Influence of Piracetam on Gliclazide—Glycated Human Serum Albumin Interaction. A Spectrofluorometric Study
Abstract
1. Introduction
2. Results and Discussion
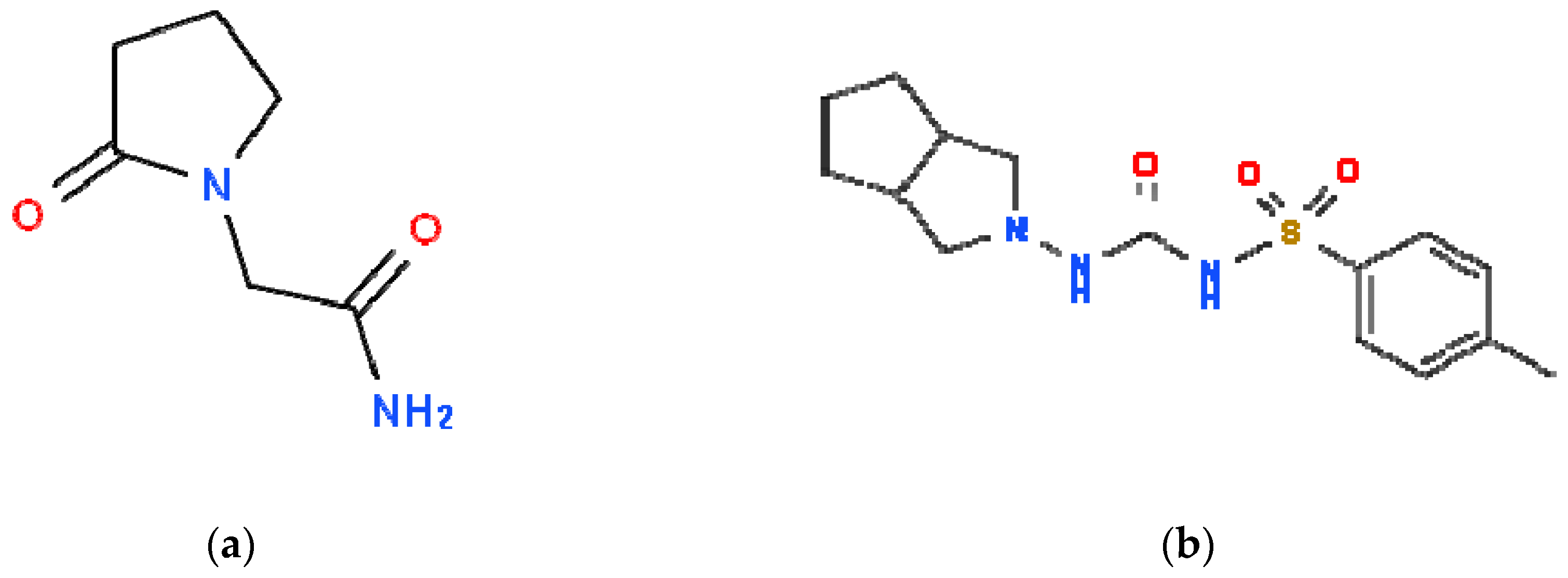
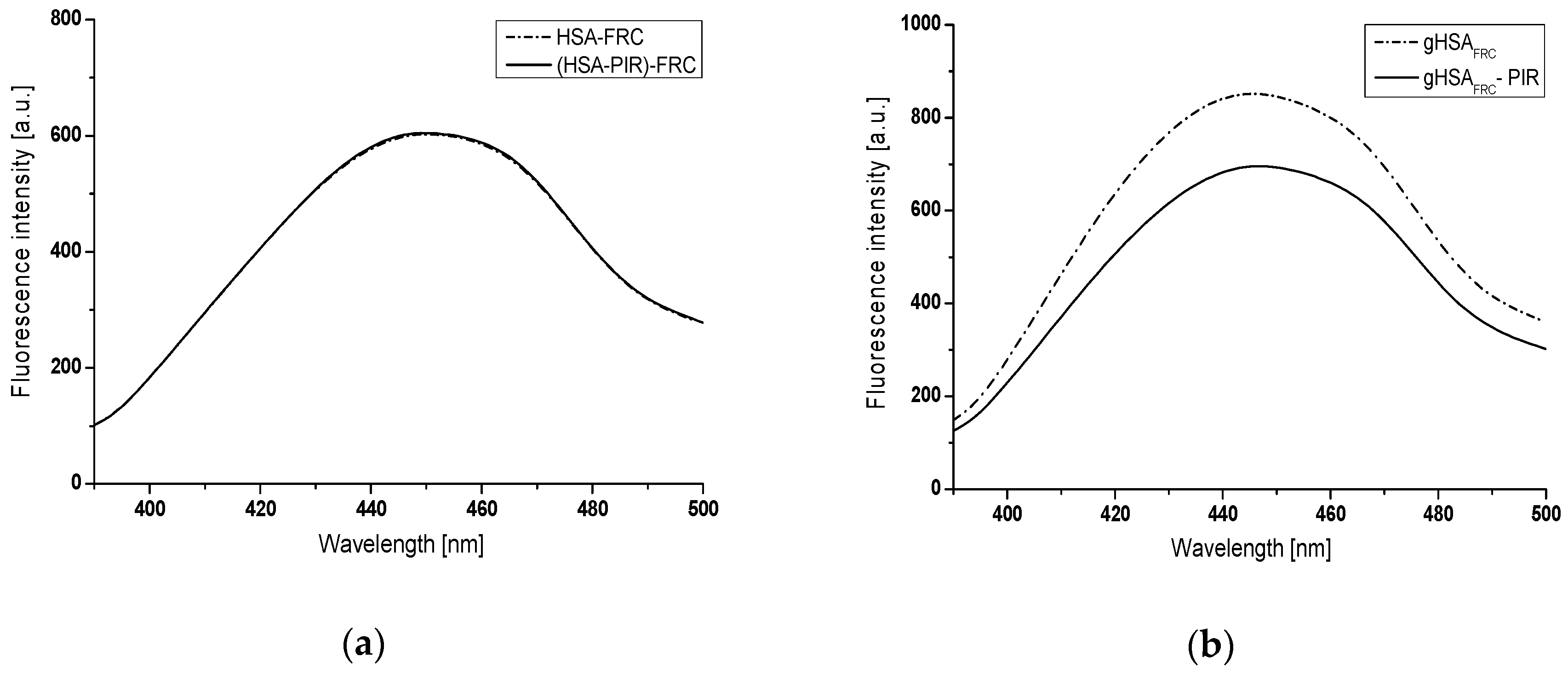
2.1. Piracetam as an Inhibitor of Human Serum Albumin In Vitro Glycation
2.2. The Interaction of Gliclazide (GLZ) with Nonglycated (HSA), Glycated