Initial Mechanisms for the Unimolecular Thermal Decomposition of 2,6-Diamino-3,5-dinitropyrazine-1-oxide
Abstract
1. Introduction
2. Computational Methods
3. Results and Discussion
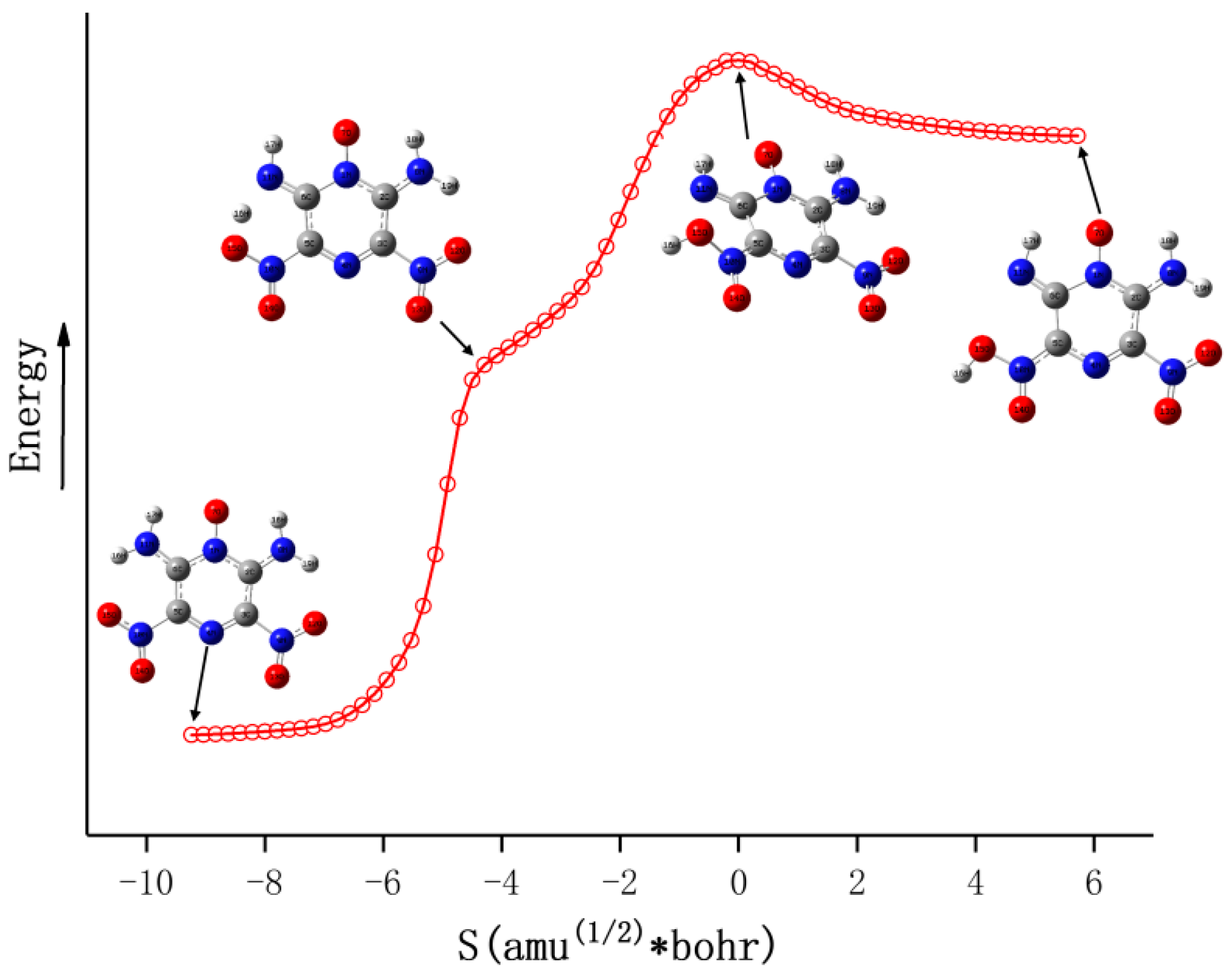
3.1. Initial Decomposition Channels
3.2. Kinetics Analysis
3.3. Molecular Dynamics Simulations
3.4. Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brill, T.B.; James, K.J. Kinetics and mechanisms of thermal decomposition of nitroaromatic explosives. Chem. Rev. 1993, 93, 2667–2692. [Google Scholar] [CrossRef]
- Pagoria, P.F. Synthesis of LLM-105. Propellants Explos. Pyrotech. 1995, 20, 38–42. [Google Scholar]
- Synthesis, Scale-up, and Characterization of 2,6-Diamino-3,5-dinitropyrazine-1-oxide. Available online: https://www.osti.gov/biblio/672328 (accessed on 27 April 1998).
