Organic Fluorescent Compounds that Display Efficient Aggregation-Induced Emission Enhancement and Intramolecular Charge Transfer
Abstract
1. Introduction
2. Results and Discussion
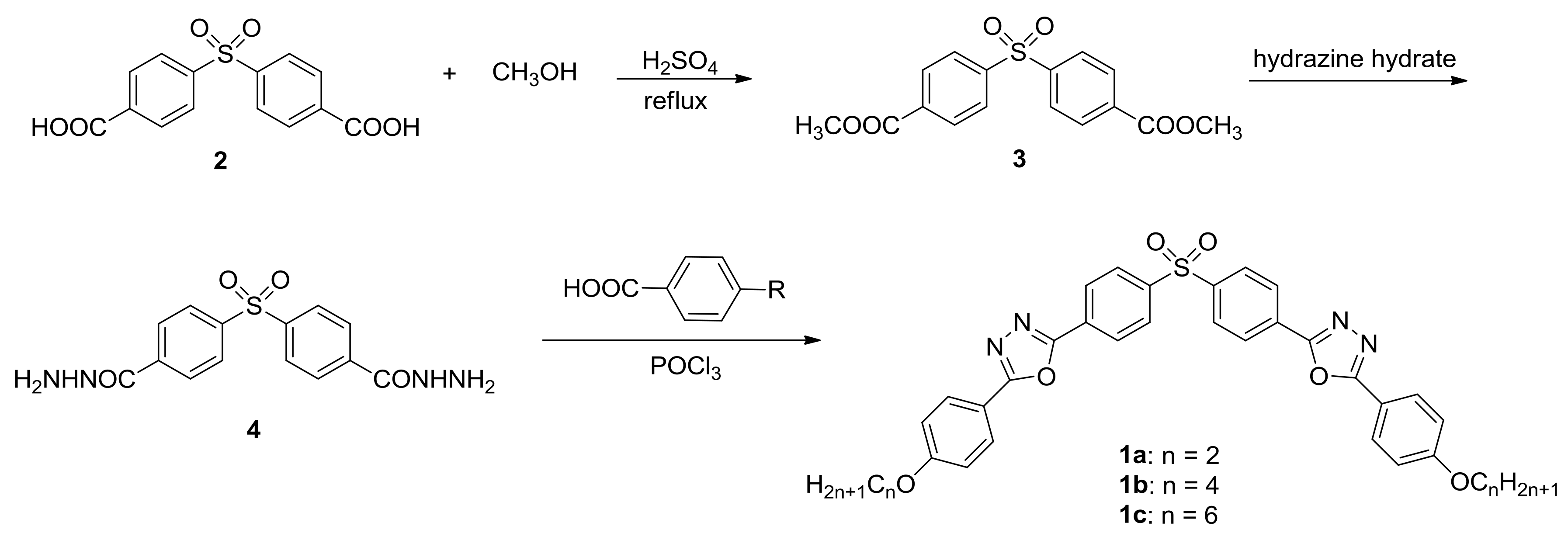
2.1. Synthesis of Target Compounds 1a–c
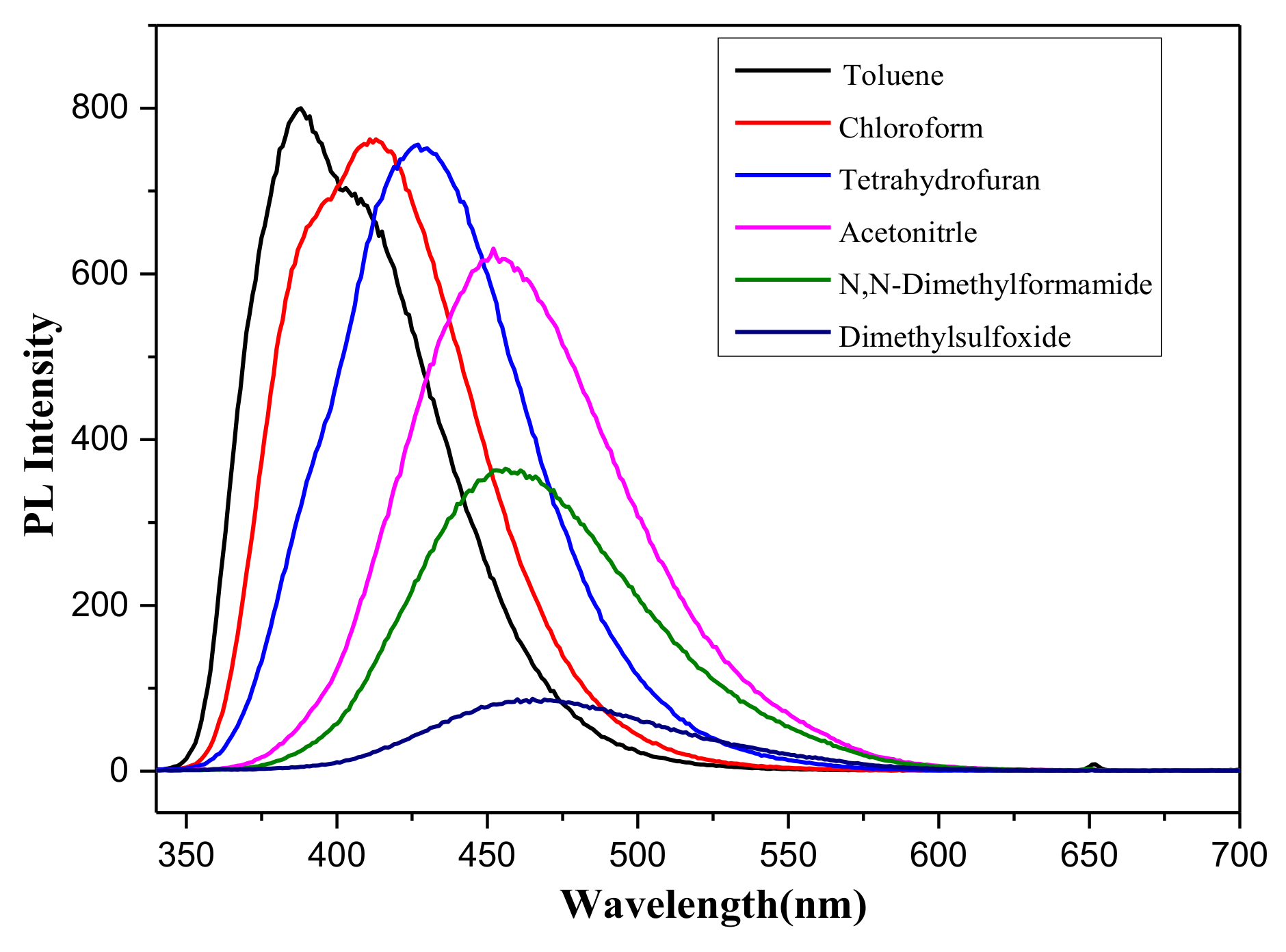
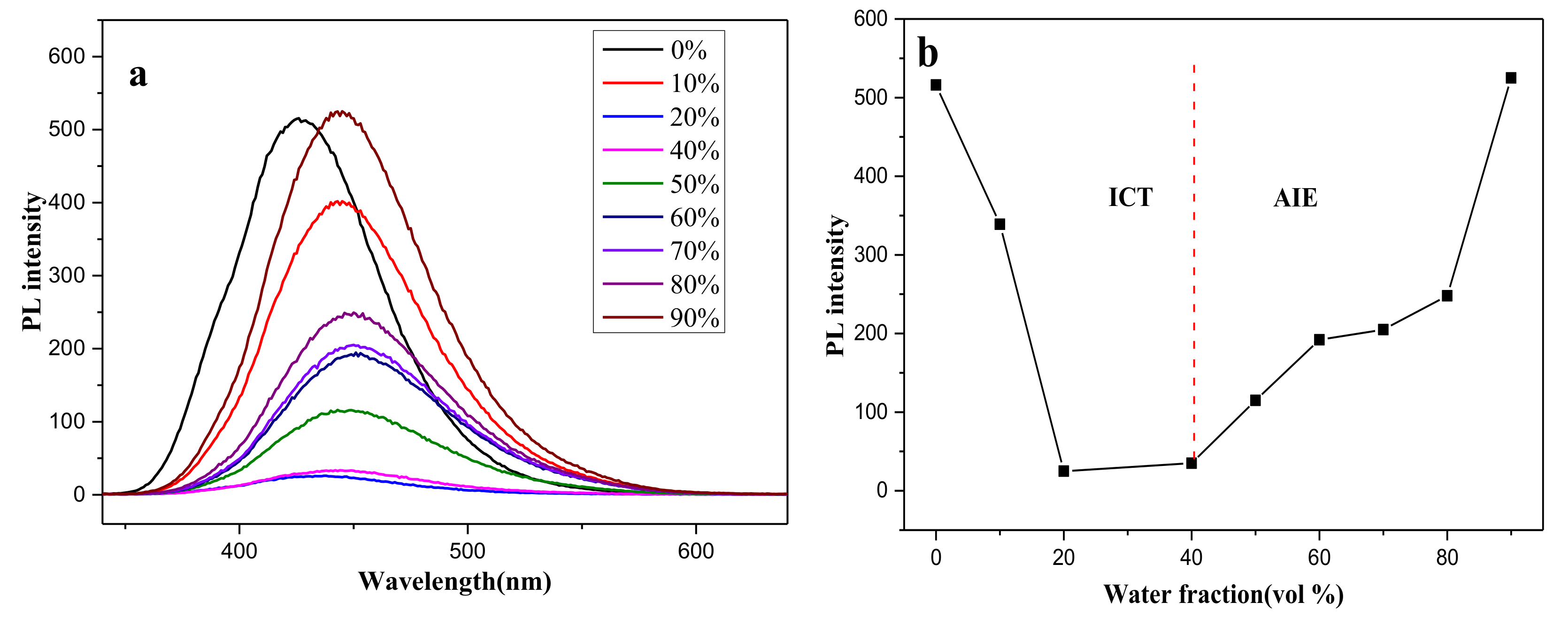
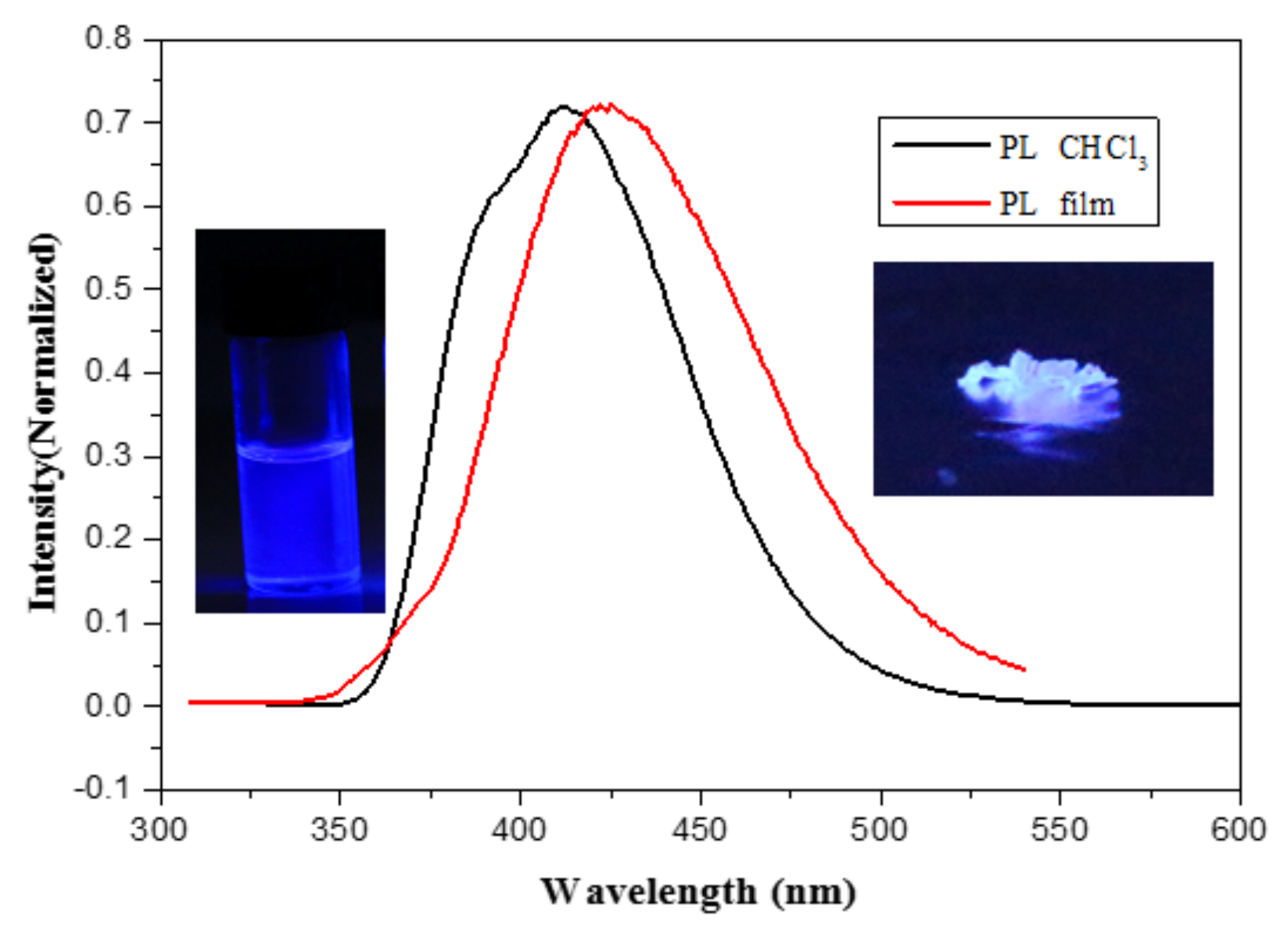
2.2. The Optical Properties of Compounds 1a–c
2.3. The Electrochemical Properties of 1a–c
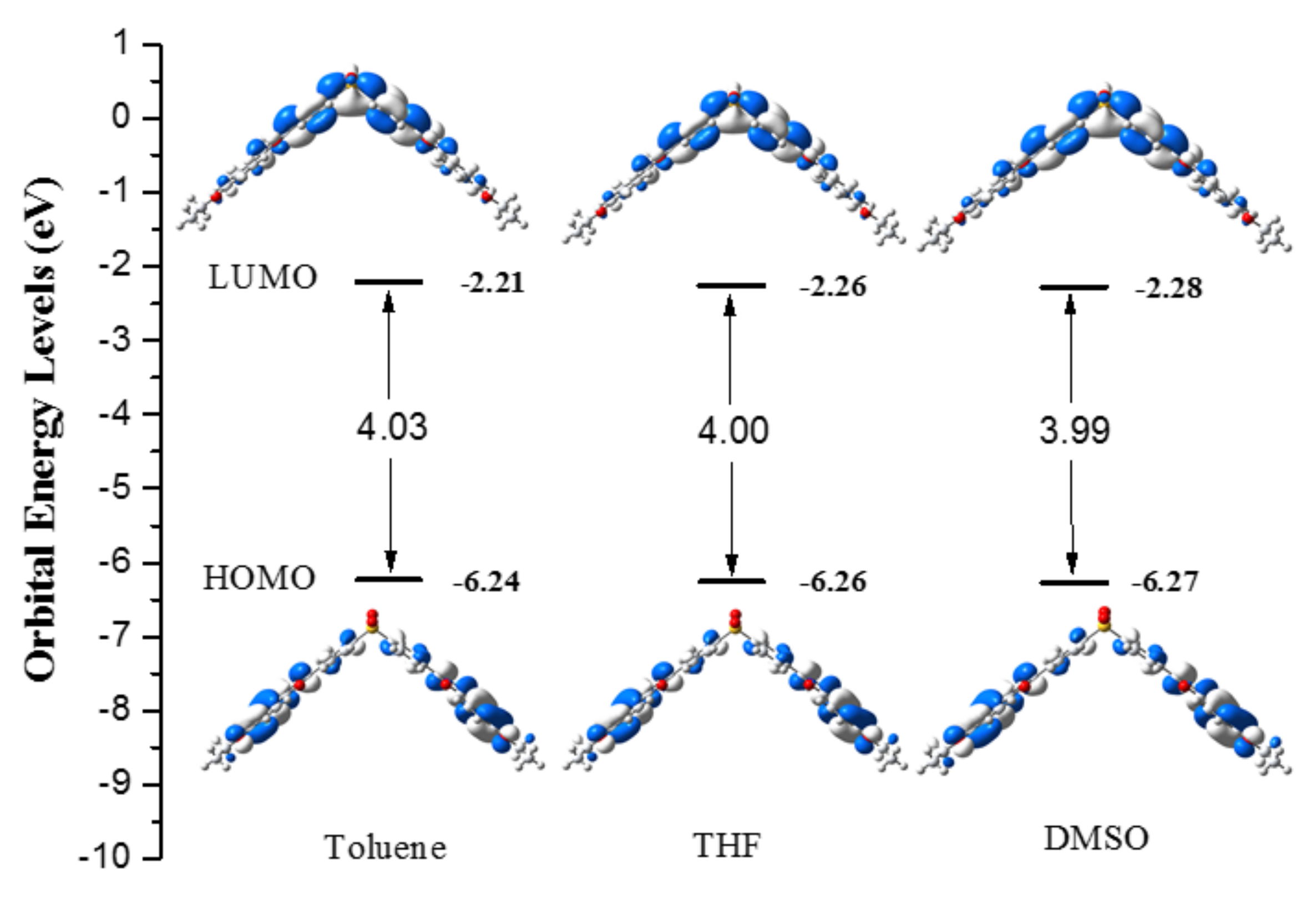
2.4. Theoretical Calculations
3. Experimental
General Information
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burroughes, J.H.; Bradley, D.D.C.; Brown, A.R.; Marks, R.N.; Mackay, K.; Friend, R.H.; Burn, P.L.; Holmes, A.B. Light-emitting diodes based on conjugated polymers. Nature 1990, 347, 539–541. [Google Scholar] [CrossRef]
- Yang, Y.M.; Zhao, Q.; Feng, W.; Li, F.Y. