Desmoglein1 Deficiency Is a Potential Cause of Cutaneous Eruptions Induced by Shuanghuanglian Injection
Abstract
:1. Introduction
2. Materials and Methods
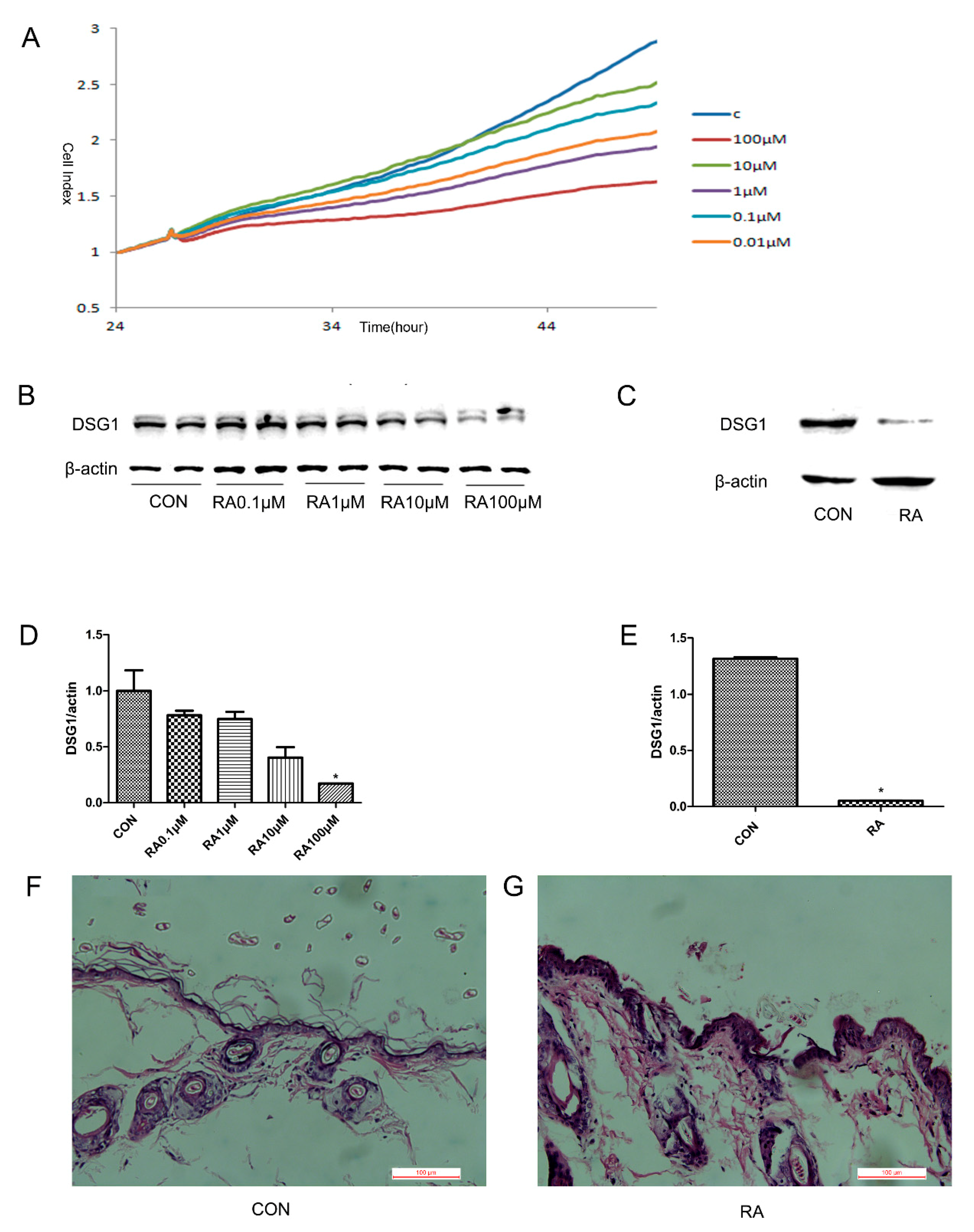
2.1. RA induced Hacat cells DSG1 Deficiency
2.2. RA Induced DSG1 Deficiency In Vivo
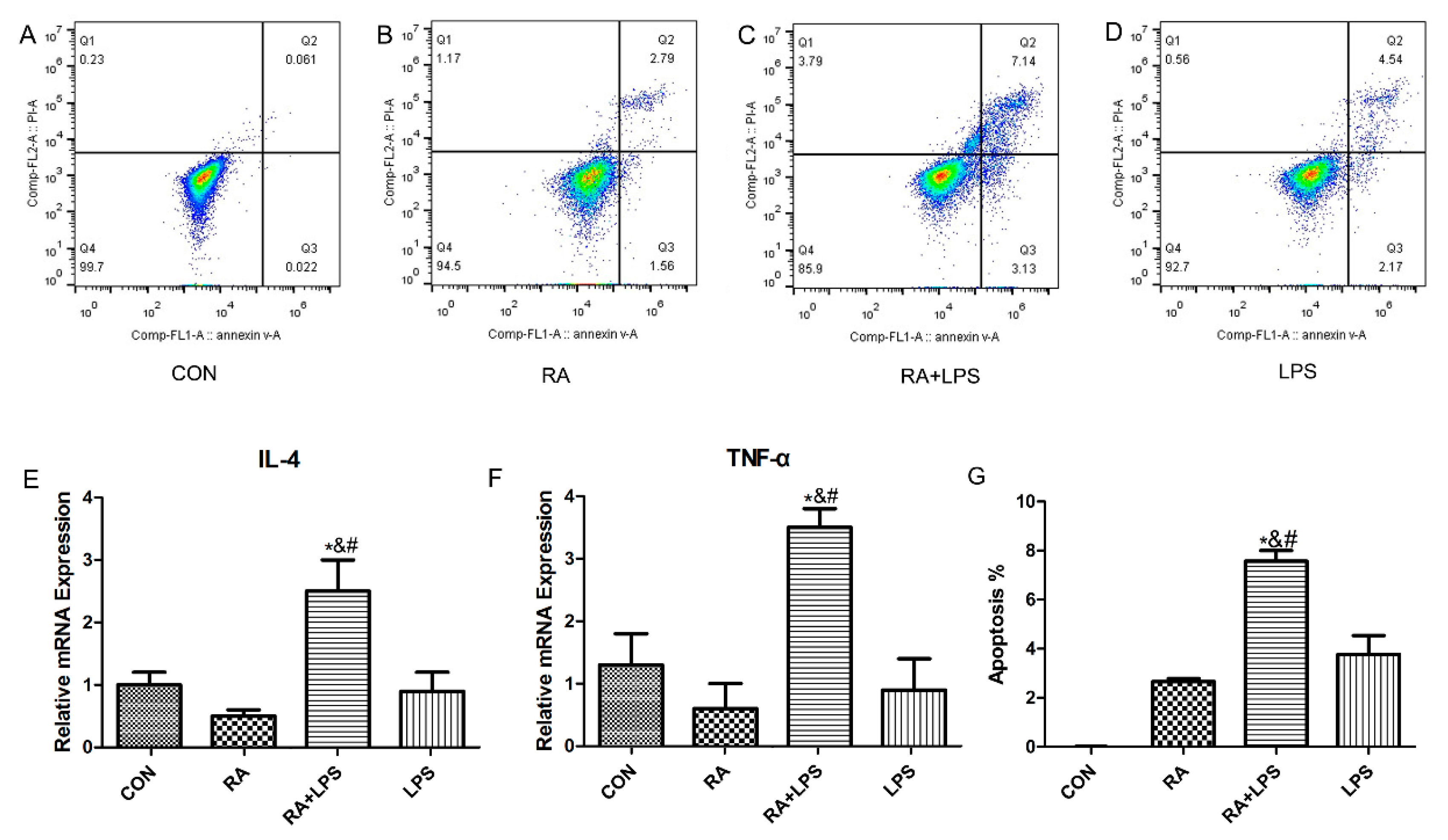
2.3. LPS Action on the DSG1-Deficient Hacat Cells
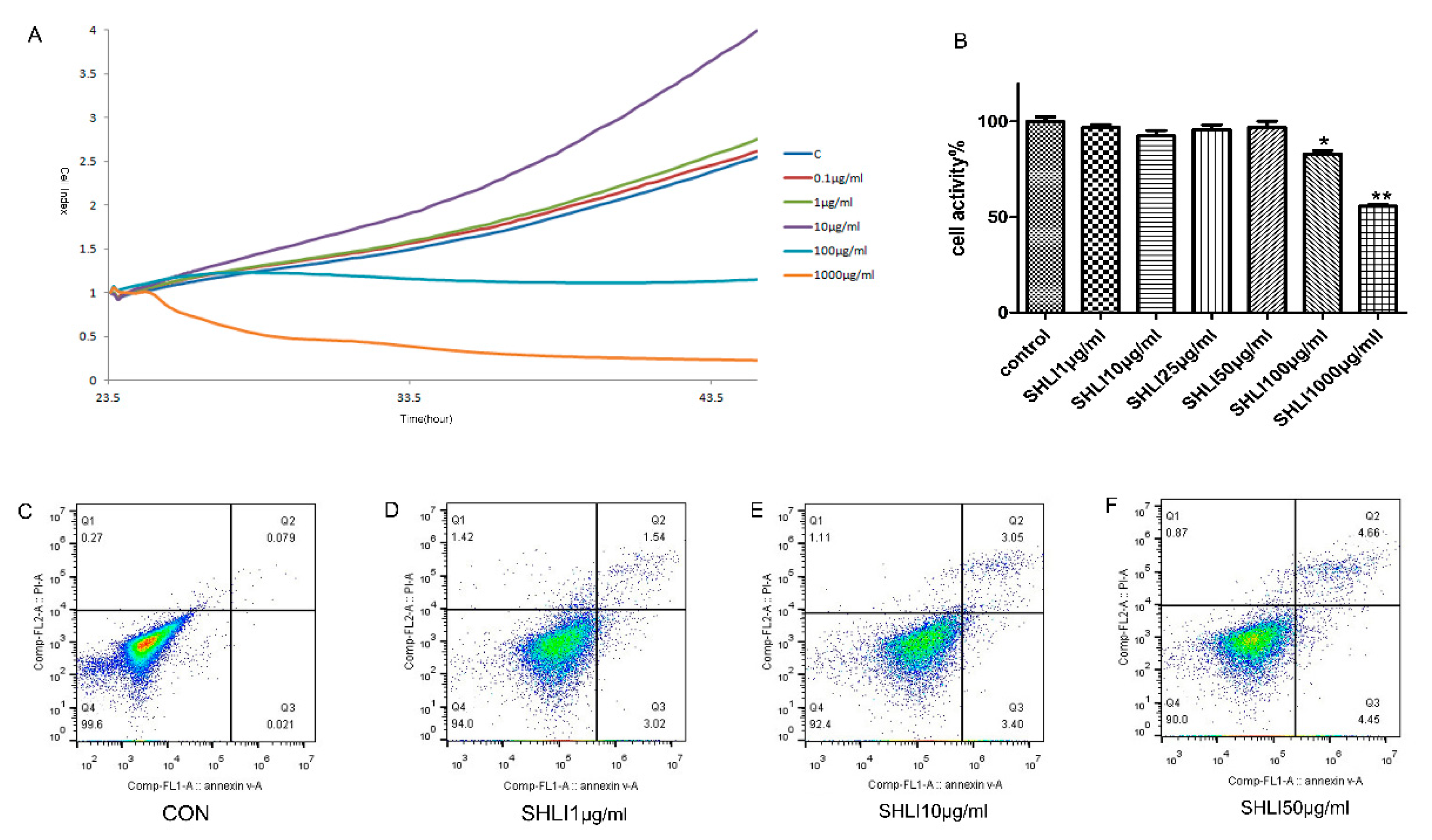
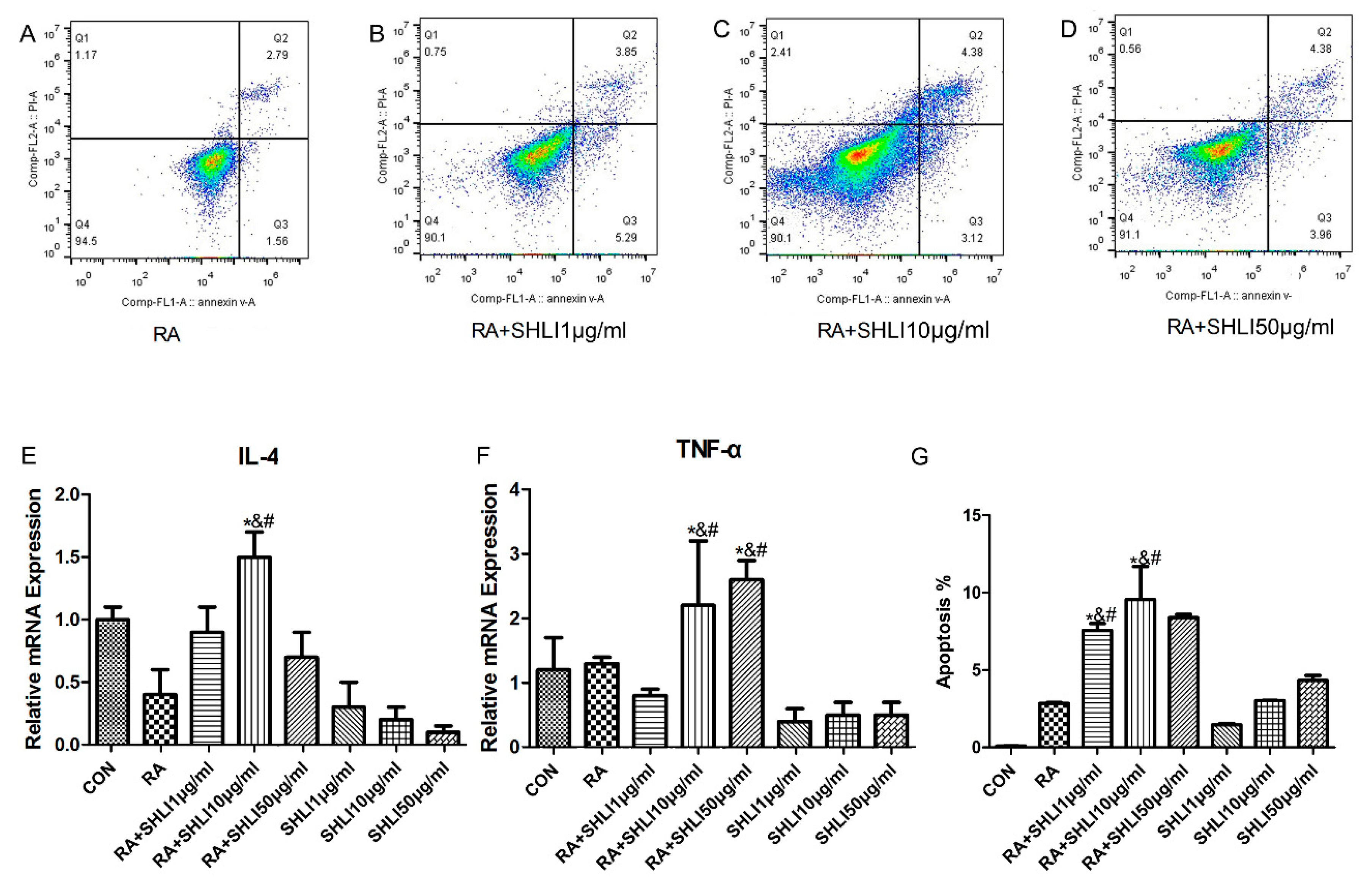
2.4. SHLI Effect on the DSG1-Deficient Hacat Cells and Mice
2.5. Cell Viability Analysis
2.6. Western Blot
2.7. Reverse Transcriptase-Polymerase Chain Reaction (PCR)
2.8. Annexin V-FITC/PI Analysis
2.9. Histopathological Analysis
2.10. Statistics Analysis
3. Results
3.1. The DSG1 Protein Expression was Downregulated by RA In Vitro and In Vivo
3.2. The Deficiency of DSG1 in Hacat Cells Increased the Level of Inflammatory Factors and Apoptosis Rate Induced by LPS
3.3. The Deficiency of DSG1 in Hacat Cells Increased the Level of Inflammatory Factors and Apoptosis Rate Induced by SHLI
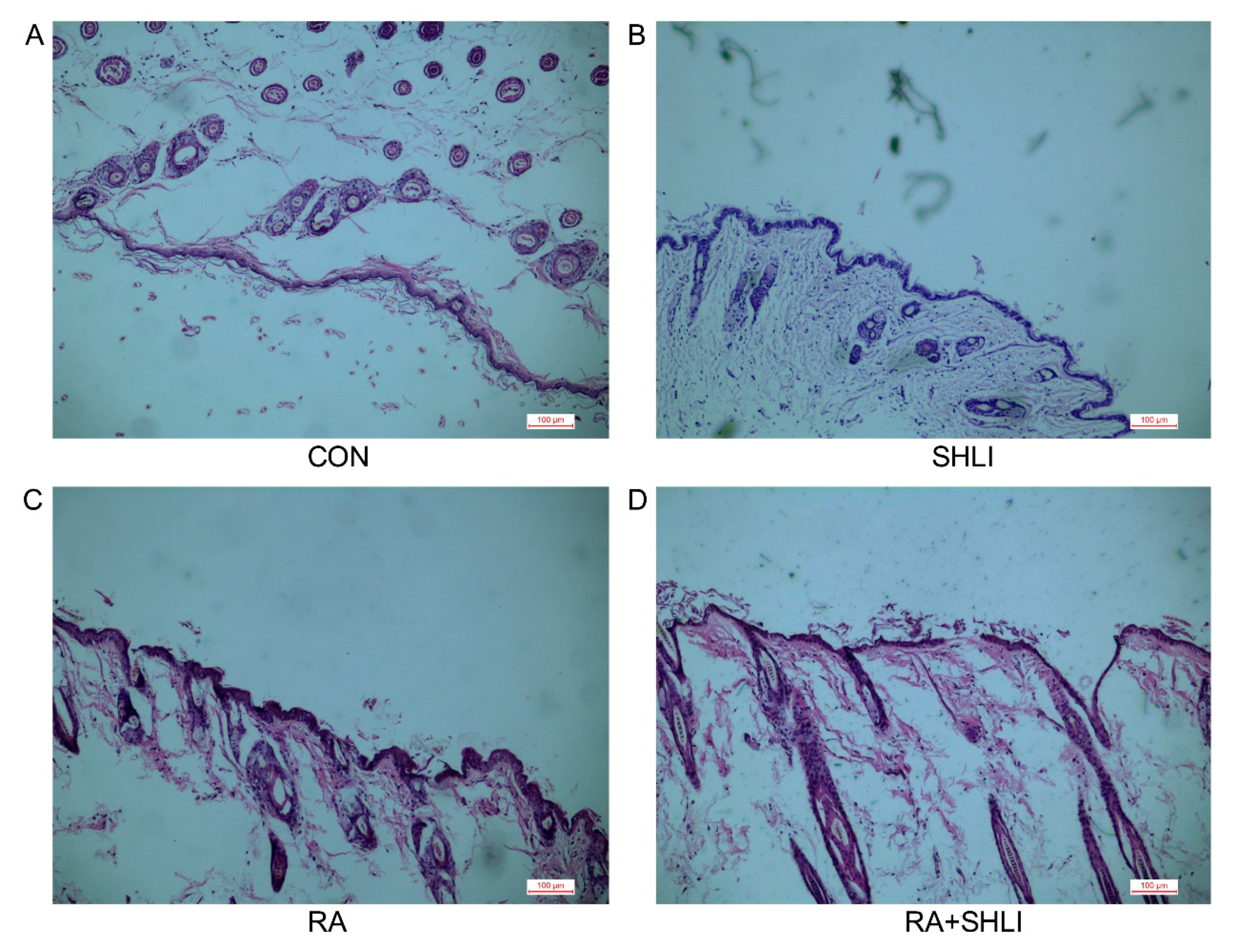
3.4. SHLI-Induced Cutaneous Adverse Reactions in Mice due to DSG1 Deficiency