- Tarver, C.M.; Urtiew, P.A. Sensitivity of 2,6-diamino-3,5-dinitropyrazine-1-oxide. J. Energetic Mater. 2005, 23, 183–203. [Google Scholar] [CrossRef]
- Crawford, M.J.; Evers, J.; Göbel, M.; Klapötke, T.M.; Mayer, P.; Oehlinger, G.; Welch, J.M. γ-FOX-7: Structure of a High Energy Density Material Immediately Prior to Decomposition. Propellants Explos. Pyrotech. 2007, 32, 478–495. [Google Scholar] [CrossRef]
- Evers, J.; Klapötke, T.M.; Mayer, P.; Oehlinger, G.; Welch, J. α-and β-FOX-7, Polymorphs of a High Energy Density Material, Studied by X-ray Single Crystal and Powder Investigations in the Temperature Range from 200 to 423 K. Inorg. Chem. 2006, 45, 4996–5007. [Google Scholar] [CrossRef]
- Gilardi, R.D.; Butcher, R.J. 2, 6-Diamino-3, 5-dinitro-1, 4-pyrazine 1-oxide. Acta Crystallogr. Sect. E: Struct. Rep. Online 2001, 57, o657–o658. [Google Scholar] [CrossRef]
- Averkive, B.B.; Antipin, M.Yu. X-ray structural study of three derivatives of dinitropyrazine. J. Mol. Struct. 2002, 606, 139–146. [Google Scholar] [CrossRef]
- Wen-Di, He. Intramolecular H-bonds in LLM-105 and its derivatives: A DFT study. J. Mol. Struct. THEOCHEM. 2005, 723, 217–222. [Google Scholar]
- Characterization of 2,6-diamino-3,5-dinitropyrazine-1-oxide (LLM-105) as an insensitive high explosive material. Available online: https://www.osti.gov/biblio/15005695 (accessed on 9 April 2002).