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.J.; Wu, H.Z.; Chen, J.R.; Yang, Z.; Yang, Z.Y.; Wu, Y.C.; Zhang, Y.; Jin, C.J.; Lu, P.Y.; Chi, Z.G.; et al. White-light emission from a single heavy atom-free molecule with room temperature phosphorescence, mechanochromism and thermochromism. Chem. Sci. 2017, 8, 1909–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ge, C.; Yao, J.; Liu, Y.; Xie, H.; Fang, J. Selective selenol fluorescent probes: Design, synthesis, structural determinants, and biological applications. J. Am. Chem. Soc. 2015, 137, 757–769. [Google Scholar] [CrossRef]
- Tang, Y.; Jin, L.; Yin, B. A dual-selective fluorescent probe for GSH and Cys detection: Emission and pH dependent selectivity. Anal. Chim. Acta 2017, 993, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Jenekhe, S.A.; Osaheni, J.A. Excimers and exciplexes of conjugated polymers. Science 1994, 265, 765–768. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.X.; Li, X.L.; Zhao, Y.; Gao, Y.; Liu, B.Q. High-Performance Blue Molecular Emitter-Free and Doping-Free Hybrid White Organic Light-Emitting Diodes: An Alternative Concept to Manipulate Charges and Excitons Based on Exciplex and Electroplex Emission. ACS Photonics 2017, 4, 1566–1575. [Google Scholar] [CrossRef]
