Waste. J. Agric. Food Chem. 2007, 55, 2848. [Google Scholar]
- Li, H.B.; Yu, Y.; Mei, Y.D.; Meng, Z.Q.; Wang, Z.Z.; Huang, W.Z.; Xiao, W.; Yao, X.S. A new hetero dimeric terpenoid derivative, japonicaside C, from the flower buds of Lonicera japonica. Nat. Prod. Res. 2017, 31, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Che, H.; Ha, D.; Wei, Y.; Zheng, S. Characterization and anti-allergic effect of a polysaccharide from the flower buds of Lonicera japonica. Carbohydr. Polym. 2012, 90, 1642–1647. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, K.I.; Lee, D.H.; Kang, S.R.; Nagappan, A.; Kim, J.A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Hah, Y.S.; et al. Polyphenolic extract isolated from Korean Lonicera japonica Thunb. induce G2/M cell cycle arrest and apoptosis in HepG2 cells: involvements of PI3K/Akt and MAPKs. Food Chem. Toxicol. 2012, 50, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Deng, L.; Cai, S.; Liu, J.; Li, W.; Du, L.; Cui, G.; Xu, X.; Lu, T. Systematic separation and purification of iridoid glycosides and crocetin derivatives from Gardenia jasminoides Ellis by high-speed counter-current chromatography. J. Chromatogr. A 2015, 26, 202–208. [Google Scholar]
- Ito, Y. New continous extraction method with a coil planet centrifuge. J. Chromatogr. A 1981, 207, 161–169. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Fisher, D. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J. Chromatogr. A 2009, 1216, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Yue, H.L.; Zhao, X.H.; Wang, Q.L.; Tao, Y.D. Separation and purification of water-soluble iridoid glucosides by high speed counter-current chromatography combined with macroporous resin column separation. J. Chromatogr. B 2013, 936, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Itoh, A.; Fujii, K.; Tomatsu, S.; Takao, C.; Tanahashi, T.; Nagakura, N.; Chen, C.C. Six secoiridoid glucosides from Adina racemosa. J. Nat. Prod. 2003, 66, 1212–1216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.F.; Zhang, Q.J.; Chen, R.Y.; Yu, D.Q. Four new N-contained iridoid glycosides from flower buds of Lonicera japonica. J. Asian Nat. Prod. Res. 2012, 14, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Song, Y.; Li, P. Capillary high-performance liquid chromatography with mass spectrometry for simultaneous determination of major flavonoids, iridoid glucosides and saponins in Flos Lonicerae. J. Chromatogr. A 2007, 1157, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Machida, K.; Asano, J.; Kikuchi, M. Caeruleosides A and B, bis-iridoid glucosides from Lonicera caerulea. Phytochemistry 1995, 39, 111–114. [Google Scholar] [CrossRef]
- Kanno, M.; Kakuda, R.; Kikuchi, M. Studies on the Constituents of Hydrangea Species, II. On the Chemical Constituents of the Leaves of Hydrangea macrophylla subsp. serrata (THUNB.) MAKINO. J. Tohoku Pharm. Univ. 2007, 54, 57–61. [Google Scholar]
- Fang, L.; Fang, Z.-Y.; Zhou, P.; Bao, J.; Yu, J.-H.; Song, J.-T.; Zhou, J.; Zhang, H. Anti-inflammatory lignans from Melodinus suaveolens. Phytochem. Lett. 2018, 26, 134–137. [Google Scholar] [CrossRef]
- Wang, P.; Yu, J.H.; Zhu, K.; Wang, Y.; Cheng, Z.Q.; Jiang, C.S.; Dai, J.G.; Wu, J.; Zhang, H. Phenolic bisabolane sesquiterpenoids from a Thai mangrove endophytic fungus, Aspergillus sp. xy02. Fitoterapia 2018, 127, 322–327. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |
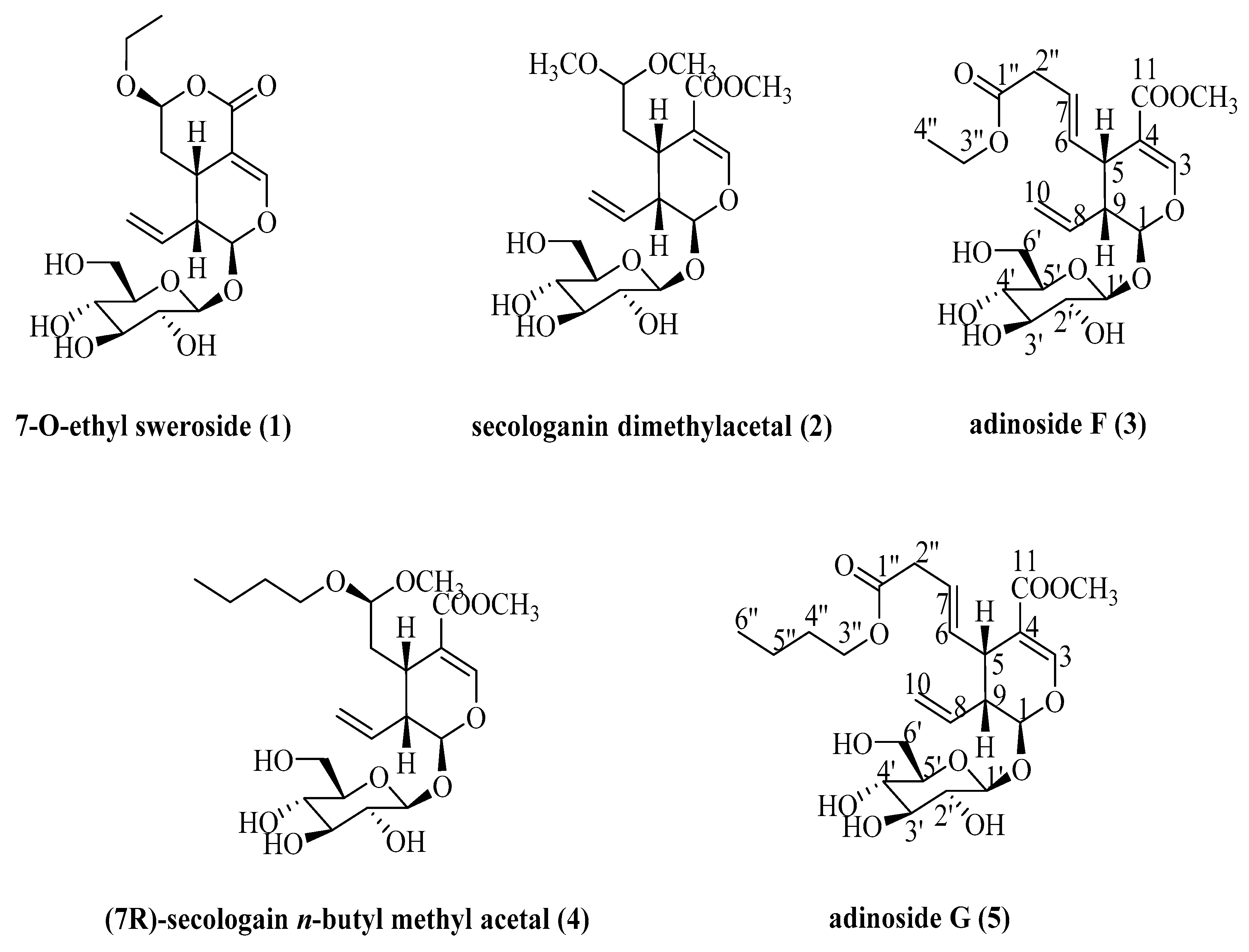
| Solvent Systems | Ratio (v/v) | K | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| ethyl acetate/n-butanol/water | 2:1:3 | 0.092 | 0.102 | 0.210 | 0.320 | 0.535 |
| ethyl acetate/n-butanol/water | 4:1:5 | 0.154 | 0.161 | 0.287 | 0.351 | 0.710 |
| ethyl acetate/n-butanol/water | 11:1:12 | 0.122 | 0.123 | 0.225 | 0.397 | 0.641 |
| ethyl acetate/n-butanol/methanol/water | 11:0.5:0.5:11 | 0.111 | 0.368 | 0.491 | 0.641 | 0.697 |
| ethyl acetate/n-butanol/methanol/water | 11:0.5:1:11 | 0.173 | 0.419 | 0.536 | 0.875 | 1.355 |
| ethyl acetate/n-butanol/methanol/water | 11:3:0.5:11 | 0.677 | 0.859 | 1.085 | 1.910 | 3.352 |
| ethyl acetate/n-butanol/methanol/water | 5:1:1:5 | 0.510 | 0.719 | 1.090 | 1.581 | 2.210 |
| No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 5.40 s | 5.50 d (5.4) | 5.51 d (8.2) | 5.50 d (5.2) | 5.50 d (8.2) |
| 3 | 7.63 d (1.2) | 7.42 d (1.2) | 7.57 s | 7.42 d (0.9) | 7.56 s |