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Naisbitt, D.J. Drug Hypersensitivity Reactions in Skin: Understanding Mechanisms and the Development of Diagnostic and Predictive Tests. Toxicology 2004, 194, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Lebrun-Vignes, B.; Valeyrie-Allanore, L. Cutaneous Adverse Drug Reactions. Rev. Med. Interne 2015, 36, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.C.; Stern, R.S. Severe Adverse Cutaneous Reactions to Drugs. N. Engl. J. Med. 1994, 331, 1272–1285. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Choi, D.; Chun, Y.J.; Noh, M. Keratinocyte-Derived Il-24 Plays a Role in the Positive Feedback Regulation of Epidermal Inflammation in Response to Environmental and Endogenous Toxic Stressors. Toxicol. Appl. Pharmacol. 2014, 280, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Rebane, A.; Zimmermann, M.; Aab, A.; Baurecht, H.; Koreck, A.; Karelson, M.; Abram, K.; Metsalu, T.; Pihlap, M.; Meyer, N.; et al. Mechanisms of Ifn-Gamma-Induced Apoptosis of Human Skin Keratinocytes in Patients with Atopic Dermatitis. J. Allergy Clin. Immunol. 2012, 129, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Folster-Holst, R.; Brautigam, M.; Sepehrmanesh, M.; Pfeiffer, S.; Jensen, J.M. Role of the Epidermal Barrier in Atopic Dermatitis. J. Dtsch. Dermatol. Ges. 2009, 7, 899–910. [Google Scholar] [CrossRef] [PubMed]
