Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review
Abstract
:1. Introduction
BBB Transport Routes
2. Drug Delivery Strategies
2.1. Monoclonal Antibody Strategy
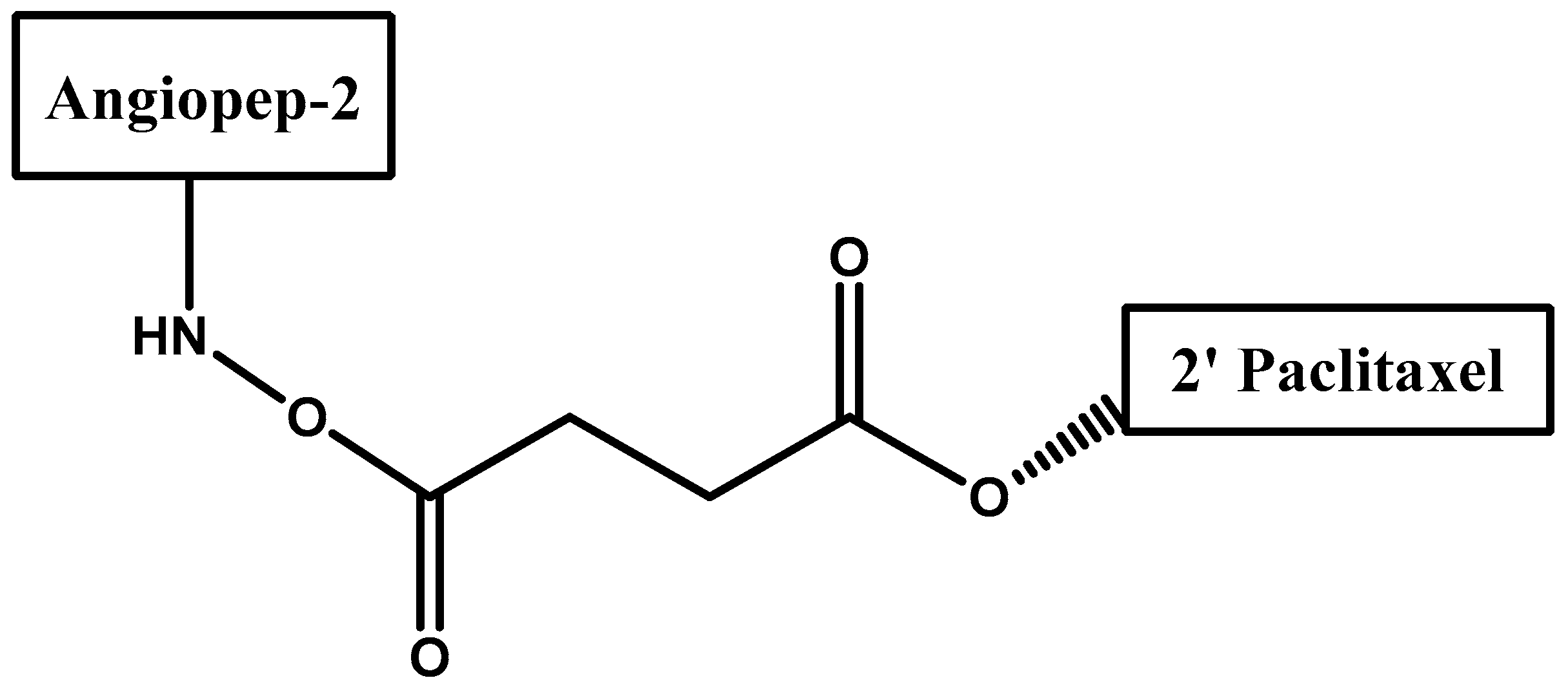
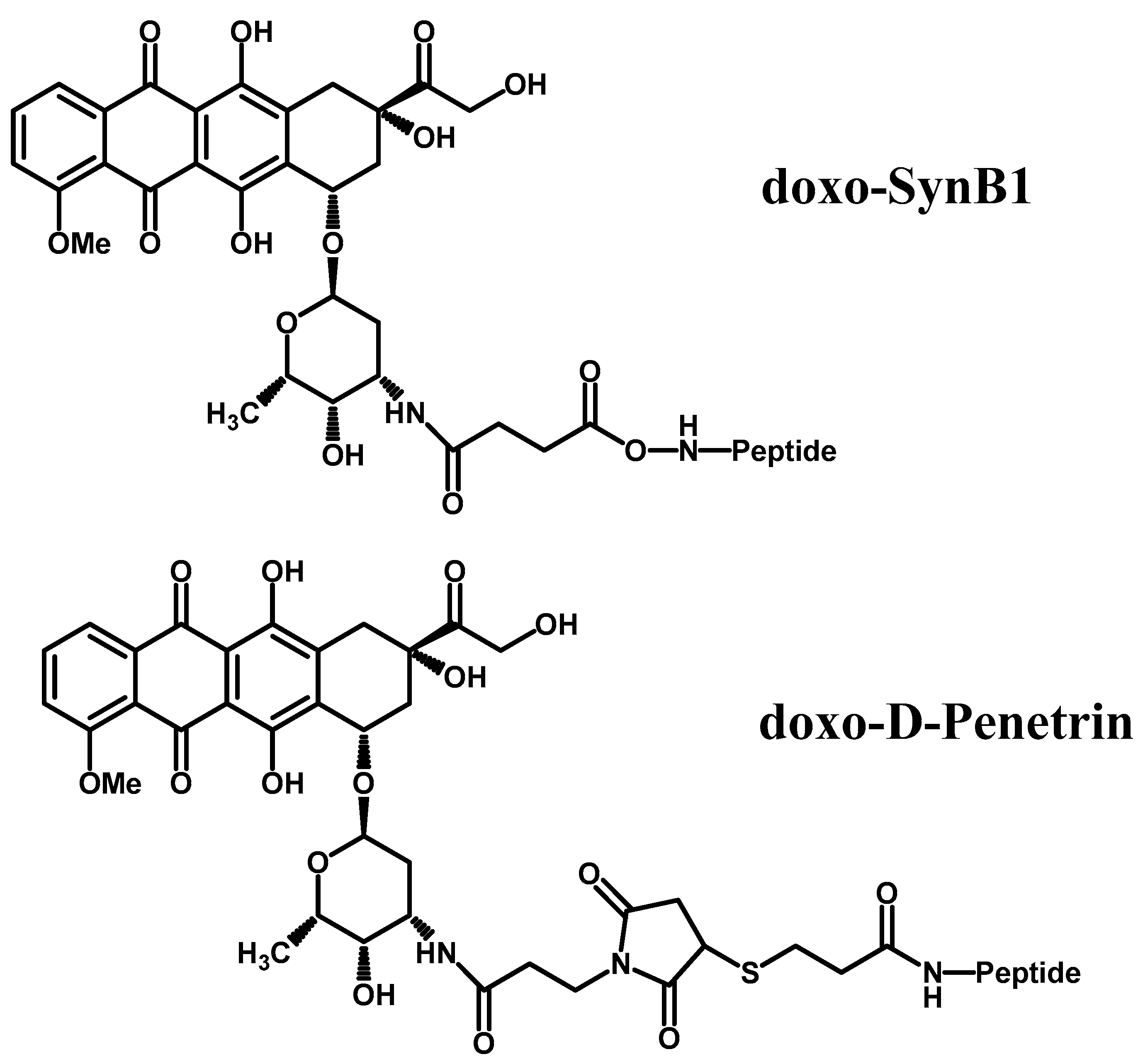
2.2. Peptide-Vector Strategy
2.3. Nanoparticles
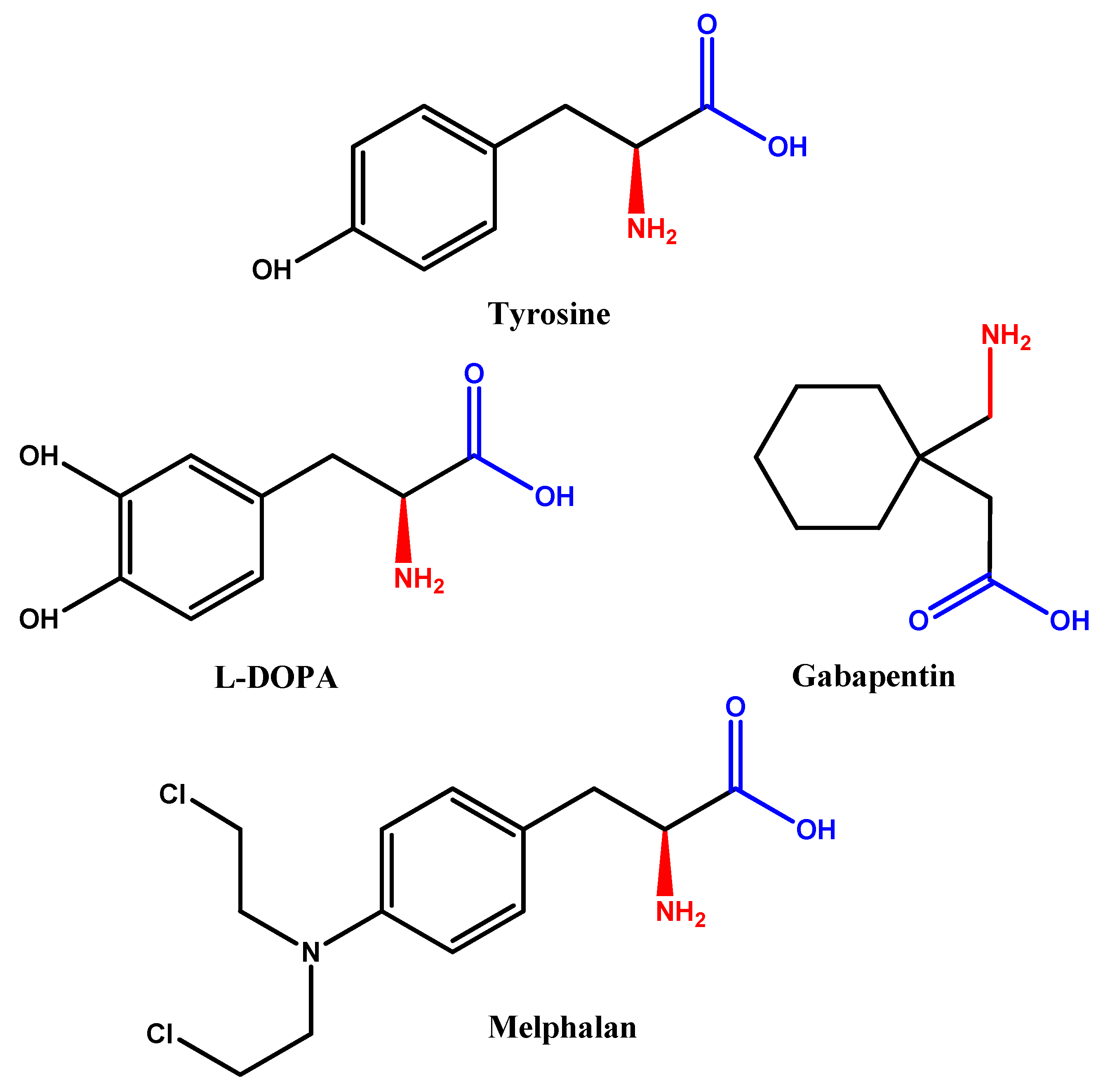
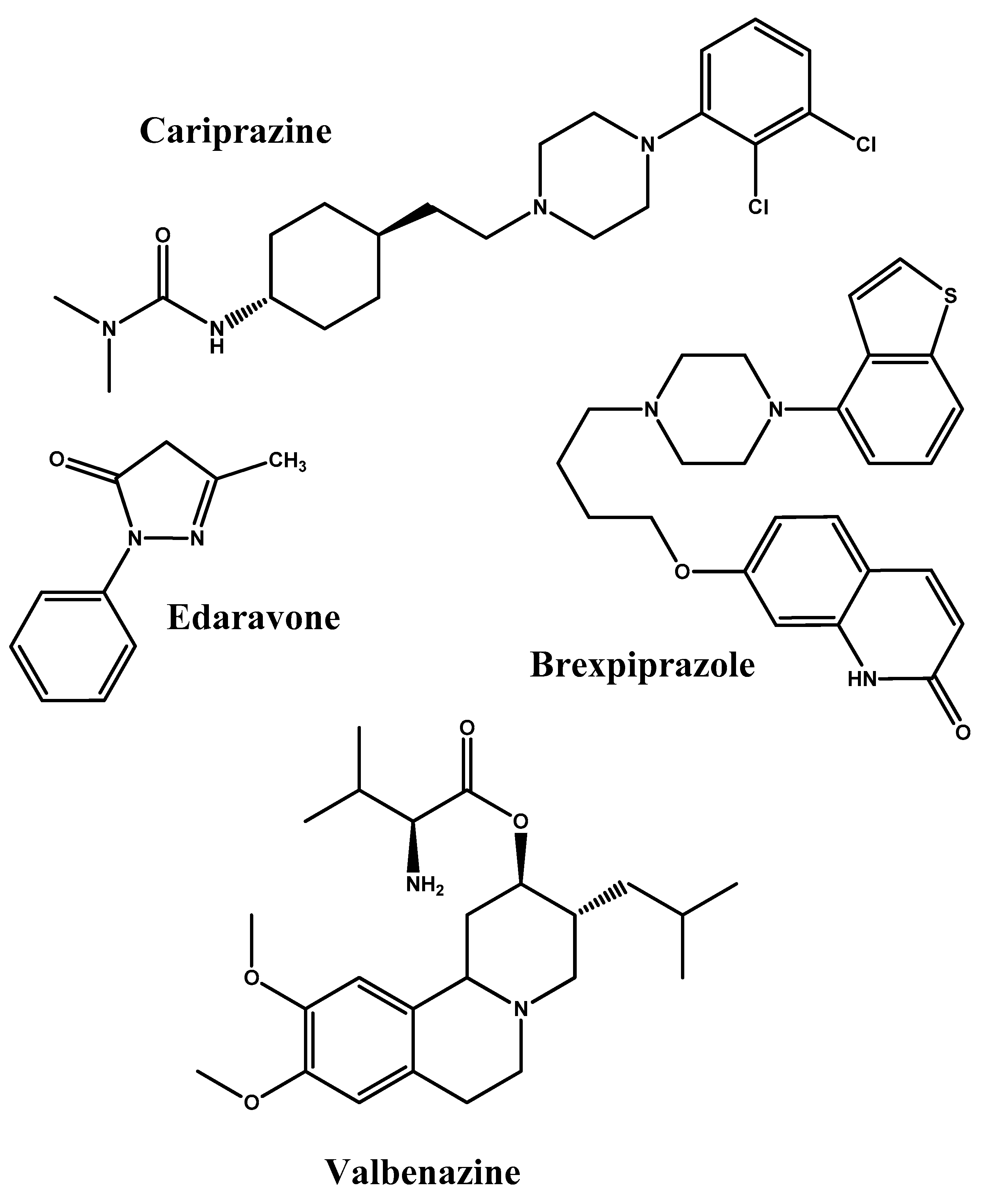
2.4. Simple Prodrug Strategy
3. Summary and Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood brain barrier |
| CNS | Central nervous system |
| LAT1 | l-type amino acid transporter 1 |
| GLUT1 | Glucose transporter 1 |
| MCT1 | Monocarboxylate transporter 1 |
| CAT1 | Cationic amino acid transporter 1 |
| ChT | Choline transporter |
| SGLT | Sodium-coupled glucose transporter |
| P-gp | P-glycoprotein |
| PTS-6 | Peptide transport system 6 |
| BCRP | Breast cancer resistant protein |
| IR | Insulin receptor |
| Tf | Transferrin |
| TfR | Transferrin receptor |
| LDLR | Low-density lipoprotein receptor |
| FcRn | Neonatal Fc receptor |
| LEPR | Leptin receptor |
| MAb | Monoclonal antibody |
| IDUA | Iduronidase |
| MPSI | Mucopolysaccharidosis type I |
| bsAb | Bispecific antibodies |
| LRP1 | Low-density lipoprotein receptor related protein 1 |
| BDNF | Brain derived neurotrophic factor |
| TRKB | Tropomyosin receptor kinase B |
| CSF | Cerebrospinal fluid |
| Dox | Doxorubicin |
| RVG | Rabies virus glycoprotein |
| TAT | Trans-activator of transcription |
| IGF2 | Insulin-like growth factor 2 |
| NAGLU | N-acetyl-alpha-glucosaminidase |
| NP | Nanoparticle |
| PEG | Poly (ethylene glycol) |
| PLA | Poly (lactic acid) |
| AuNP | Gold nanoparticle |
| AgNP | Silver nanoparticle |
| SWCNT | Single-walled carbon nanotube |
| CD | Carbon dot |
| PLGA | Poly (lactic co-glycolic acid) |
| Lf | Lactoferrin |
| UDCA | Ursodeoxycholic acid |
| AZT | Azidothymidine |
| ICAM-1 | Intracellular adhesion molecule 1 |
References
- Ronaldson, P.T.; Davis, T.P. Targeting blood–brain barrier changes during inflammatory pain: An opportunity for optimizing CNS drug delivery. Ther. Deliv. 2011, 2, 1015–1041. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Mariscal, L.; Nava, P.; Hernandez, S. Critical role of tight junctions in drug delivery across epithelial and endothelial cell layers. J. Membr. Biol. 2005, 207, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Matsuhisa, K.; Kondoh, M.; Takahashi, A.; Yagi, K. Tight junction modulator and drug delivery. Expert Opin. Drug Deliv. 