| 4 | - | - | - | - | - |
| 5 | 3.30 m | 2.91 m | 3.38 m | 2.93 m | 3.37 m |
| 6 | 1.72 m, 1.82 m | 1.63 ddd (12.8, 8.0, 4.5), 2.06 m | 5.54 m | 1.63 m, 2.07 m | 5.54 m |
| 7 | 5.41 s | 4.49 dd (7.1, 4.5) | 5.55 m | 4.54 dd (6.8, 4.8) | 5.50 m |
| 8 | 5.48 ddd (17.3, 10.5, 9.9) | 5.73 ddd (17.4, 10.2, 9.0) | 5.72 ddd (17.3, 10.3, 8.5) | 5.72 ddd (17.2, 10.3, 9.0) | 5.71 ddd (17.3, 10.5, 8.6) |
| 9 | 2.63 m | 2.67 m | 2.59 m | 2.67 m | 2.58 m |
| 10 | 5.27 m, 5.29 m | 5.26 brd (10.2), 5.30 brd (17.4) | 5.19 brd (10.6), 5.22 brd (17.3) | 5.26 brd (10.2), 5.30 brd (17.2) | 5.18 brd (10.5), 5.22 brd (17.3) |
| 11 | - | - | - | - | - |
| 1′ | 4.74 d (7.9) | 4.67 d (7.9) | 4.73 d (7.8) | 4.67 d (7.9) | 4.72 d (7.7) |
| 2′ | 3.45 m | 3.19 dd (8.7, 7.9) | 3.20 dd (8.8, 7.8) | 3.18 dd (8.9, 7.9) | 3.19 dd (8.8, 7.6) |
| 3′ | 3.60 t (8.5) | 3.10–3.40 m | 3.10–3.45 m | 3.10–3.40 m | 3.10–3.45 m |
| 4′ | 3.41 t (8.5) | 3.10–3.40 m | 3.10–3.45 m | 3.10–3.40 m | 3.10–3.45 m |
| 5′ | 3.65 m | 3.10–3.40 m | 3.10–3.45 m | 3.10–3.40 m | 3.10–3.45 m |
| 6′ | 3.66 m, 3.97 m | 3.66 dd (12.0, 6.0), 3.89 dd (12.0, 2.1) | 3.67 m, 3.89 dd (11.8, 1.7) | 3.65 dd (11.9, 6.0), 3.89 dd (11.9, 2.0) | 3.66 m, 3.88 dd (11.9, 1.8) |
| 11-OMe | - | 3.69 s | 3.68 s | 3.69 s | 3.67 s |
| 1′′ | 3.92 m, 3.87 m | - | - | 3.60 m, 3.40 m | - |
| 2′′ | - | - | 3.06 m, 3.05 m | 1.54 m | 3.06 m |
| 3′′ | - | - | 4.13 m, 4.11 m | 1.41 m | 4.07 m |
| 4′′ | - | - | 1.24 t (7.4) | 0.94 t (7.4) | 1.61 m |
| 5′′ | - | - | - | - | 1.39 m |
| 6′′ | - | - | - | - | 0.95 t (7.4) |
| 7-OMea | - | 3.28 s | - | 3.29 s | - |
| 7-OMeb | - | 3.29 s | - | - | - |
| No | 1 | 2 | 3 | 4 | 5 | No | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 97.1 | 97. 8 | 97. 4 | 97. 9 | 97.4 | 4′ | 70.2 | 71. 5 | 71. 5 | 71. 6 | 71.5 |
| 3 | 152.3 | 153. 2 | 154.2 | 153. 3 | 154.2 | 5′ | 76.1 | 78. 4 | 78. 5 | 78. 4 | 78.5 |
| 4 | 104.7 | 111.7 | 109.5 | 111.7 | 109.5 | 6′ | 65.3 | 62.8 | 62.8 | 62.8 | 62.8 |
| 5 | 22.0 | 29. 4 | 39.6 | 29. 3 | 39.6 | 11-OMe | - | 51.7 | 51.8 | 51.7 | 51.7 |
| 6 | 29.5 | 33. 2 | 133. 5 | 33. 1 | 133.4 | 1′′ | 62.3 | - | 173.5 | 66.3 | 173.6 |
| 7 | 100.1 | 104.4 | 126.6 | 104.0 | 126.6 | 2′′ | 15.2 | - | 38.6 | 33.7 | 38.6 |