- Jurakic Toncic, R.; Marinovic, B. The Role of Impaired Epidermal Barrier Function in Atopic Dermatitis. Acta Dermatovenerol. Croat. 2016, 24, 95–109. [Google Scholar] [PubMed]
- Garrod, D.R.; Merritt, A.J.; Nie, Z. Desmosomal Cadherins. Curr. Opin. Cell Biol. 2002, 14, 537–545. [Google Scholar] [CrossRef]
- Berika, M.; Garrod, D. Desmosomal Adhesion in Vivo. Cell Commun. Adhes. 2014, 21, 65–75. [Google Scholar] [CrossRef] [PubMed]
- Elias, P.M.; Matsuyoshi, N.; Wu, H.; Lin, C.; Wang, Z.H.; Brown, B.E.; Stanley, J.R. Desmoglein Isoform Distribution Affects Stratum Corneum Structure and Function. J. Cell Biol. 2001, 153, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Amagai, M. Autoimmune and Infectious Skin Diseases That Target Desmogleins. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Getsios, S.; Simpson, C.L.; Kojima, S.; Harmon, R.; Sheu, L.J.; Dusek, R.L.; Cornwell, M.; Green, K.J. Desmoglein 1-Dependent Suppression of Egfr Signaling Promotes Epidermal Differentiation and Morphogenesis. J. Cell Biol. 2009, 185, 1243–1258. [Google Scholar] [CrossRef] [PubMed]