in the Presence of Fructose (gHSAFRC), and Glycated in the Presence of Both Fructose and Piracetam (gHSAFRC-PIR) Human Serum Albumin
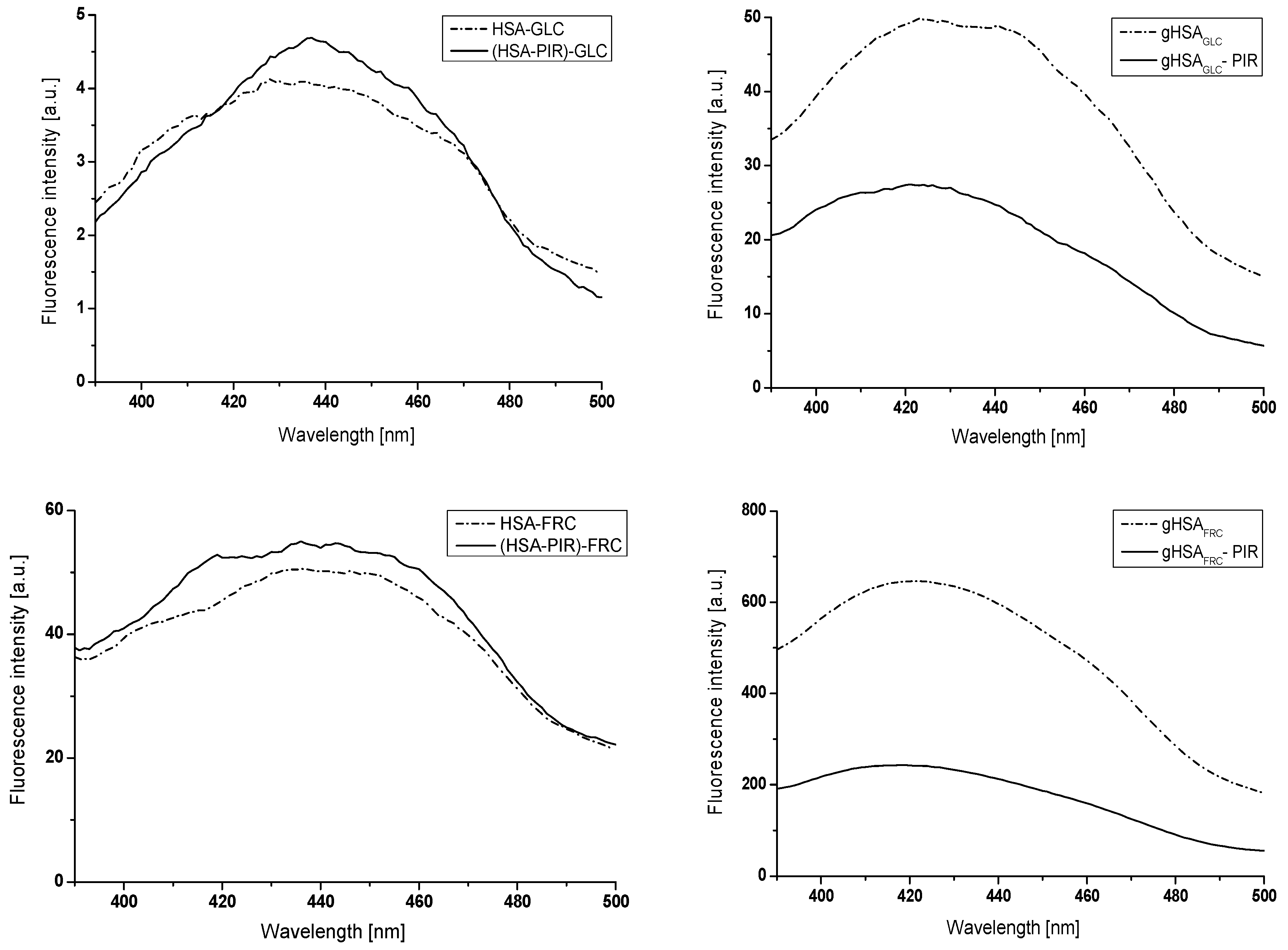
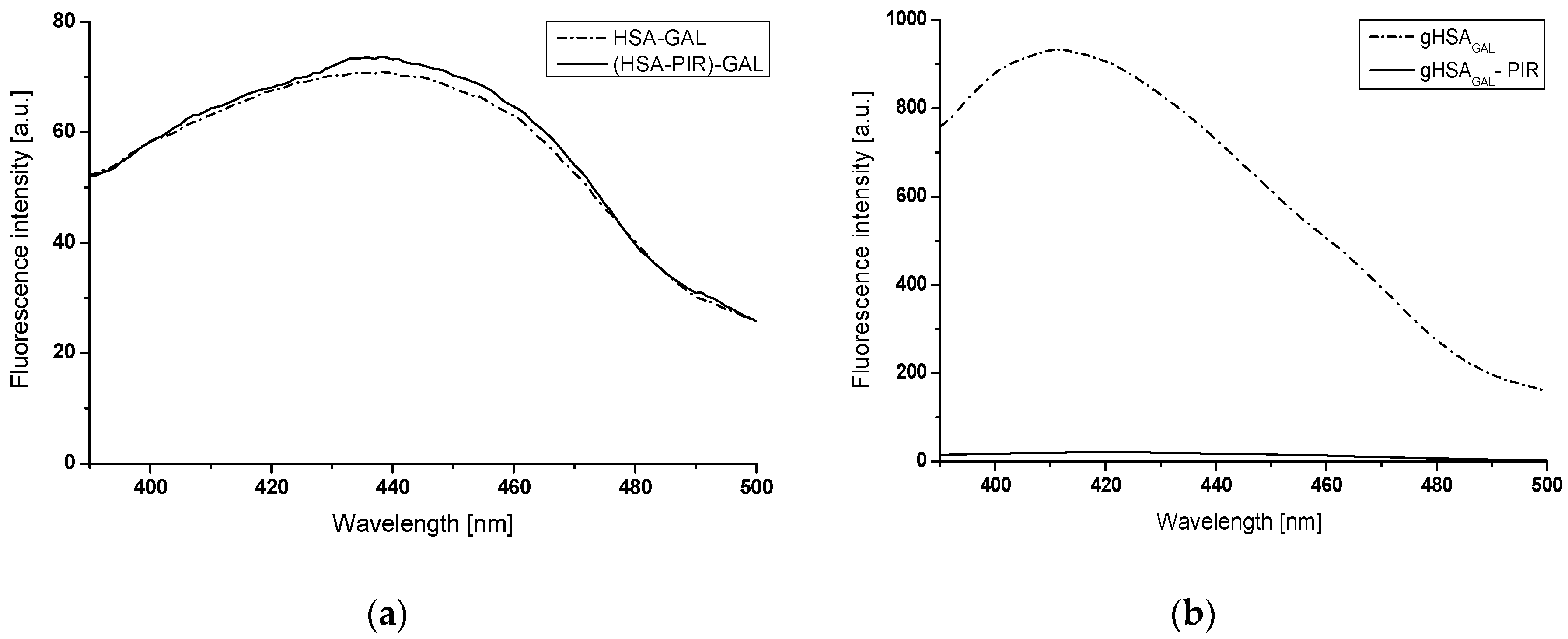
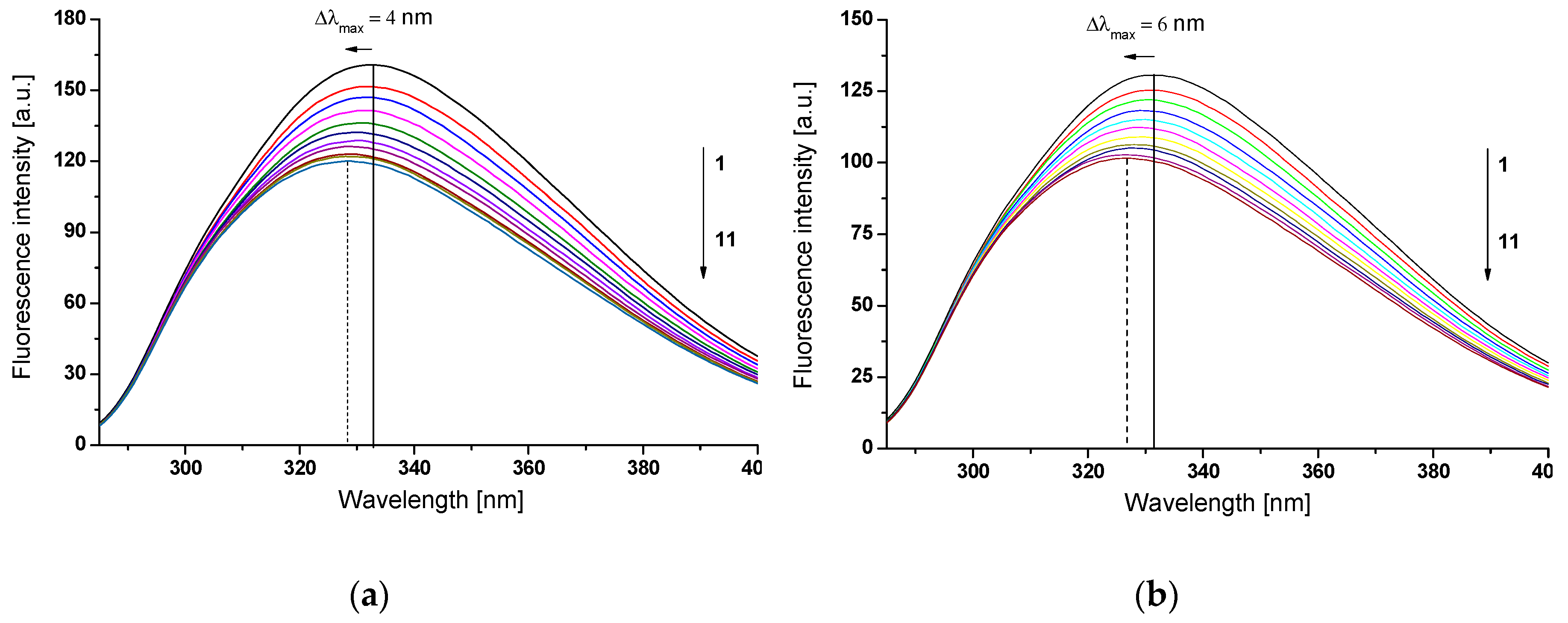
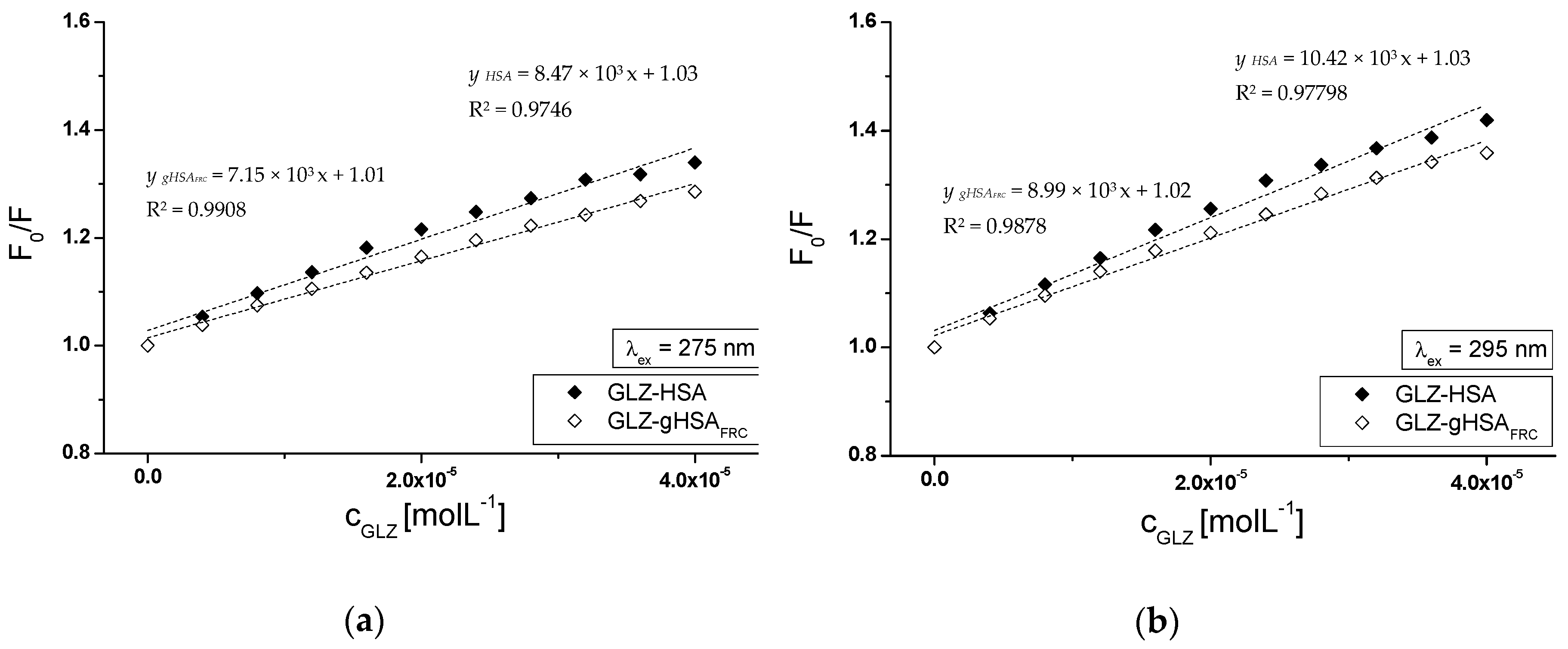
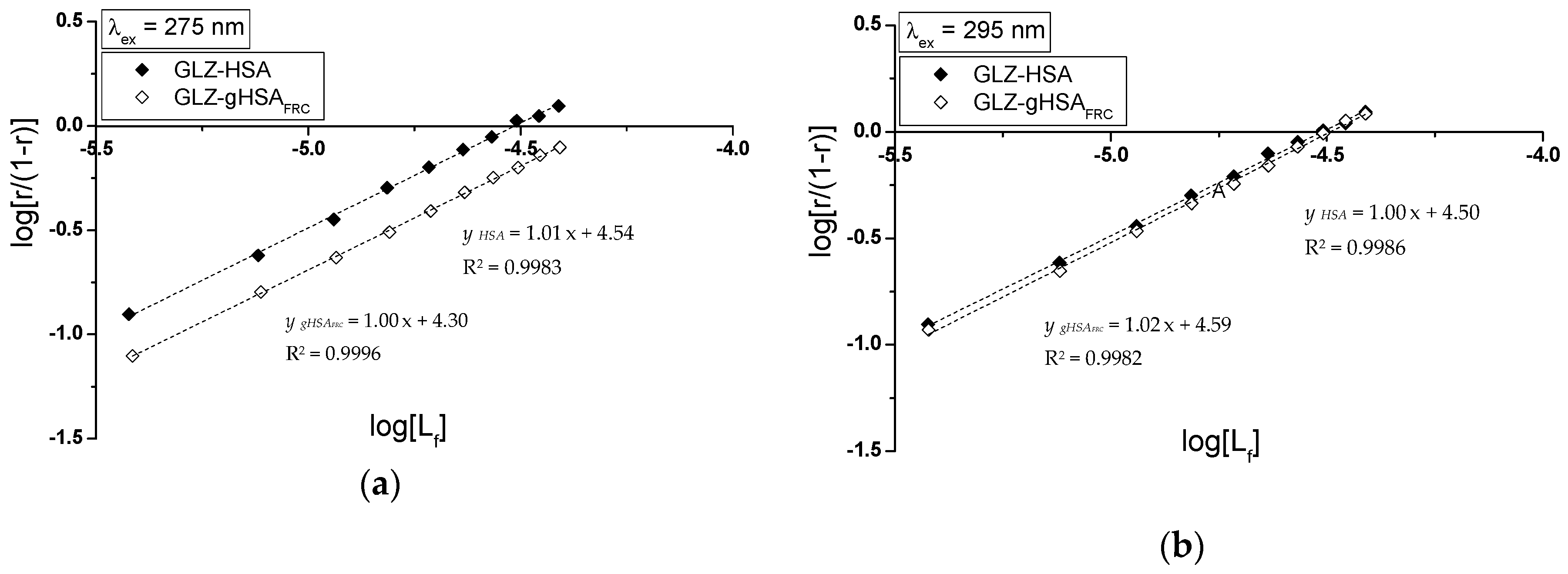
2.2.1. The Influence of Glycation on Gliclazide–Serum Albumin Interaction