- Luo, D.X.; Yang, Y.F.; Xiao, Y.; Zhao, Y.; Yang, Y.B.; Liu, B.Q. Regulating Charge and Exciton Distribution in High-Performance Hybrid White Organic Light-Emitting Diodes with n-Type Interlayer Switch. Nano-Micro Lett. 2017, 9, 37. [Google Scholar] [CrossRef]
- Tang, B.Z.; Zhan, X.W.; Yu, G.; Lee, P.P.S.; Liu, Y.Q.; Zhu, D.B. Efficient blue emission from siloles. J. Mater. Chem. 2001, 11, 2974–2978. [Google Scholar] [CrossRef]
- Liu, B.Q.; Nie, H.; Lin, G.W.; Hu, S.B.; Gao, D.Y.; Zou, J.H.; Xu, M.; Wang, L.; Zhao, Z.J.; Ning, H.L.; et al. High-Performance Doping-Free Hybrid White OLEDs Based on Blue Aggregation-Induced Emission Luminogens. ACS Appl. Mater. Interfaces 2017, 9, 34162–34171. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Q.; Nie, H.; Zhou, X.B.; Hu, S.B.; Luo, D.X.; Gao, D.Y.; Zou, J.H.; Xu, M.; Wang, L.; Zhao, Z.J.; et al. Manipulation of Charge and Exciton Distribution Based on Blue Aggregation-Induced Emission Fluorophors: A Novel Concept to Achieve High-Performance Hybrid White Organic Light-Emitting Diodes. Adv. Funct. Mater. 