- Garrod, D.; Chidgey, M. Desmosome Structure, Composition and Function. Biochim. Biophys. Acta 2008, 1778, 572–587. [Google Scholar] [CrossRef] [PubMed]
- Samuelov, L.; Sarig, O.; Harmon, R.M.; Rapaport, D.; Ishida-Yamamoto, A.; Isakov, O.; Koetsier, J.L.; Gat, A.; Goldberg, I.; Bergman, R.; et al. Desmoglein 1 Deficiency Results in Severe Dermatitis, Multiple Allergies and Metabolic Wasting. Nat. Genet. 2013, 45, 1244–1248. [Google Scholar] [CrossRef] [PubMed]
- Sumitomo, T.; Mori, Y.; Nakamura, Y.; Honda-Ogawa, M.; Nakagawa, S.; Yamaguchi, M.; Matsue, H.; Terao, Y.; Nakata, M.; Kawabata, S. Streptococcal Cysteine Protease-Mediated Cleavage of Desmogleins Is Involved in the Pathogenesis of Cutaneous Infection. Front. Cell. Infect. Microbiol. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lee, S.E.; Chang, J.Y.; Kim, S.C. Retinoid Induces the Degradation of Corneodesmosomes and Downregulation of Corneodesmosomal Cadherins: Implications on the Mechanism of Retinoid-Induced Desquamation. Ann. Dermatol. 