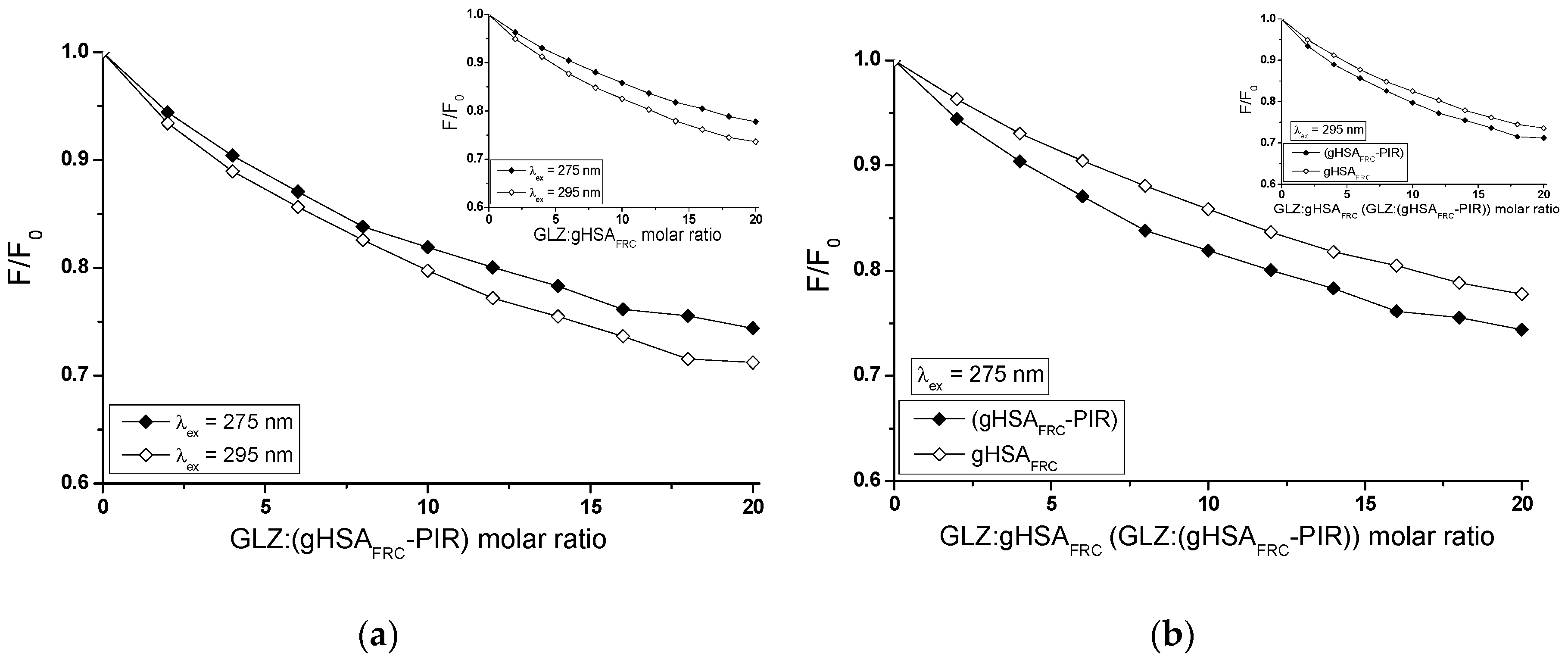
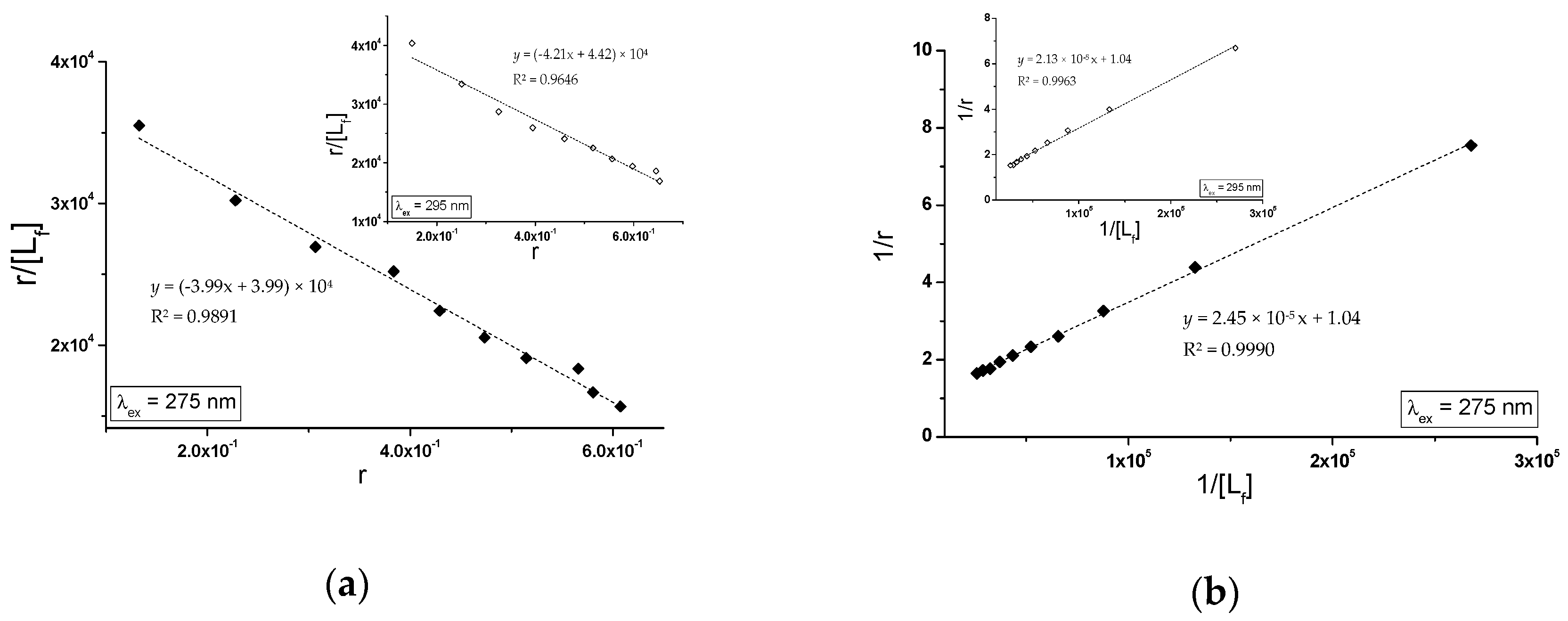
2.2.2. The Influence of Piracetam on the Gliclazide-Albumin Interaction
3. Materials and Methods
3.1. Chemicals
3.2. In Vitro Glycation of Human Serum Albumin
3.3. Instruments and Measurement Conditions
3.4. Emission Fluorescence Measurement of Human Serum Albumin and Advanced Glycation End-Products (AGEs)
3.5. Emission Fluorescence Measurement of Nonmodified and Modified Human Serum Albumin in the Presence of Piracetam and Gliclazide
3.6. The Analyzis of Drug–Albumin Interaction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Online version of IDF Diabetes Atlas. Available online: www.idf.org/diabetesatlas (accessed on 1 November 2018).