2016, 26, 776–783. [Google Scholar] [CrossRef]
- Mei, J.; Leung, L.C.; Ryan, T.K.; Kwok, R.T.K.; Lam, W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Gu, P.Y.; Zhang, Y.H.; Liu, G.Y.; Ge, J.F.; Xu, Q.F.; Zhang, Q.C.; Lu, J.M. A new V-shaped organic fluorescent compound integrated with crystallization-induced emission enhancement and intramolecular charge transfer. Chem. Asian J. 2013, 8, 2161–2166. [Google Scholar] [CrossRef] [PubMed]
- Shao, A.D.; Guo, Z.Q.; Zhu, S.J.; Zhu, S.Q.; Shi, P.; Tian, H.; Zhu, W.H. Insight into aggregation-induced emission characteristics of red-emissive quinoline-malononitrile by cell tracking and real-time trypsin detection. Chem. Sci. 2014, 5, 1383–1389. [Google Scholar] [CrossRef]
- Gu, P.Y.; Lu, C.J.; Hu, Z.J.; Li, N.J.; Zhao, T.T.; Xu, Q.F.; Xu, Q.H.; Zhang, J.D.; Lu, J.M. The AIEE effect and two-photon absorption (TPA) enhancement induced by polymerization: Synthesis of a monomer with ICT and AIE effects and its homopolymer by ATRP and a study of their photophysical properties. J. Mater. Chem. C 2013, 1, 2599–2606. [Google Scholar] [CrossRef]
- Qin, W.; Ding, D.; Liu, J.Z.; Yuan, W.Z.; Hu, Y.; Liu, B.; Tang, B.Z. Biocompatible nanoparticles with aggregation-induced emission characteristics as far-red/near-infrared fluorescent bioprobes for in vitro and in vivo imaging applications. Adv. Funct. Mater. 2012, 22, 771–779. [Google Scholar] [CrossRef]
- Kartens, T.; Kobs, K. Rhodamine B and rhodamine 101 as reference substances for fluorescence quantum yield measurements. J. Phys. Chem. 1980, 84, 1871–1872. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B. Fox, GAUSSIAN 09 (Revision B.01); Gaussian, Inc.: Wallingford, UK, 2010. [Google Scholar]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.T.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. Accuracy of AH n equilibrium geometries by single determinant molecular orbital theory. Mol. Phys. 1974, 27, 209–214. [Google Scholar] [CrossRef]