2009, 6, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Why Is the Global CNS Pharmaceutical Market So Under-Penetrated? Elsevier: New York, NY, USA, 2002. [Google Scholar]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J. Blood–brain barrier structure and function and the challenges for CNS drug delivery. J. Inherit. Metab. Dis. 2013, 36, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Oldendorf, W.H. Lipid solubility and drug penetration of the blood brain barrier. Proc. Soc. Exp. Biol. Med. 1974, 147, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Reichel, A. Pharmacokinetics of CNS penetration. In Blood-Brain Barrier in Drug Discovery: Optimizing Brain Exposure of CNS Drugs and Minimizing Brain Side Effects for Peripheral Drugs; Wiley: Hoboken, NJ, USA, 2015; pp. 7–41. [Google Scholar]
- Misra, A.; Ganesh, S.; Shahiwala, A.; Shah, S.P. Drug delivery to the central nervous system: A review. J. Pharm. Pharm. Sci. 2003, 6, 252–273. [Google Scholar] [PubMed]
- Reichel, A. Addressing central nervous system (CNS) penetration in drug discovery: Basics and implications of the evolving new concept. Chem. Biodivers. 2009, 6, 2030–2049. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Shityakov, S.; Förster, C. In silico predictive model to determine vector-mediated transport properties for the blood–brain barrier choline transporter. Adv. Appl. Bioinf. Chem. AABC 2014, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.S.; Hirayama, B.A.; Timbol, G.; Liu, J.; Diez-Sampedro, A.; Kepe, V.; Satyamurthy, N.; Huang, S.C.; Wright, E.M.; Barrio, J.R. Regional distribution of SGLT activity in rat brain in vivo. Am. J. Physiol. Cell Physiol. 2013, 304, C240–C247. [Google Scholar] [CrossRef] [PubMed]
- On, N.H.; Miller, D.W. Transporter-based delivery of anticancer drugs to the brain: Improving brain penetration by minimizing drug efflux at the blood-brain barrier. Curr. Pharm. Des. 2014, 20, 1499–1509. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S. Regulation of ABC transporters blood-brain barrier: The good, the bad, and the ugly. Adv. Cancer Res. 2015, 125, 43–70. [Google Scholar] [PubMed]
- Oldendorf, W.H. Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. Am. J. Physiol. 1971, 221, 1629–1639. [Google Scholar] [CrossRef] [PubMed]
- Giddings, S.; Chirgwin, J.; Permutt, M. Evaluation of rat insulin messenger RNA in pancreatic and extrapancreatic tissues. Diabetologia 1985, 28, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Skarlatos, S.; Yoshikawa, T.; Pardridge, W.M. Transport of [125i] transferrin through the rat blood-brain barrier. Brain Res. 1995, 683, 164–171. [Google Scholar] [CrossRef]
- Zhang, Y.; Pardridge, W.M. Rapid transferrin efflux from brain to blood across the blood–brain barrier. J. Neurochem. 2001, 76, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Schlachetzki, F.; Zhu, C.; Pardridge, W.M. Expression of the neonatal fc receptor (FCRN) at the blood–brain barrier. J. Neurochem. 2002, 81, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Golden, P.L.; Maccagnan, T.J.; Pardridge, W.M. Human blood-brain barrier leptin receptor. Binding and endocytosis in isolated human brain microvessels. J. Clin. Investig. 