- Thornalley, P.J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems-role in ageing and disease. Drug Metab. Drug Interact. 2008, 23, 125–150. [Google Scholar] [CrossRef]
- Rondeau, P.; Bourdon, E. The glycation of albumin: structural and functional impacts. Biochimie 2011, 93, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Ansari, N.A.; Dash, D. Amadori glycated proteins: role in production of autoantibodies in diabetes mellitus and effect of inhibitors on non-enzymatic glycation. Aging Dis. 2013, 4, 50–56. [Google Scholar] [PubMed]
- Golab, K.; Gburek, J.; Konopska, B.; Krotkiewski, H.; Warwas, M. Binding of glycated ovocystatin to rat renal brush border membranes. Anim. Sci. J. 2013, 84, 702–707. [Google Scholar] [CrossRef] [PubMed]
- Sho-ichi Yamagishi, S.; Nakamura, K.; Imaizumi, T. Advanced Glycation End Products (AGEs) and Diabetic Vascular Complications. Curr. Diabetes Rev. 2005, 1, 93–106. [Google Scholar] [CrossRef]
- Sharma, S.D.; Pandey, B.N.; Mishra, K.P.; Sivakami, S. Amadori product and age formation during nonenzymatic glycosylation of bovine serum albumin in vitro. J. Biochem. Mol. Biol. Biophys. 2002, 6, 233–242. [Google Scholar] [PubMed]
- Kankova, K. Diabetic threesome (hyperglicaemia, renal function and nutrition) and advanced glycation end products: evidence for the multiple-hit agent. Proc. Nutr. Soc. 2008, 67, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.P. Clinical, pathophysiological and structure/function consequences of modification of albumin by Amadori-glucose adducts. Biochim. Biophys. Acta 2013, 1830, 5480–5485. [Google Scholar] [CrossRef]
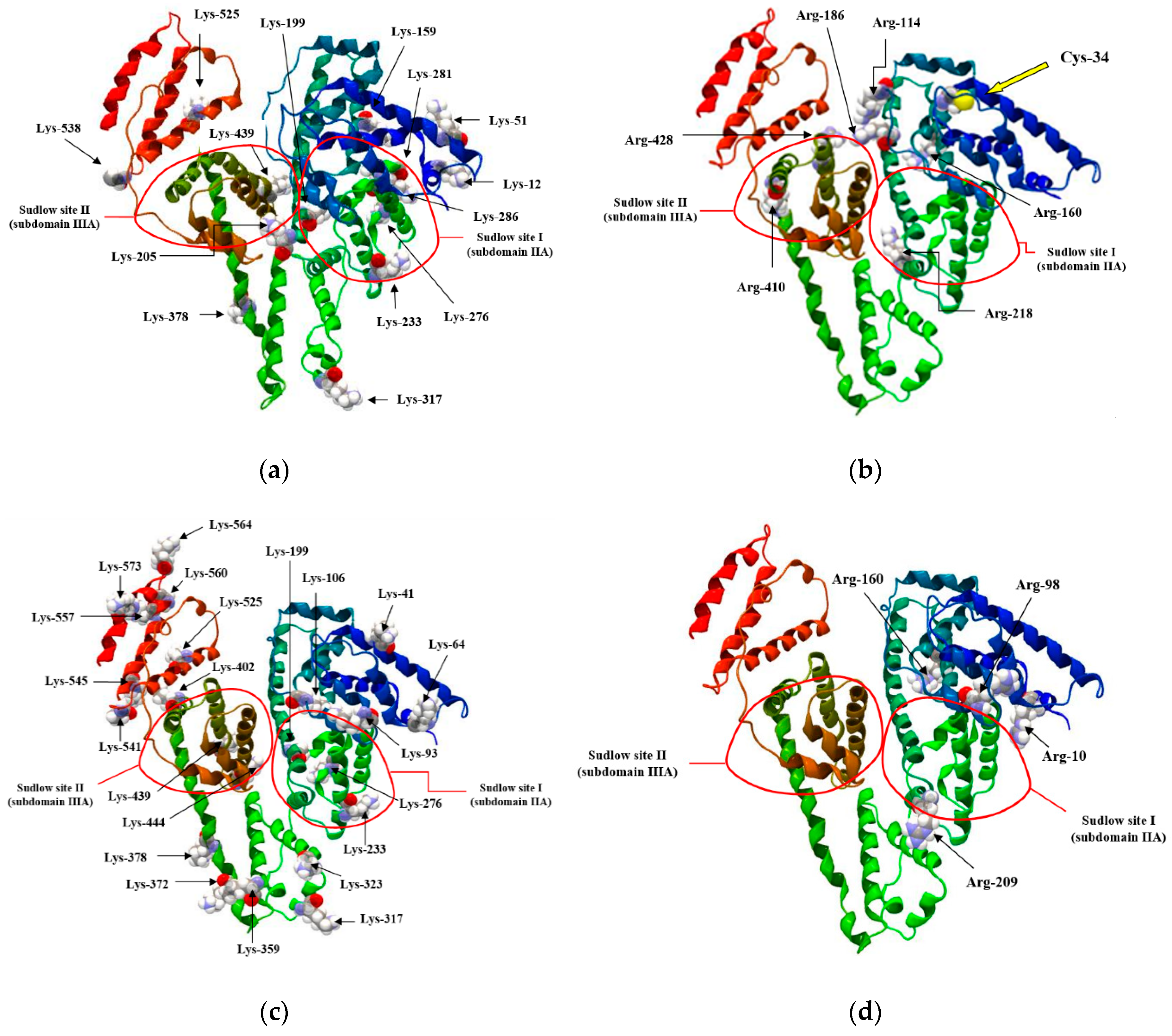
- Artali, R.; Bombieri, G.; Calabi, L.; Del Pra, A. A molecular dynamics study of human serum albumin binding sites. Farmaco 2005, 60, 485–495. [Google Scholar] [CrossRef]
- Peters, T. All about Albumin. In Biochemistry, Genetics and Medical Applications; Academic Press: San Diego, CA, USA, 1995; pp. 1–40. [Google Scholar]
- Carter, D.C.; Ho, J.X. Structure of serum Albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar]
- He, X.M.; Carter, D.C. Atomic structure and chemistry of human serum albumin. Nature 1992, 358, 209–215. [Google Scholar] [CrossRef]
- Kragh-Hansen, U.; Minchiotti, L.; Galiano, M.; Peters, T. Human serum albumin isoforms: genetic and molecular aspects and functional consequences. Biochim. Biophys. Acta 2013, 1830, 5405–5417. [Google Scholar] [CrossRef] [PubMed]
- Sudlow, G.; Birkett, D.J.; Wade, D.N. The Characterization of Two Specific Drug Binding Sites on Human Serum Albumin. Mol. Pharmacol. 1975, 11, 824–832. [Google Scholar]
- Maciążek-Jurczyk, M.; Szkudlarek, A.; Chudzik, M.; Pożycka, J.; Sułkowska, A. Alteration of human serum albumin binding properties induced by modifications: A review. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2017, 188, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Szkudlarek, A.; Pentak, D.; Ploch, A.; Pożycka, J.; Maciążek-Jurczyk, M. In Vitro Investigation of the Interaction of Tolbutamide and Losartan with Human Serum Albumin in Hyperglycemia States. Molecules 2017, 22, 2249. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.S.; Rainbow, S.; Mukherjee, S. Serum levels of low molecular weight advanced glycation products in diabetic subjects. Diabet. Med. 2003, 20, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Nawale, R.B.; Mourya, V.K.; Bhise, S.B. Non-enzymatic glycation of proteins: a cause for complication in diabetes. Indian Biochem. Biophys. 2006, 43, 337–344. [Google Scholar]