- Lin, B.C.; Cheng, C.P.; You, Z.Q.; Hsu, C.P. Charge Transport Properties of Tris(8-hydroxyquinolinato) aluminum(III): Why It Is an Electron Transporter. J. Am. Chem. Soc. 2005, 127, 66–67. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
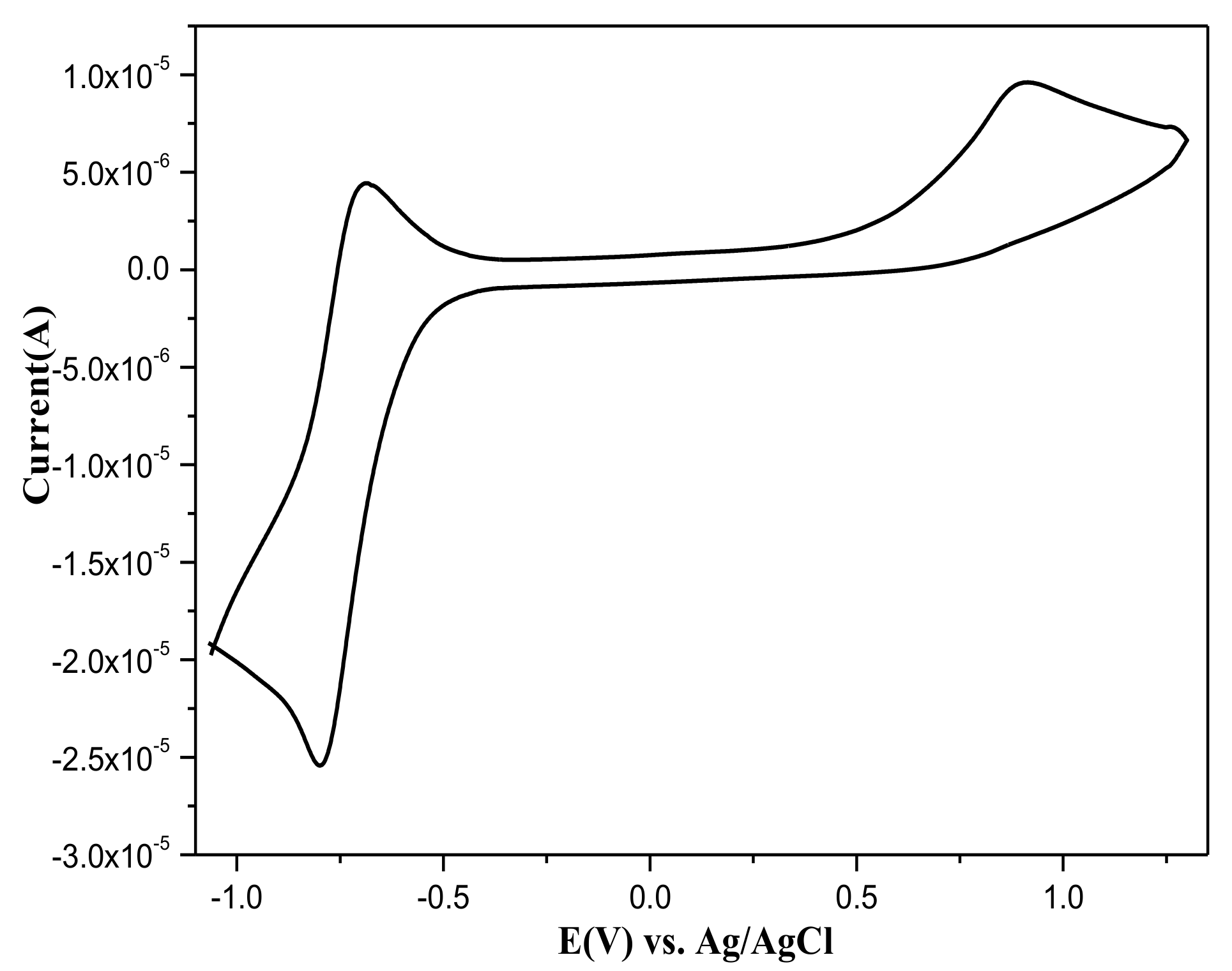
| Comp. | a λmax,Abs (nm) | a λmax,em (nm) | b λmax,em (nm) | cΦF | Ered/peak (V) | E1ox/peak (V) | dEgopt (eV) | e Tg/T5d (°C) |
|---|---|---|---|---|---|---|---|---|
| 1a | 323 | 412 | 420 | 0.42 | −0.73 | 0.62 | 4.18 | 119/396 |
| 1b | 326 | 411 | 423 | 0.36 | −0.74 | 0.75 | 4.21 | 156/383 |
| 1c | 328 | 411 | 423 | 0.32 | −0.76 | 0.78 | 4.26 | 145/386 |
| Comp. | IP (v) | IP (a) | HEP | EA (v) | EA (a) | EEP | λhole | λelectron | Δ |
|---|---|---|---|---|---|---|---|---|---|
| 1a | 6.21 | 6.07 | 5.90 | 2.35 | 2.49 | 2.63 | 0.31 | 0.28 | 0.03 |
| 1b | 6.18 | 6.07 | 5.90 | 2.29 | 2.49 | 2.63 | 0.28 | 0.34 | 0.06 |
| 1c | 6.17 | 6.07 | 5.90 | 2.29 | 2.49 | 2.63 | 0.27 | 0.34 | 0.07 |