2011, 23, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Chen, J.; Li, J.J.; Wang, X.; Zhai, H.L.; Zhang, X.Y. High-Performance Liquid Chromatography with Photodiode Array Detection and Chemometrics Method for the Analysis of Multiple Components in the Traditional Chinese Medicine Shuanghuanglian Oral Liquid. J. Sep. Sci. 2015, 38, 4187–4195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, L.; Yuan, Q.; Cui, X.; Shang, H.; Zhang, B.; Li, Y. Adverse Drug Reactions of Shuanghuanglian Injection: A Systematic Review of Public Literatures. J. Evid.-Based Med. 2010, 3, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Liang, A.H.; Li, C.Y.; Zhang, Y.S.; Zhao, Y.; Han, J.Y.; Lu, Y.T. Influence of Solvent and Drug Preparation Time on Shuanghuanglian Injections Induce Pseudo-Allergic Reaction. Zhongguo Zhong Yao Za Zhi 2015, 40, 2723–2736. [Google Scholar] [PubMed]
- Yi, Y.; Zhang, Y.S.; Li, C.Y.; Zhao, H.Y.; Xiao, H.B.; Li, G.Q.; Lu, Y.T.; Han, J.Y.; Zhao, Y.; Wang, H.J.; et al. Study of Screening Pseudoallergenic Substances of Shuanghuanglian Injection. Zhongguo Zhong Yao Za Zhi 2015, 40, 2727–2731. [Google Scholar] [PubMed]
- Abassi, Y.A.; Jackson, J.A.; Zhu, J.; O’Connell, J.; Wang, X.; Xu, X. Label-Free, Real-Time Monitoring of Ige-Mediated Mast Cell Activation on Microelectronic Cell Sensor Arrays. J. Immunol. Methods 