- Wu, C.H.; Huang, S.M.; Lin, J.A.; Yen, G.C. Inhibition of advanced glycation endproduct formation by foodstuffs. Food Funct. 2011, 2, 224–234. [Google Scholar] [CrossRef]
- Ahmed, N.; Babaei-Jadidi, R.; Howell, S.K.; Thornalley, P.J.; Beisswenger, P.J. Glycated and oxidized protein degradation products are indicators of fasting and postprandial hyperglycemia in diabetes. Diabetes Care 2005, 28, 2465–2471. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Randell, E.W.; Vasdev, S.C.; Gill, V.D.; Han, Y.; Gadag, V.; Raouf, A.A.; El Said, H. Plasma protein advanced glycation end products, carboxymethyl cysteine and carboxyethyl cysteine, are elevated and related to nephropathy in patients with diabetes. Mol. Cell Biochem. 2007, 302, 35–42. [Google Scholar] [CrossRef]
- Goh, S.Y.; Cooper, M.E. The role of Advanced Glycation End Products in progression and complications of diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1143–1152. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S. Role of advanced glycation end products (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp. Gerontol. 2011, 46, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Chevion, A.; Chevion, M.; Hofmann, M.; Quehenberger, P.; Illmer, T.; Luther, T.; Berentshtein, E.; Tritschler, H.; Müller, M.; et al. Advanced glycation end product (AGEs) induced activation of NKκB is suppressed by α-lipoic acid in cultured endothelial cells. Diabetes 1997, 46, 1481–1490. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, N.; Thornalley, P.J. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes. Metab. 2007, 9, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Ames, J.M.; Smith, R.D.; Baynes, J.W.; Metz, T. A perspective on the Maillard reaction and the analysis of protein glycation by mass spectrometry: probing the pathogenesis of chronic disease. J. Proteome. Res. 2009, 8, 754–769. [Google Scholar] [CrossRef] [PubMed]
- Basta, G. Receptor for advanced glycation endproducts and atherosclerosis: From basic mechanisms to clinical implications. Atherosclerosis 2008, 196, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Zuwała-Jagiełło, J.; Simon, K.; Pazgan-Simon, M.; Kość-Czarny, A.; Warwas, M. Advanced glycation end products in serum of patients with chronic hepatitis and liver cirrhosis. Adv. Clin. Exp. Med. 2006, 15, 259–264. [Google Scholar]
- Van Reekum, R.; Black, S.E.; Conn, D.; Clarke, D. Cognition-enhancing drugs in dementia: a guide to the near future. Can. J. Psychiatry 1997, 42, 35S–50S. [Google Scholar]
- Winblad, B. Piracetam: a review of pharmacological properties and clinical uses. CNS Drug Rev. 2005, 11, 169–182. [Google Scholar] [CrossRef]
- Ballagi-Pordany, G.; Koszeghy, A.; Koltai, M.Z.; Aranyi, Z.; Pogatsa, G. Divergent cardiac effects of the first and second generation hypoglycemic sulfonylurea compounds. Diabetes Res. Clin. Pract. 1990, 8, 109–114. [Google Scholar] [CrossRef]
- Stryer, L. Fluorescence spectroscopy of proteins. Science 1978, 162, 526–533. [Google Scholar] [CrossRef]
- Valeur, B. Molecular Fluorescence: Principles and Applications; Wiley VCH: London/Weinheim, UK, 2002. [Google Scholar]