- Zhang, C.; Wang, X.; Huang, H. π-stacked Interactions in Explosive Crystals: Buffers against External Mechanical Stimuli. J. Am.Chem. Soc. 2008, 130, 8359–8365. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, A.; Zhang, C.; Jiang, D.; Zhu, Y.; Zhang, C. Crystal Packing of Low-Sensitivity and High-Energy Explosives. Cryst. Growth Des. 2014, 14, 4703–4713. [Google Scholar] [CrossRef]
- Gump, J.C.; Stoltz, C.A.; Mason, B.P.; Freedman, B.G.; Ball, J.R.; Peiris, S.M. Equations of state of 2,6-diamino-3,5-dinitropyrazine-1-oxide. J. Appl. Phys. 2011, 110, 073523. [Google Scholar] [CrossRef]
- Manaa, M.R.; Kuo, I.F.; Fried, L.E. First-Principles High-Pressure Unreacted Equation of State and Heat of Formation of Crystal 2,6-Diamino-3,5-dinitropyrazine-1-oxide (LLM-105). J. Chem. Phys. 2014, 141, 064702. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, E.; Riad Manaa, M.; Zaug, J.M.; Kuo, I.-F.W.; Pagoria, P.F.; Kalkan, B.; Crowhurst, J.C.; Armstrong, M.R. The high pressure structure and equation of state of 2, 6-diamino-3, 5-dinitropyrazine-1-oxide (LLM-105) up to 20 GPa: X-ray diffraction measurements and first principles molecular dynamics simulations. J. Chem. Phys. 2015, 143, 144506. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiong, Y.; Li, H.; Zhang, C. Reversible Hydrogen Transfer as New Sensitivity Mechanism for Energetic Materials against External Stimuli: A Case of the Insensitive 2, 6-Diamino-3, 5-dinitropyrazine-1-oxide. J. Chem. Phys. C 2018, 122, 1109–1118. [Google Scholar] [CrossRef]
- Byrd, E.F.; Rice, B.M. Improved prediction of heats of formation of energetic materials using quantum mechanical calculations. J. Chem. Phys. A 2006, 110, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Zheng, L. Some Key Techniques of Measuring Propellants and Explosives by Temperature-dependent FTIR. Chin. J. Energ. Mater. 2007, 15, 676–680. [Google Scholar]