| Alq3 | IP (5.8) | EA (3.0) | 0.242 | 0.276 | 0.034 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, R.; Zhao, B.; Xia, Y.; Li, D. Organic Fluorescent Compounds that Display Efficient Aggregation-Induced Emission Enhancement and Intramolecular Charge Transfer. Molecules 2018, 23, 1446. https://doi.org/10.3390/molecules23061446
Hou R, Zhao B, Xia Y, Li D. Organic Fluorescent Compounds that Display Efficient Aggregation-Induced Emission Enhancement and Intramolecular Charge Transfer. Molecules. 2018; 23(6):1446. https://doi.org/10.3390/molecules23061446
Chicago/Turabian StyleHou, Ruibin, Baohua Zhao, Yan Xia, and Dongfeng Li. 2018. "Organic Fluorescent Compounds that Display Efficient Aggregation-Induced Emission Enhancement and Intramolecular Charge Transfer" Molecules 23, no. 6: 1446. https://doi.org/10.3390/molecules23061446
APA StyleHou, R., Zhao, B., Xia, Y., & Li, D. (2018). Organic Fluorescent Compounds that Display Efficient Aggregation-Induced Emission Enhancement and Intramolecular Charge Transfer. Molecules, 23(6), 1446. https://doi.org/10.3390/molecules23061446