- Maciążek-Jurczyk, M.; Maliszewska, M.; Pożycka, J.; Równicka-Zubik, J.; Góra, A.; Sułkowska, A. Tamoxifen and curcumin binding to serum albumin. Spectroscopic study. J. Mol. Struct. 2013, 1044, 194–200. [Google Scholar]
- Monti, S.; Ottani, S.; Manoli, F. Chiral recognition of 2-(3-benzoylphenyl)propionic acid (ketoprofen) by serum albumin: an investigation with microcalorimetry, circular dichroism and molecular modeling. Phys. Chem. Chem. Phys. 2009, 11, 9104–9113. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Takahashi, H.; Tsuchiya, S. New fluorescence of nonenzymatically glucosylated human serum albumin. FEBS lett. 1984, 176, 27–31. [Google Scholar] [CrossRef]
- Mendez, D.L.; Jensen, R.A.; McElroy, L.A.; Pena, J.M.; Esquerra, R.M. The effect of non-enzymatic glycation on the unfolding of human serum albumin. Arch. Biochem. Biophys. 2005, 444, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, R.; Anguizola, J.; Joseph, K.S.; Hage, D.S. High-performance affinity chromatography and the analysis of drug interactions with modified proteins: binding of gliclazide with glycated human serum albumin. Anal. Bioanal. Chem. 2011, 401, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Seedher, N.; Kanojia, M. Fluorescence spectroscopic studies on the complexation of antidiabetic drugs with glycosylated serum albumin. J. Appl. Spectrosc. 2013, 80, 754–760. [Google Scholar] [CrossRef]
- Holm, T.; Raghavan, C.T.; Nahomi, R.; Nagaraj, R.H.; Kessel, L. Effects of photobleaching on selected advanced glycation end products in the human lens. BMC Res. Notes 2015, 8, 5. [Google Scholar] [CrossRef]
- Eftink, M.R.; Ghiron, C.A. Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; pp. 130–135. [Google Scholar]
- Koyama, H.; Sugioka, N.; Uno, A.; Mori, S.; Nakajima, K. Effects of glycosylation of hypoglycaemic drug binding to serum albumin. Biopharm. Drug. Dispos. 1997, 18, 791–801. [Google Scholar] [CrossRef]
- Joseph, K.S.; Anguizola, J.; Jackson, A.J.; Hage, D.S. Chromatographic analysis of acetohexamide binding to glycated human serum albumin. J. Chromatogr. B 2010, 878, 2775–2781. [Google Scholar] [CrossRef] [PubMed]
- Shaklai, N.; Garlick, R.L.; Bunn, H.F. Nonenzymatic glycosylation of human serum albumin alters its conformation and function. J. Biol. Chem. 1984, 259, 3812–3817. [Google Scholar] [PubMed]
- Grigoryan, K.R.; Ghazaryan, A.G. Quenching mechanism of human serum albumin fluorescence by Gangleron. Chem. Biol. 2013, 2, 6–10. [Google Scholar]
- Szkudlarek, A.; Pentak, D.; Ploch, A.; Pożycka, J.; Maciążek-Jurczyk, M. Effect of Temperature on Tolbutamide Binding to Glycated Serum Albumin. Molecules 2017, 22, 569. [Google Scholar] [CrossRef] [PubMed]
- Taira, Z.; Terada, H. Specific and non-specific ligand binding to serum albumin. Biochem. Pharmacol. 1985, 34, 1999–2005. [Google Scholar] [PubMed]
- Kirby, E.P. Fluorescence Instrumentation and Methodology. In Excited States of Proteins and Nucleic Acids; Steiner, R.F., Weinryb, I., Eds.; Springer: Boston, MA, USA, 1971. [Google Scholar]
- Rub, M.A.; Khan, J.M.; Asiri, A.M.; Khan, R.H.; Din, K. Study on the interaction between amphiphilic drug and bovine serum albumin: A thermodynamic and spectroscopic description. J. Lumin. 2014, 155, 39–46. [Google Scholar] [CrossRef]
- Curry, S.; Brick, P.; Franks, N. Fatty acid binding to human serum albumin: New insights from crystallographic studies. Biochim. Biophys. Acta 1999, 1441, 131–140. [Google Scholar] [CrossRef]