- Wang, Y.B.; Ge, Z.X.; Wang, B.Z. Preparation and thermo performance studying of fine LLM-105. Chin. J. Energ. Mater. 2011, 19, 523–526. [Google Scholar]
- Politzer, P.; Murray, J.S.; Seminario, J.M.; Lane, P.; Grice, M.E.; Concha, M.C. Computational characterization of energetic materials. J. Mol. Struct. THEOCHEM 2001, 573, 1–10. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Some perspectives on sensitivity to initiation of detonation. Green Energ. Mater. 2014, 3, 45–62. [Google Scholar]
- Miao, M.; Dreger, Z.A.; Patterson, J.E.; Gupta, Y.M. Shock wave induced decomposition of RDX: Quantum chemistry calculations. J. Phys. Chem. 2008, 112, 7383–7390. [Google Scholar] [CrossRef]
- Tsyshevsky, R.V.; Kuklja, M.M. Decomposition mechanisms and kinetics of novel energetic molecules BNFF-1 and ANFF-1: Quantum-chemical modeling. Molecules 2013, 18, 8500–8517. [Google Scholar] [CrossRef]
- Owens, F.J. Calculation of energy barriers for bond rupture in some energetic molecules. J. Mol. Struct. THEOCHEM 1996, 370, 11–16. [Google Scholar] [CrossRef]
- Yuan, B.; Bernstein, E.R. Initial mechanisms for the unimolecular decomposition of electronically excited bisfuroxan based energetic materials. J. Chem. Phys. 2017, 146, 014301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09, Revision, D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Accounts 2008, 120, 215–241. [Google Scholar]