1997, 99, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm. Sin. B 2016, 6, 268–286. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Cloyd, J.C.; Siegel, R.A. A review of intranasal formulations for the treatment of seizure emergencies. J. Control. Release 2016, 237, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. J. Control. Release 2017. [Google Scholar] [CrossRef] [PubMed]
- Gomes, P.; Soares-da-Silva, P. L-dopa transport properties in an immortalised cell line of rat capillary cerebral endothelial cells, RBE 4. Brain Res. 1999, 829, 143–150. [Google Scholar] [CrossRef]
- Cornford, E.M.; Young, D.; Paxton, J.W.; Finlay, G.J.; Wilson, W.R.; Pardridge, W.M. Melphalan penetration of the blood-brain barrier via the neutral amino acid transporter in tumor-bearing brain. Cancer Res. 1992, 52, 138–143. [Google Scholar] [PubMed]
- Taylor, C.P. Mechanisms of action of gabapentin. Rev. Neurol. 1997, 153, S39–S45. [Google Scholar] [PubMed]
- Frankel, J.S.; Schwartz, T.L. Brexpiprazole and cariprazine: Distinguishing two new atypical antipsychotics from the original dopamine stabilizer aripiprazole. Ther. Adv. Psychopharmacol. 2017, 7, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Meuth, S.; Gobel, K.; Wiendl, H. Immune therapy of multiple sclerosis-future strategies. Curr. Pharm. Des. 2012, 18, 4489–4497. [Google Scholar] [CrossRef] [PubMed]
- Rothstein, J.D. Edaravone: A new drug approved for ALS. Cell 2017, 171, 725. [Google Scholar] [CrossRef] [PubMed]
- Müller, T. Valbenazine Granted Breakthrough Drug Status for Treating Tardive Dyskinesia; Taylor & Francis: Abingdon, UK, 2015. [Google Scholar]
- Pardridge, W.M.; Kang, Y.-S.; Buciak, J.L.; Yang, J. Human insulin receptor monoclonal antibody undergoes high affinity binding to human brain capillaries in vitro and rapid transcytosis through the blood–brain barrier in vivo in the primate. Pharm. Res. 1995, 12, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yang, J.; Pardridge, W.M. Drug targeting of a peptide radiopharmaceutical through the primate blood-brain barrier in vivo with a monoclonal antibody to the human insulin receptor. J. Clin. Investig. 1997, 100, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Zhang, Y.; Zhang, Y.; Xia, C.f.; Wang, Y.; Pardridge, W.M. Genetic engineering of a lysosomal enzyme fusion protein for targeted delivery across the human blood-brain barrier. Biotechnol. Bioeng. 2008, 99, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Lu, J.Z.; Hui, E.K.W.; Sumbria, R.K.; Pardridge, W.M. Pharmacokinetics and brain uptake in the rhesus monkey of a fusion protein of arylsulfatase a and a monoclonal antibody against the human insulin receptor. Biotechnol. Bioeng. 2013, 110, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Boado, R.J.; Hui, E.K.