- Lehrer, S.S. Solute Perturbation of Protein Fluorescence. The Quenching of the Tryptophyl Fluorescence of Model Compounds and of Lysozyme by Iodide Ion. Biochemistry 1971, 10, 3254–3263. [Google Scholar] [CrossRef]
- Hiratsuka, T. Conformational changes in the 23-kilodalton NH2-terminal peptide segment of myosin ATPase associated with ATP hydrolysis. J. Biol. Chem. 1990, 265, 18786–18790. [Google Scholar]
- Klotz, I.M.; Hunston, D.L. Properties of graphical representations of multiple classes of binding sites. Biochemistry 1971, 10, 3065–3069. [Google Scholar] [CrossRef]
- Wilkinson, K.D. Quantitative analysis of protein-protein interactions. Methods Mol. Biol. 2004, 261, 15–32. [Google Scholar] [PubMed]
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |

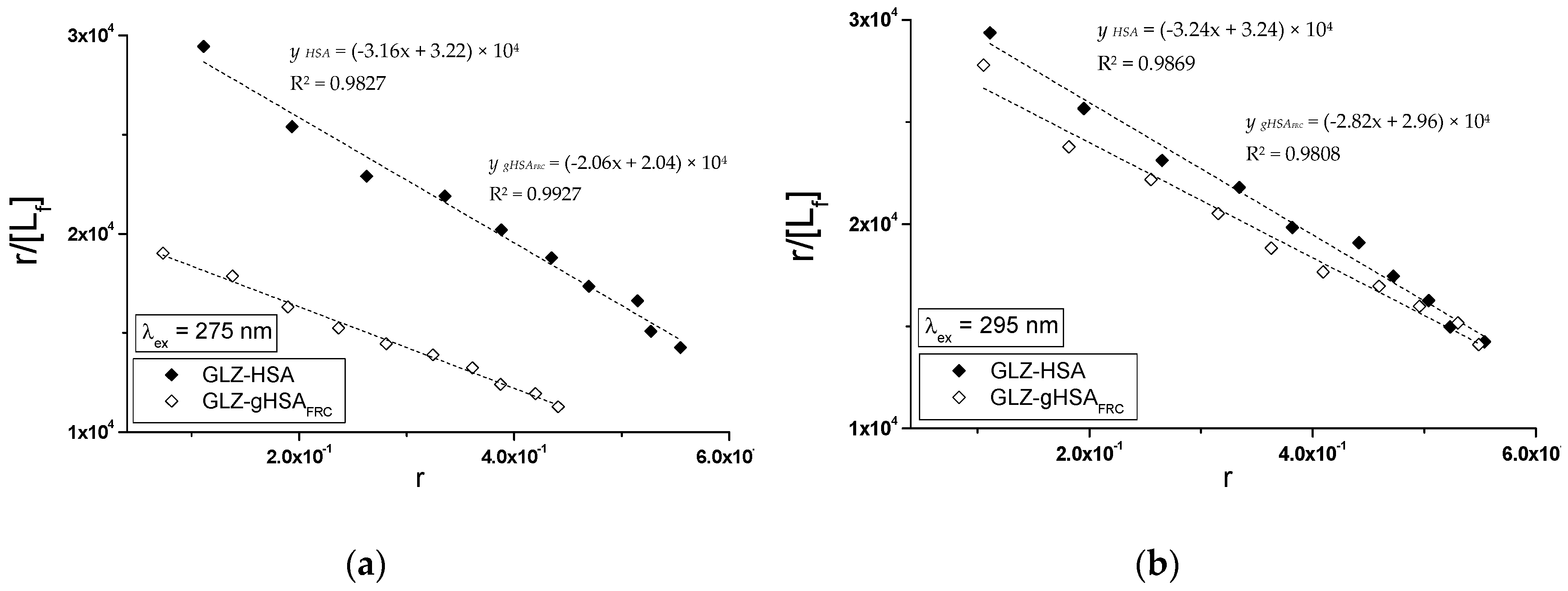
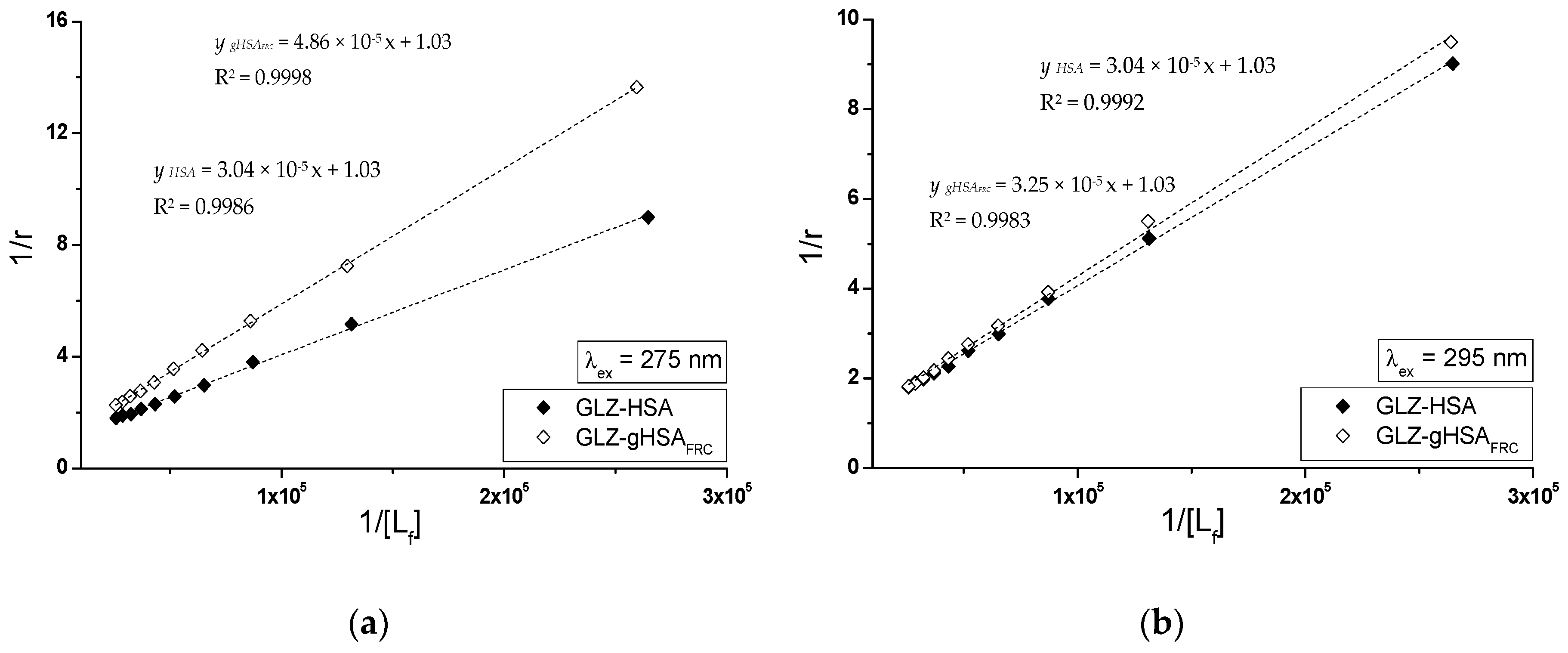

| [Calbumin] | λex = 335 nm | λex = 370 nm | λex = 485 nm | |
|---|---|---|---|---|
| 2 × 10−6 mol∙L−1 | 1.81 | 2.28 | 1.24 | |
| 2.66 | 3.11 | 3.42 | ||
| 45.70 | 30.28 | 29.56 | ||
| 5 × 10−4 mol∙L−1 | 1.17 | 1.11 | 1.10 | |
| 1.22 | 1.22 | 1.12 | ||
| 1.38 | 1.48 | 1.13 |
| λex = 275 nm | ± RSD *) × 103 (mol−1∙L) | a ± RSD *) × 1012(mol−1∙L∙s−1) |
| GLZ-HSA | 8.47 ± 0.43 | 1.37 ± 0.07 |
| GLZ-gHSAFRC | 7.15 ± 0.22 | 1.15 ± 0.04 |
| λex = 295 nm | ± RSD *) × 103 (mol−1∙L) | a ± RSD *) × 1012 (mol−1∙L∙s−1) |
| GLZ-HSA | 10.42 ± 0.49 | 1.68 ± 0.08 |
| GLZ-gHSAFRC | 8.99 ± 0.32 | 1.45 ± 0.05 |
| Scatchard Method | Klotz Method | Hill Method | |||
|---|---|---|---|---|---|
| λex = 275 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) |
| GLZ-HSA | 3.16 ± 0.14 | 1.02 ± 0.06 | 3.38 ± 0.09 | 0.97 ± 0.04 | 1.01 ± 0.01 |
| GLZ-gHSAFRC | 2.06 ± 0.06 | 0.99 ± 0.04 | 2.11 ± 0.04 | 0.97 ± 0.02 | 1.00 ± 0.01 |
| λex = 295 nm | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) |
| GLZ-HSA | 3.24 ± 0.12 | 1.00 ± 0.05 | 3.38 ± 0.06 | 0.97 ± 0.03 | 1.00 ± 0.01 |
| GLZ-gHSAFRC | 2.82 ± 0.13 | 1.05 ± 0.07 | 3.17 ± 0.10 | 0.97 ± 0.04 | 1.02 ± 0.02 |
| Scatchard Method | Klotz Method | Hill Method | |||
|---|---|---|---|---|---|
| (gHSAFRC-PIR) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) × 104 (mol−1∙L) | ± RSD *) | ± RSD *) |
| λex = 275 nm | 3.99 ± 0.14 | 1.00 ± 0.05 | 4.23 ± 0.07 | 0.97 ± 0.02 | 1.00 ± 0.01 |
| λex = 295 nm | 4.21 ± 0.27 | 1.05 ± 0.10 | 4.88 ± 0.12 | 0.96 ± 0.04 | 1.04 ± 0.03 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szkudlarek, A.; Pożycka, J.; Maciążek-Jurczyk, M. Influence of Piracetam on Gliclazide—Glycated Human Serum Albumin Interaction. A Spectrofluorometric Study. Molecules 2019, 24, 111. https://doi.org/10.3390/molecules24010111
Szkudlarek A, Pożycka J, Maciążek-Jurczyk M. Influence of Piracetam on Gliclazide—Glycated Human Serum Albumin Interaction. A Spectrofluorometric Study. Molecules. 2019; 24(1):111. https://doi.org/10.3390/molecules24010111
Chicago/Turabian StyleSzkudlarek, Agnieszka, Jadwiga Pożycka, and Małgorzata Maciążek-Jurczyk. 2019. "Influence of Piracetam on Gliclazide—Glycated Human Serum Albumin Interaction. A Spectrofluorometric Study" Molecules 24, no. 1: 111. https://doi.org/10.3390/molecules24010111
APA StyleSzkudlarek, A., Pożycka, J., & Maciążek-Jurczyk, M. (2019). Influence of Piracetam on Gliclazide—Glycated Human Serum Albumin Interaction. A Spectrofluorometric Study. Molecules, 24(1), 111. https://doi.org/10.3390/molecules24010111