- Grimme, S. Semiempirical hybrid density functional with perturbative second-order correlation. J. Chem. Phys. 2006, 124, 034108–034116. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Eyring, H. The activated complex in chemical reactions. J. Chem. Phys. 1935, 3, 107–115. [Google Scholar] [CrossRef]
- Evans, M.G.; Polanyi, M. Some applications of the transition state method to the calculation of reaction velocities, especially in solution. Trans. Faraday Soc. 1935, 31, 875–894. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.A.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. Phys. Rev. B: Condens. Matter 2002, 14, 2717–2744. [Google Scholar]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B: Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Pfrommer, B.G.; Côté, M.; Louie, S.G.; Cohen, M.L. Relaxation of crystals with the quasi-Newton method. J. Comput. Phys. 1997, 131, 233–240. [Google Scholar] [CrossRef]
- Velardez, G.F.; Alavi, S.; Thompson, D.L. Theoretical predictions of the initial decomposition steps of dimethylnitramine. J. Chem. Phys. 2005, 123, 074313. [Google Scholar] [CrossRef] [PubMed]
- Behrens Jr, R.; Bulusu, S. Thermal decomposition of energetic materials. 2. Deuterium isotope effects and isotopic scrambling in condensed-phase decomposition of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine. J. Phys. Chem. 1991, 95, 5838–5845. [Google Scholar] [CrossRef]