2004, 292, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Kayalar, O.; Oztay, F. Retinoic Acid Induced Repair in the Lung of Adult Hyperoxic Mice, Reducing Transforming Growth Factor-Beta1 (Tgf-Beta1) Mediated Abnormal Alterations. Acta Histochem. 2014, 116, 810–819. [Google Scholar] [CrossRef] [PubMed]
- Tohyama, M.; Hashimoto, K. Immunological Mechanisms of Epidermal Damage in Toxic Epidermal Necrolysis. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Frank, J.; Cserhalmi-Friedman, P.B.; Ahmad, W.; Panteleyev, A.A.; Aita, V.M.; Christiano, A.M. Characterization of the Desmosomal Cadherin Gene Family: Genomic Organization of Two Desmoglein Genes on Human Chromosome 18q12. Exp. Dermatol. 2001, 10, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Posadas, S.J.; Padial, A.; Torres, M.J.; Mayorga, C.; Leyva, L.; Sanchez, E.; Alvarez, J.; Romano, A.; Juarez, C.; Blanca, M. Delayed Reactions to Drugs Show Levels of Perforin, Granzyme B, and Fas-L to Be Related to Disease Severity. J. Allergy Clin. Immunol. 2002, 109, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Paquet, P.; Paquet, F.; al Saleh, W.; Reper, P.; Vanderkelen, A.; Pierard, G.E. Immunoregulatory Effector Cells in Drug-Induced Toxic Epidermal Necrolysis. Am. J. Dermatopathol. 