-W.; Lu, J.Z.; Pardridge, W.M. Agt-181: Expression in cho cells and pharmacokinetics, safety, and plasma iduronidase enzyme activity in rhesus monkeys. J. Biotechnol. 2009, 144, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Giugliani, R.; Nestrasil, I.; Chen, S.; Pardridge, W.; Rioux, P. Intravenous infusion of iduronidase-igg and its impact on the central nervous system in children with hurler syndrome. Mol. Genet. Metab. 2017, 120, S55–S56. [Google Scholar] [CrossRef]
- Jefferies, W.A.; Brandon, M.R.; Hunt, S.V.; Williams, A.F.; Gatter, K.C.; Mason, D.Y. Transferrin receptor on endothelium of brain capillaries. Nature 1984, 312, 162–163. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Engelhardt, B.; Lesley, J.; Bickel, U.; Pardridge, W.M. Targeting rat anti-mouse transferrin receptor monoclonal antibodies through blood-brain barrier in mouse. J. Pharmacol. Exp. Ther. 2000, 292, 1048–1052. [Google Scholar] [PubMed]
- Bickel, U.; Yoshikawa, T.; Landaw, E.M.; Faull, K.F.; Pardridge, W.M. Pharmacologic effects in vivo in brain by vector-mediated peptide drug delivery. Proc. Natl. Acad. Sci. USA 1993, 90, 2618–2622. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Vandesquille, M.; Koukouli, F.; Dudeffant, C.; Youssef, I.; Lenormand, P.; Ganneau, C.; Maskos, U.; Czech, C.; Grueninger, F. Camelid single-domain antibodies: A versatile tool for in vivo imaging of extracellular and intracellular brain targets. J. Control. Release 2016, 243, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.-T.; Bullock, K.M.; Erickson, M.A.; Zhang, J.; Banks, W.A. Alpha synuclein is transported into and out of the brain by the blood–brain barrier. Peptides 2014, 62, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Deane, R.; Wu, Z.; Sagare, A.; Davis, J.; Yan, S.D.; Hamm, K.; Xu, F.; Parisi, M.; LaRue, B.; Hu, H.W.; et al. Lrp/amyloid β-peptide interaction mediates differential brain efflux of β isoforms. Neuron 2004, 43, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Benchenane, K.; Berezowski, V.; Ali, C.; Fernández-Monreal, M.; López-Atalaya, J.P.; Brillault, J.; Chuquet, J.; Nouvelot, A.; MacKenzie, E.T.; Bu, G.; et al. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor–related protein-mediated transcytosis. Circulation 2005, 111, 2241–2249. [Google Scholar] [CrossRef] [PubMed]
- Régina, A.; Demeule, M.; Ché, C.; Lavallée, I.; Poirier, J.; Gabathuler, R.; Béliveau, R.; Castaigne, J.-P. Antitumour activity of ang1005, a conjugate between paclitaxel and the new brain delivery vector angiopep-2. Br. J. Pharmacol. 2009, 155, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Demeule, M.; Currie, J.C.; Bertrand, Y.; Ché, C.; Nguyen, T.; Régina, A.; Gabathuler, R.; Castaigne, J.P.; Béliveau, R. Involvement of the low-density lipoprotein receptor-related protein in the transcytosis of the brain delivery vector angiopep-2. J. Neurochem. 