- Chakraborty, D.; Muller, R.P.; Dasgupta, S.; Goddard, W.A. Mechanism for unimolecular decomposition of HMX (1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine), an ab initio study. J. Phys. Chem. A. 2001, 105, 1302–1314. [Google Scholar] [CrossRef]
- Zhang, L.; Zybin, S.V.; van Duin, A.C.T.; Dasgupta, S.; Goddard III, W.A.; Kober, E.M. Carbon cluster formation during thermal decomposition of octahydro-1, 3, 5, 7-tetranitro-1, 3, 5, 7-tetrazocine and 1, 3, 5-triamino-2, 4, 6-trinitrobenzene high explosives from ReaxFF reactive molecular dynamics simulations. J. Phys. Chem. A. 2009, 113, 10619–10640. [Google Scholar] [CrossRef] [PubMed]
- Guirguis, R.; Hsu, D.; Bogan, D.; Oran, E. A mechanism for ignition of high-temperature gaseous nitromethane—The key role of the nitro group in chemical explosives. Combust. Flame 1985, 61, 51–62. [Google Scholar] [CrossRef]
- Glascoe, E.A.; Zaug, J.M.; Armstrong, M.R.; Crowhurst, J.C.; Grant, C.D.; Fried, L.E. Nanosecond Time-Resolved and Steady-State Infrared Studies of Photoinduced Decomposition of TATB at Ambient and Elevated Pressure. J. Phys. Chem. A. 2009, 113, 5881–5887. [Google Scholar] [CrossRef]
- Shao, J.; Cheng, X.; Yang, X. Density functional calculations of bond dissociation energies for removal of the nitrogen dioxide moiety in some nitroaromatic molecules. J. Mol. Struct. 2005, 755, 127–130. [Google Scholar] [CrossRef]
- Rice, B.M.; Sahu, S.; Owens, F.J. Density functional calculations of bond dissociation energies for NO2 scission in some nitroaromatic molecules. J. Mol. Struct. 2002, 583, 69–72. [Google Scholar] [CrossRef]