2000, 22, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Correia, O.; Delgado, L.; Barbosa, I.L.; Campilho, F.; Fleming-Torrinha, J. Increased Interleukin 10, Tumor Necrosis Factor Alpha, and Interleukin 6 Levels in Blister Fluid of Toxic Epidermal Necrolysis. J. Am. Acad. Dermatol. 2002, 47, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Nassif, A.; Moslehi, H.; le Gouvello, S.; Bagot, M.; Lyonnet, L.; Michel, L.; Boumsell, L.; Bensussan, A.; Roujeau, J.C. Evaluation of the Potential Role of Cytokines in Toxic Epidermal Necrolysis. J. Investig. Dermatol. 2004, 123, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Tapia, B.; Padial, A.; Sanchez-Sabate, E.; Alvarez-Ferreira, J.; Morel, E.; Blanca, M.; Bellon, T. Involvement of Ccl27-Ccr10 Interactions in Drug-Induced Cutaneous Reactions. J. Allergy Clin. Immunol. 2004, 114, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Caproni, M.; Torchia, D.; Schincaglia, E.; Volpi, W.; Frezzolini, A.; Schena, D.; Marzano, A.; Quaglino, P.; de Simone, C.; Parodi, A.; et al. Expression of Cytokines and Chemokine Receptors in the Cutaneous Lesions of Erythema Multiforme and Stevens-Johnson Syndrome/Toxic Epidermal Necrolysis. Br. J. Dermatol. 