2008, 106, 1534–1544. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Pardridge, W.M. Neuroprotection with noninvasive neurotrophin delivery to the brain. Proc. Natl. Acad. Sci. USA 1999, 96, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pardridge, W.M. Conjugation of brain-derived neurotrophic factor to a blood–brain barrier drug targeting system enables neuroprotection in regional brain ischemia following intravenous injection of the neurotrophin. Brain Res. 2001, 889, 49–56. [Google Scholar] [CrossRef]
- Zhang, Y.; Pardridge, W.M. Neuroprotection in transient focal brain ischemia after delayed intravenous administration of brain-derived neurotrophic factor conjugated to a blood-brain barrier drug targeting system. Stroke 2001, 32, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Delivery of biologics across the blood–brain barrier with molecular trojan horse technology. BioDrugs 2017, 31, 503–519. [Google Scholar] [CrossRef] [PubMed]
- Baldrick, P. Safety evaluation of biological drugs: What are toxicology studies in primates telling us? Regul. Toxicol. Pharmacol. 2011, 59, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, C.; Clair, P.; Lefauconnier, J.-M.; Kaczorek, M.; Scherrmann, J.-M.; Temsamani, J. New advances in the transport of doxorubicin through the blood-brain barrier by a peptide vector-mediated strategy. Mol. Pharmacol. 2000, 57, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Lentz, T.L. Rabies virus binding to an acetylcholine receptor α-subunit peptide. J. Mol. Recognit. 1990, 3, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Wu, H.; McBride, J.L.; Jung, K.-E.; Kim, M.H.; Davidson, B.L.; Lee, S.K.; Shankar, P.; Manjunath, N. Transvascular delivery of small interfering RNA to the central nervous system. Nature 2007, 448, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Böckenhoff, A.; Cramer, S.; Wölte, P.; Knieling, S.; Wohlenberg, C.; Gieselmann, V.; Galla, H.-J.; Matzner, U. Comparison of five peptide vectors for improved brain delivery of the lysosomal enzyme arylsulfatase a. J. Neurosci. 2014, 34, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Sarko, D.; Beijer, B.; Boy, R.G.; Nothelfer, E.-M.; Leotta, K.; Eisenhut, M.; Altmann, A.; Haberkorn, U.; Mier, W. The pharmacokinetics of cell-penetrating peptides. Mol. Pharm. 2010, 7, 2224–2231. [Google Scholar] [CrossRef] [PubMed]
- Kan, S.-H.; Aoyagi-Scharber, M.; Le, S.Q.; Vincelette, J.; Ohmi, K.; Bullens, S.; Wendt, D.J.; Christianson, T.M.; Tiger, P.M.; Brown, J.R. Delivery of an enzyme-IGFII fusion protein to the mouse brain is therapeutic for mucopolysaccharidosis type IIIB. Proc. Natl. Acad. Sci. USA 2014, 111, 14870–14875. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Peng, Z.; Seven, E.S.; Leblanc, R.M. Crossing the blood-brain barrier with nanoparticles. J. Control. Release 2017. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Thamphiwatana, S.; Angsantikul, P.; Zhang, L. Nanoparticle approaches against bacterial infections. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2014, 6, 532–547. [Google Scholar] [CrossRef] [PubMed]