- Gonzalez, A.C.; Larson, C.W.; McMillen, D.F.; Golden, D.M. Mechanism of decomposition of nitroaromatics. Laser-powered homogeneous pyrolysis of substituted nitrobenzenes. J. Phys. Chem. 1985, 89, 4809–4814. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Savin, A.; Jepsen, O.; Flad, J.; Andersen, O.K.; Preuss, H.; von Schnering, H.G. Electron localization in solid-state structures of the elements: The diamond structure. Angew. Chem. Int. Ed. 1992, 31, 187–188. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Meaning and Functional Form of the Electron Localization Function. Acta Phys.-Chim. Sin. 2011, 27, 2786–2792. [Google Scholar]
- Manaa, M.R.; Reed, E.J.; Fried, L.E.; Goldman, N. Nitrogen-rich heterocycles as reactivity retardants in shocked insensitive explosives. J. Am. Chem. Soc. 2009, 131, 5483–5487. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Relationships between impact sensitivities and molecular surface electrostatic potentials of nitroaromatic and nitroheterocyclic molecules. Mol. Phys. 1995, 85, 1–8. [Google Scholar] [CrossRef]
- Rice, B.M.; Hare, J.J. A quantum mechanical investigation of the relation between impact sensitivity and the charge distribution in energetic molecules. J. Phys. Chem. A. 2002, 106, 1770–1783. [Google Scholar] [CrossRef]
- Zhang, C.; Shu, Y.; Huang, Y.; Zhao, X.; Dong, H. Investigation of correlation between impact sensitivities and nitro group charges in nitro compounds. J. Phys. Chem. B 2005, 109, 8978–8982. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

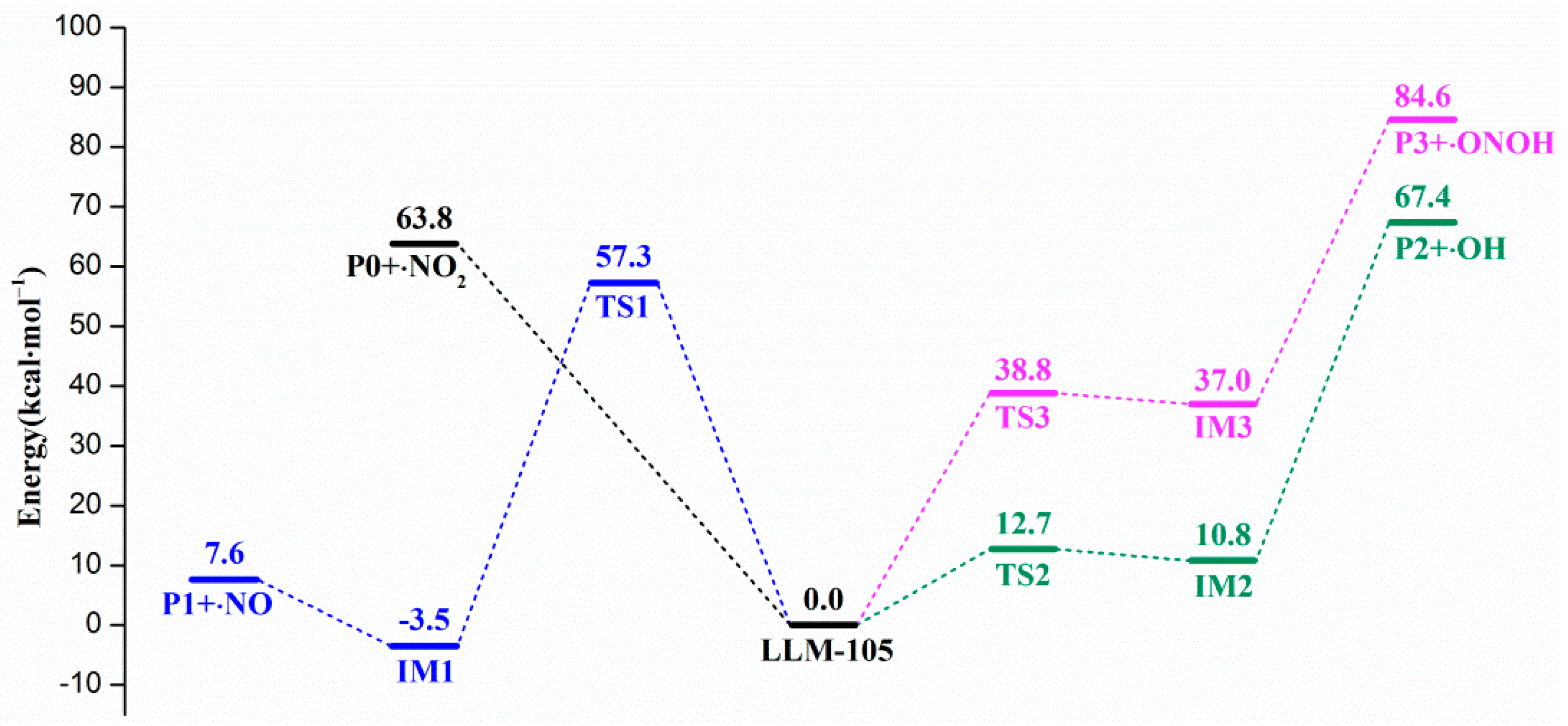
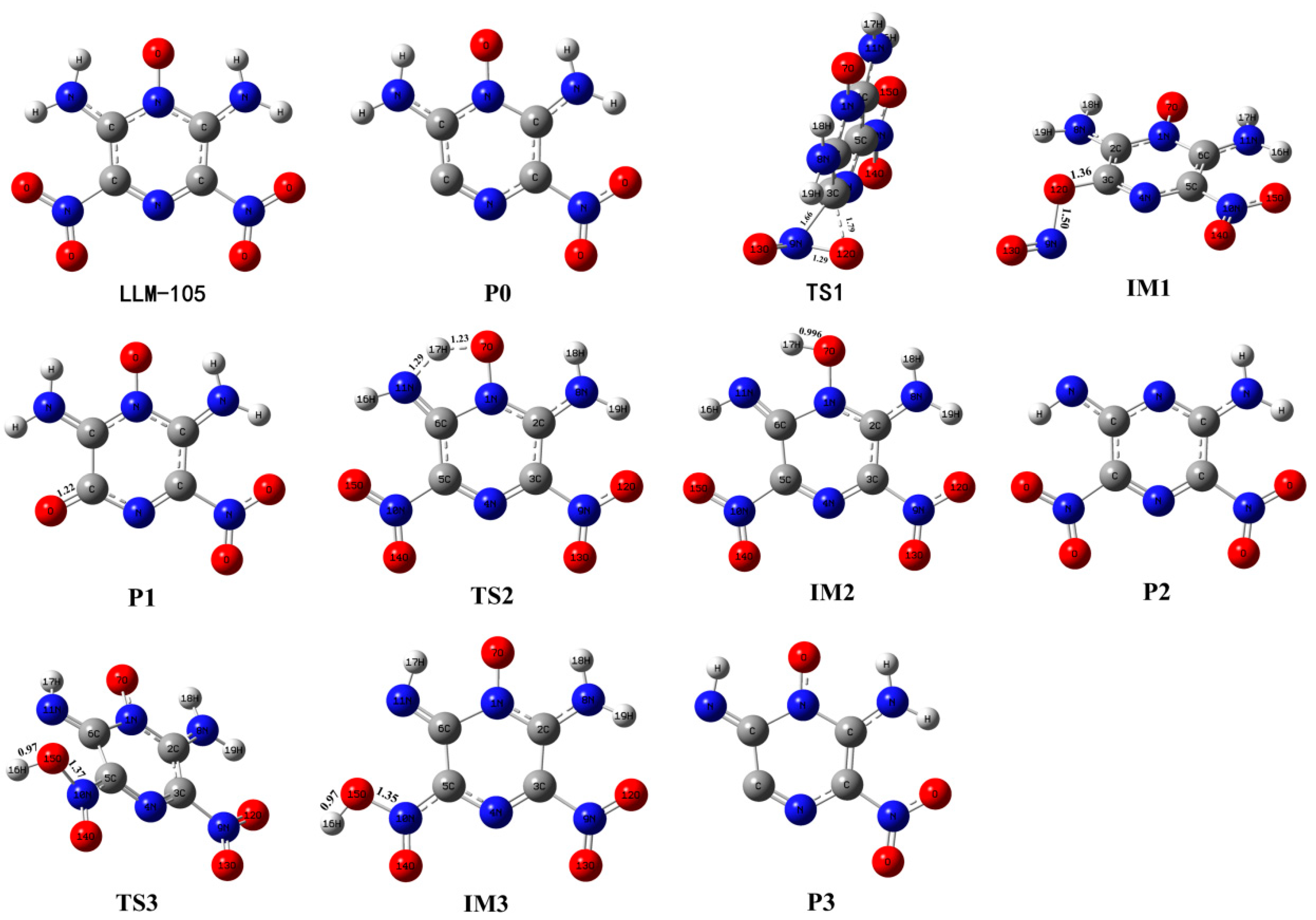