2006, 155, 722–728. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Zhang, X.; Fan, S.; Song, L.; Yang, Z.; Zhuang, P.; Zhang, Y. Desmoglein1 Deficiency Is a Potential Cause of Cutaneous Eruptions Induced by Shuanghuanglian Injection. Molecules 2018, 23, 1477. https://doi.org/10.3390/molecules23061477
Zhang Y, Zhang X, Fan S, Song L, Yang Z, Zhuang P, Zhang Y. Desmoglein1 Deficiency Is a Potential Cause of Cutaneous Eruptions Induced by Shuanghuanglian Injection. Molecules. 2018; 23(6):1477. https://doi.org/10.3390/molecules23061477
Chicago/Turabian StyleZhang, Yidan, Xiujun Zhang, Shanshan Fan, Lili Song, Zhen Yang, Pengwei Zhuang, and Yanjun Zhang. 2018. "Desmoglein1 Deficiency Is a Potential Cause of Cutaneous Eruptions Induced by Shuanghuanglian Injection" Molecules 23, no. 6: 1477. https://doi.org/10.3390/molecules23061477
APA StyleZhang, Y., Zhang, X., Fan, S., Song, L., Yang, Z., Zhuang, P., & Zhang, Y. (2018). Desmoglein1 Deficiency Is a Potential Cause of Cutaneous Eruptions Induced by Shuanghuanglian Injection. Molecules, 23(6), 1477. https://doi.org/10.3390/molecules23061477