- Yhee, J.Y.; Son, S.; Lee, H.; Kim, K. Nanoparticle-based combination therapy for cancer treatment. Curr. Pharm. Des. 2015, 21, 3158–3166. [Google Scholar] [CrossRef] [PubMed]
- Xia, H.; Anderson, B.; Mao, Q.; Davidson, B.L. Recombinant human adenovirus: Targeting to the human transferrin receptor improves gene transfer to brain microcapillary endothelium. J. Virol. 2000, 74, 11359–11366. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Gao, X.; Kang, T.; Jiang, M.; Miao, D.; Gu, G.; Hu, Q.; Song, Q.; Yao, L.; Tu, Y. B6 peptide-modified peg-pla nanoparticles for enhanced brain delivery of neuroprotective peptide. Bioconjugate Chem. 2013, 24, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Choi, W.I.; Kim, Y.H.; Tae, G. Brain-targeted delivery of protein using chitosan-and RVG peptide-conjugated, pluronic-based nano-carrier. Biomaterials 2013, 34, 1170–1178. [Google Scholar] [CrossRef] [PubMed]
- Mittal, D.; Md, S.; Hasan, Q.; Fazil, M.; Ali, A.; Baboota, S.; Ali, J. Brain targeted nanoparticulate drug delivery system of rasagiline via intranasal route. Drug Deliv. 2016, 23, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dai, Q.; Morshed, R.A.; Fan, X.; Wegscheid, M.L.; Wainwright, D.A.; Han, Y.; Zhang, L.; Auffinger, B.; Tobias, A.L. Blood-brain barrier permeable gold nanoparticles: An efficient delivery platform for enhanced malignant glioma therapy and imaging. Small 2014, 10, 5137–5150. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.J.; Davis, M.E. Increased brain uptake of targeted nanoparticles by adding an acid-cleavable linkage between transferrin and the nanoparticle core. Proc. Natl. Acad. Sci. USA 2015, 112, 12486–12491. [Google Scholar] [CrossRef] [PubMed]
- Trickler, W.J.; Lantz, S.M.; Murdock, R.C.; Schrand, A.M.; Robinson, B.L.; Newport, G.D.; Schlager, J.J.; Oldenburg, S.J.; Paule, M.G.; Slikker, W., Jr. Silver nanoparticle induced blood-brain barrier inflammation and increased permeability in primary rat brain microvessel endothelial cells. Toxicol. Sci. 2010, 118, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Salvati, E.; Re, F.; Sesana, S.; Cambianica, I.; Sancini, G.; Masserini, M.; Gregori, M. Liposomes functionalized to overcome the blood–brain barrier and to target amyloid-β peptide: The chemical design affects the permeability across an in vitro model. Int. J. Nanomed. 2013, 8, 1749–1758. [Google Scholar]