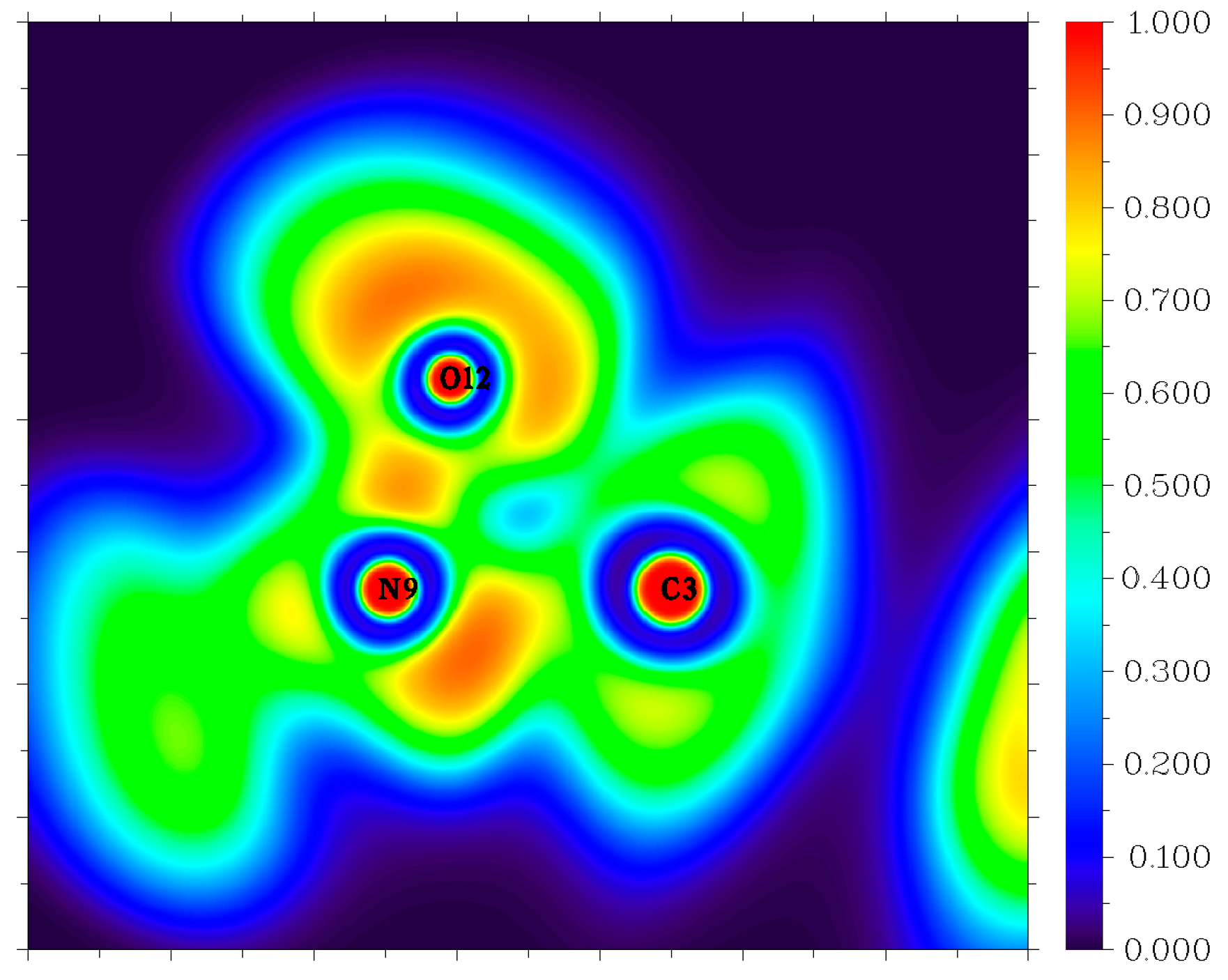
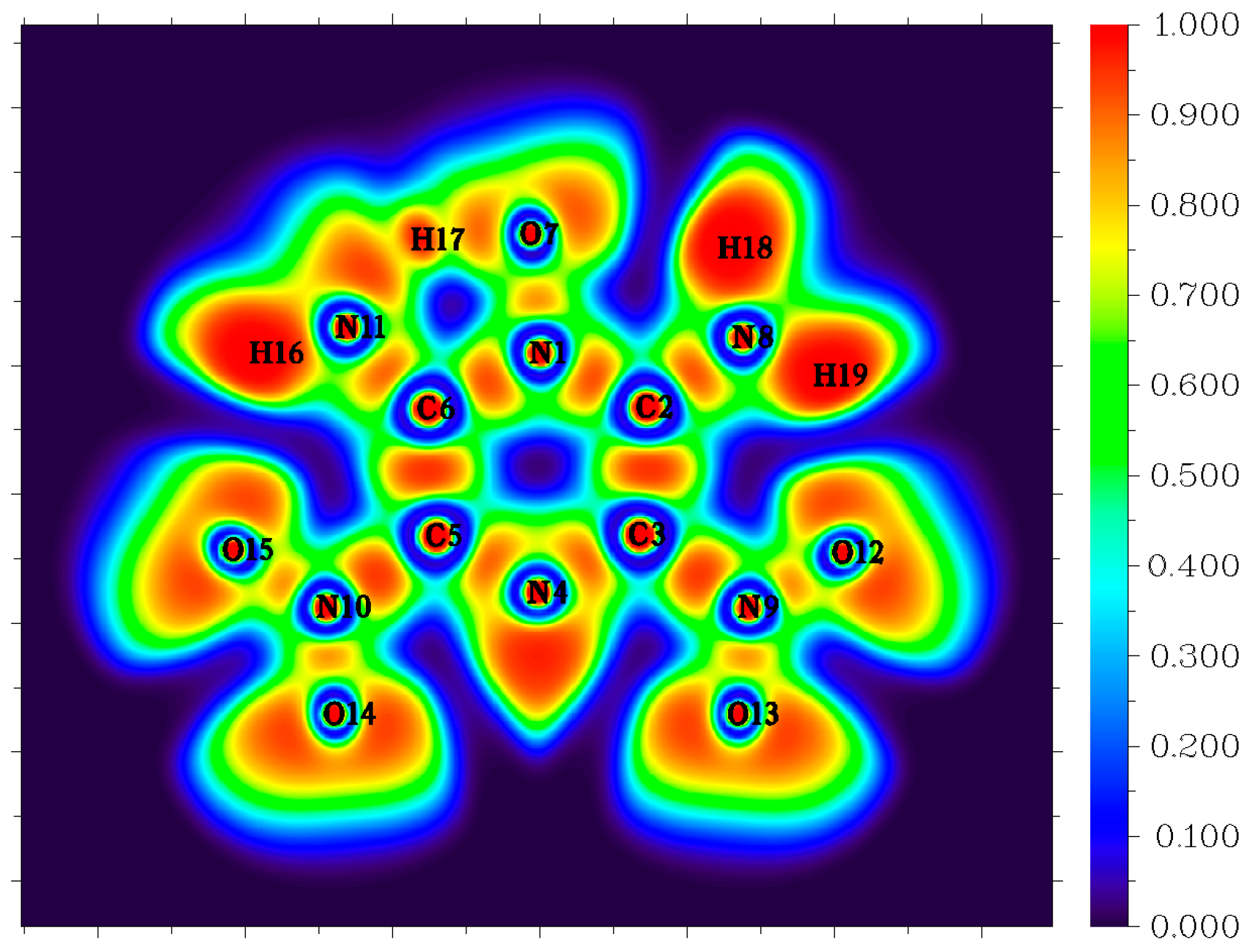
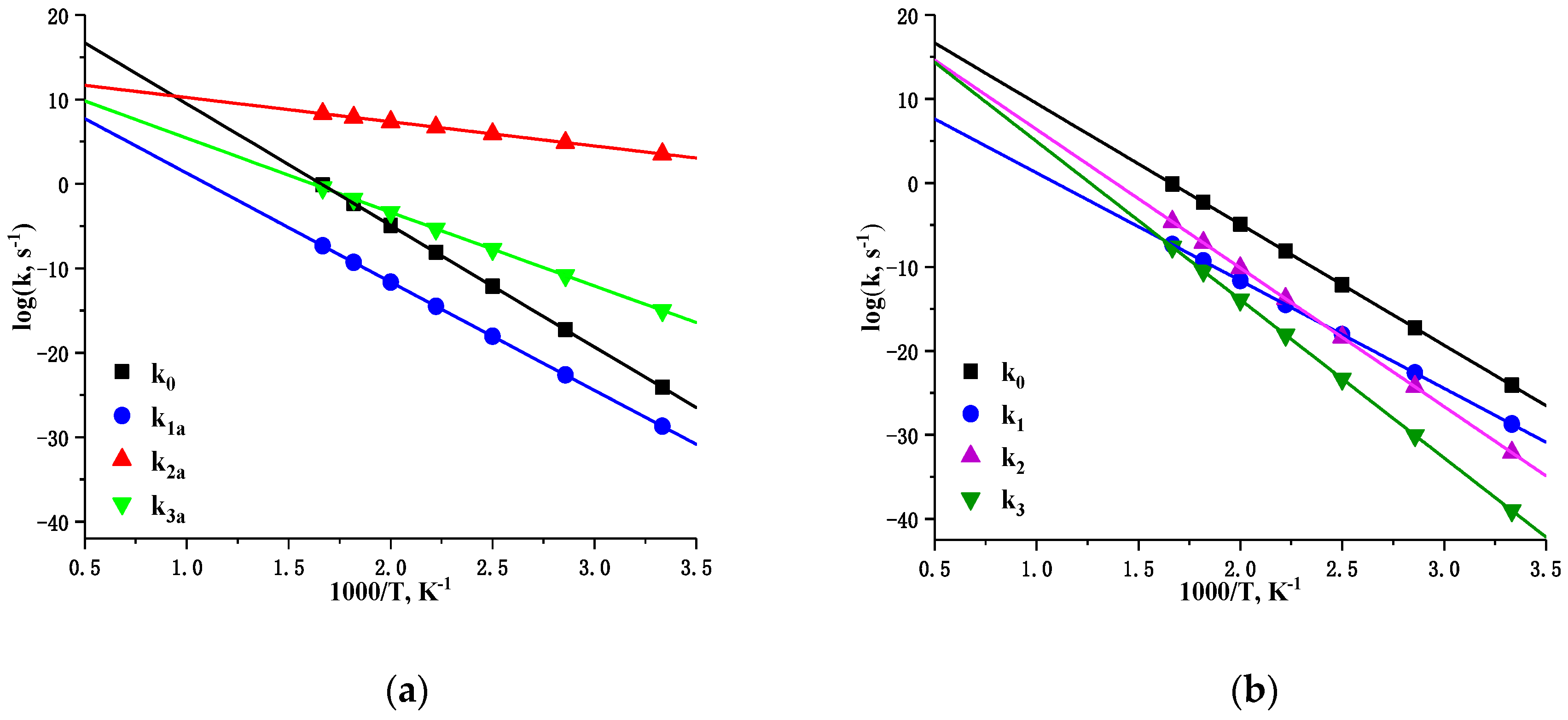

| Reaction | Log(A, s−1) | Ea, kcal mol−1 | |
|---|---|---|---|
| LLM-105→P0+•NO2 | (k0) | 23.9 | 65.8 |
| LLM-105→IM1 | (k1a) | 14.0 | 58.7 |
| LLM-105→P1+•NO | (k1) | 14.0 | 58.7 |
| LLM-105→IM2 | (k2a) | 13.1 | 13.1 |
| LLM-105→P2+•OH | (k2) | 22.9 | 75.5 |
| LLM-105→IM3 | (k3a) | 14.2 | 40.0 |
| LLM-105→P3+•ONOH | (k3) | 23.8 | 86.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, N.; Gan, Q.; Yu, Q.; Zhang, X.; Li, R.; Qian, S.; Feng, C. Initial Mechanisms for the Unimolecular Thermal Decomposition of 2,6-Diamino-3,5-dinitropyrazine-1-oxide. Molecules 2019, 24, 125. https://doi.org/10.3390/molecules24010125
Cheng N, Gan Q, Yu Q, Zhang X, Li R, Qian S, Feng C. Initial Mechanisms for the Unimolecular Thermal Decomposition of 2,6-Diamino-3,5-dinitropyrazine-1-oxide. Molecules. 2019; 24(1):125. https://doi.org/10.3390/molecules24010125
Chicago/Turabian StyleCheng, Nianshou, Qiang Gan, Qian Yu, Xuemei Zhang, Rong Li, Shichuan Qian, and Changgen Feng. 2019. "Initial Mechanisms for the Unimolecular Thermal Decomposition of 2,6-Diamino-3,5-dinitropyrazine-1-oxide" Molecules 24, no. 1: 125. https://doi.org/10.3390/molecules24010125
APA StyleCheng, N., Gan, Q., Yu, Q., Zhang, X., Li, R., Qian, S., & Feng, C. (2019). Initial Mechanisms for the Unimolecular Thermal Decomposition of 2,6-Diamino-3,5-dinitropyrazine-1-oxide. Molecules, 24(1), 125. https://doi.org/10.3390/molecules24010125