| 8 | 131.6 | 135. 8 | 135. 8 | 135. 8 | 135.8 | 3′′ | - | - | 61.8 | 20.5 | 65.6 |
| 9 | 42.4 | 45.3 | 46.3 | 45.4 | 46.3 | 4′′ | - | - | 14.5 | 14.3 | 31.8 |
| 10 | 121.2 | 119. 8 | 118. 8 | 119. 8 | 118.8 | 5′′ | - | - | - | - | 20.2 |
| 11 | 169.1 | 169. 1 | 168. 8 | 169. 1 | 168.7 | 6′′ | - | - | - | - | 14.1 |
| 1′ | 98.8 | 100.1 | 100.2 | 100.1 | 100.2 | 7-OMea | - | 53.9 | - | 53.8 | - |
| 2′ | 73.5 | 74. 6 | 74. 7 | 74. 7 | 74.8 | 7-OMeb | - | 52.5 | - | - | - |
| 3′ | 75.8 | 78. 0 | 78. 0 | 78. 1 | 78.0 |
| Compound | IC50 (µM) |
|---|---|
| 1 | 5.90 ± 0.71 |
| 2 | 6.50 ± 1.10 |
| 3 | 4.52 ± 0.55 |
| 4 | 6.11 ± 0.93 |
| 5 | 5.35 ± 0.51 |
| Ginkgolide B | 2.21 ± 0.40 |
| Compound | MIC (µg/mL) |
|---|---|
| 1 | 17.5 ± 3.1 |
| 2 | 23.4 ± 4.0 |
| 3 | 15.4 ± 2.1 |
| 4 | 26.0 ± 3.7 |
| 5 | 13.7 ± 1.9 |
| Penicillin | 0.4 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, R.; Fang, L.; Li, J.; Zhao, Z.; Zhang, H.; Zhang, Y. Separation of Five Iridoid Glycosides from Lonicerae Japonicae Flos Using High-Speed Counter-Current Chromatography and Their Anti-Inflammatory and Antibacterial Activities. Molecules 2019, 24, 197. https://doi.org/10.3390/molecules24010197
Yang R, Fang L, Li J, Zhao Z, Zhang H, Zhang Y. Separation of Five Iridoid Glycosides from Lonicerae Japonicae Flos Using High-Speed Counter-Current Chromatography and Their Anti-Inflammatory and Antibacterial Activities. Molecules. 2019; 24(1):197. https://doi.org/10.3390/molecules24010197
Chicago/Turabian StyleYang, Ran, Lei Fang, Jia Li, Zhenhua Zhao, Hua Zhang, and Yongqing Zhang. 2019. "Separation of Five Iridoid Glycosides from Lonicerae Japonicae Flos Using High-Speed Counter-Current Chromatography and Their Anti-Inflammatory and Antibacterial Activities" Molecules 24, no. 1: 197. https://doi.org/10.3390/molecules24010197
APA StyleYang, R., Fang, L., Li, J., Zhao, Z., Zhang, H., & Zhang, Y. (2019). Separation of Five Iridoid Glycosides from Lonicerae Japonicae Flos Using High-Speed Counter-Current Chromatography and Their Anti-Inflammatory and Antibacterial Activities. Molecules, 24(1), 197. https://doi.org/10.3390/molecules24010197