- Li, S.; Amat, D.; Peng, Z.; Vanni, S.; Raskin, S.; De Angulo, G.; Othman, A.M.; Graham, R.M.; Leblanc, R.M. Transferrin conjugated nontoxic carbon dots for doxorubicin delivery to target pediatric brain tumor cells. Nanoscale 2016, 8, 16662–16669. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Miyanji, E.H.; Zhou, Y.; Pardo, J.; Hettiarachchi, S.D.; Li, S.; Blackwelder, P.L.; Skromne, I.; Leblanc, R.M. Carbon dots: Promising biomaterials for bone-specific imaging and drug delivery. Nanoscale 2017, 9, 17533–17543. [Google Scholar] [CrossRef] [PubMed]
- Fabbro, A.; Prato, M.; Ballerini, L. Carbon nanotubes in neuroregeneration and repair. Adv. Drug Deliv. Rev. 2013, 65, 2034–2044. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.M.; Foo, J.B.; Fakurazi, S.; Hussein, M.Z. Release behaviour and toxicity evaluation of levodopa from carboxylated single-walled carbon nanotubes. Beilstein J. Nanotechnol. 2015, 6, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; You, H.; Yang, X.; Lin, B.; Zhu, Z.; Lu, Z.; Li, X.; Zhao, Y.; Mao, L.; Shen, S. Functional single-walled carbon nanotubes ‘CAR’ for targeting dopamine delivery into the brain of parkinsonian mice. Nanoscale 2017, 9, 10832–10845. [Google Scholar] [CrossRef] [PubMed]
- Shityakov, S.; Forster, C. Multidrug resistance protein p-gp interaction with nanoparticles (fullerenes and carbon nanotube) to assess their drug delivery potential: A theoretical molecular docking study. Int. J. Comput. Biol. Drug Des. 2013, 6, 343–357. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ebrahimi, A.; Li, J.; Cui, Q. Fullerene–biomolecule conjugates and their biomedicinal applications. Int. J. Nanomed. 2014, 9, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Tsao, N.; Wu, C.-M.; Hsu, H.-P.; Liu, C.-C.; Luh, T.-Y.; Chou, C.-K.; Lei, H.-Y. Inhibition of the increased permeability of blood-brain barrier in escherichia coli-induced meningitis by carboxyfullerene. Fullerene Sci. Technol. 2001, 9, 307–320. [Google Scholar] [CrossRef]
- Bi, C.; Wang, A.; Chu, Y.; Liu, S.; Mu, H.; Liu, W.; Wu, Z.; Sun, K.; Li, Y. Intranasal delivery of rotigotine to the brain with lactoferrin-modified peg-plga nanoparticles for parkinson’s disease treatment. Int. J. Nanomed. 2016, 11, 6547–6559. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.; Muro, S. Lysosomal enzyme replacement therapies: Historical development, clinical outcomes, and future perspectives. Adv. Drug Deliv. Rev. 2017, 118, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Hsu, J.; Northrup, L.; Bhowmick, T.; Muro, S. Enhanced delivery of α-glucosidase for pompe disease by icam-1-targeted nanocarriers: Comparative performance of a strategy for three distinct lysosomal storage disorders. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Garnacho, C.; Dhami, R.; Simone, E.; Dziubla, T.; Leferovich, J.; Schuchman, E.H.; Muzykantov, V.; Muro, S. Delivery of acid sphingomyelinase in normal and niemann-pick disease mice using intercellular adhesion molecule-1-targeted polymer nanocarriers. J. Pharmacol. Exp. Ther. 2008, 325, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Garnacho, C.; Muro, S. Icam-1 targeting, intracellular trafficking, and functional activity of polymer nanocarriers coated with a fibrinogen-derived peptide for lysosomal enzyme replacement. J. Drug Target. 2017, 25, 786–795. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Sawalha, M.; Khawaja, Y.; Najjar, A.; Karaman, R. Dopamine and levodopa prodrugs for the treatment of parkinson’s disease. Molecules 2017, 23, 40. [Google Scholar] [CrossRef] [PubMed]
- Sinokrot, H.; Smerat, T.; Najjar, A.; Karaman, R. Advanced prodrug strategies in nucleoside and non-nucleoside antiviral agents: A review of the recent five years. Molecules 2017, 22, 1736. [Google Scholar] [CrossRef] [PubMed]
- Najjar, A.; Rajabi, N.; Karaman, R. Recent approaches to platinum (iv) prodrugs: A variety of strategies for enhanced delivery and efficacy. Curr. Pharm. Des. 2017, 23, 2366–2376. [Google Scholar] [CrossRef] [PubMed]
- Denora, N.; Laquintana, V.; Lopedota, A.; Serra, M.; Dazzi, L.; Biggio, G.; Pal, D.; Mitra, A.K.; Latrofa, A.; Trapani, G. Novel l-dopa and dopamine prodrugs containing a 2-phenyl-imidazopyridine moiety. Pharm. Res. 2007, 24, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Nieto, O.; Fontenla, J.A.; Rivas, E.; de Ceballos, M.L.; Fernández-Mayoralas, A. Synthesis of glycosyl derivatives as dopamine prodrugs: Interaction with glucose carrier glut-1. Org. Biomol. Chem. 2003, 1, 767–771. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; Nieto, O.; Rivas, E.; Montenegro, G.; Fontenla, J.A.; Fernández-Mayoralas, A. Synthesis and biological studies of glycosyl dopamine derivatives as potential antiparkinsonian agents. Carbohydr. Res. 2000, 327, 353–365. [Google Scholar] [CrossRef]
- Bonina, F.; Puglia, C.; Rimoli, M.G.; Melisi, D.; Boatto, G.; Nieddu, M.; Calignano, A.; Rana, G.L.; Caprariis, P.D. Glycosyl derivatives of dopamine and l-dopa as anti-parkinson prodrugs: Synthesis, pharmacological activity and in vitro stability studies. J. Drug Target. 2003, 11, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, L.; Viggiano, D.; Viggiano, A.; Abignente, E.; Rimoli, M.G.; Melisi, D.; Curcio, A.; Nieddu, M.; Boatto, G.; Carboni, E. Galactosylated dopamine enters into the brain, blocks the mesocorticolimbic system and modulates activity and scanning time in naples high excitability rats. Neuroscience 2008, 152, 234–244. [Google Scholar] [CrossRef] [PubMed]
- More, S.S.; Vince, R. Design, synthesis and biological evaluation of glutathione peptidomimetics as components of anti-parkinson prodrugs. J. Med. Chem. 2008, 51, 4581–4588. [Google Scholar] [CrossRef] [PubMed]
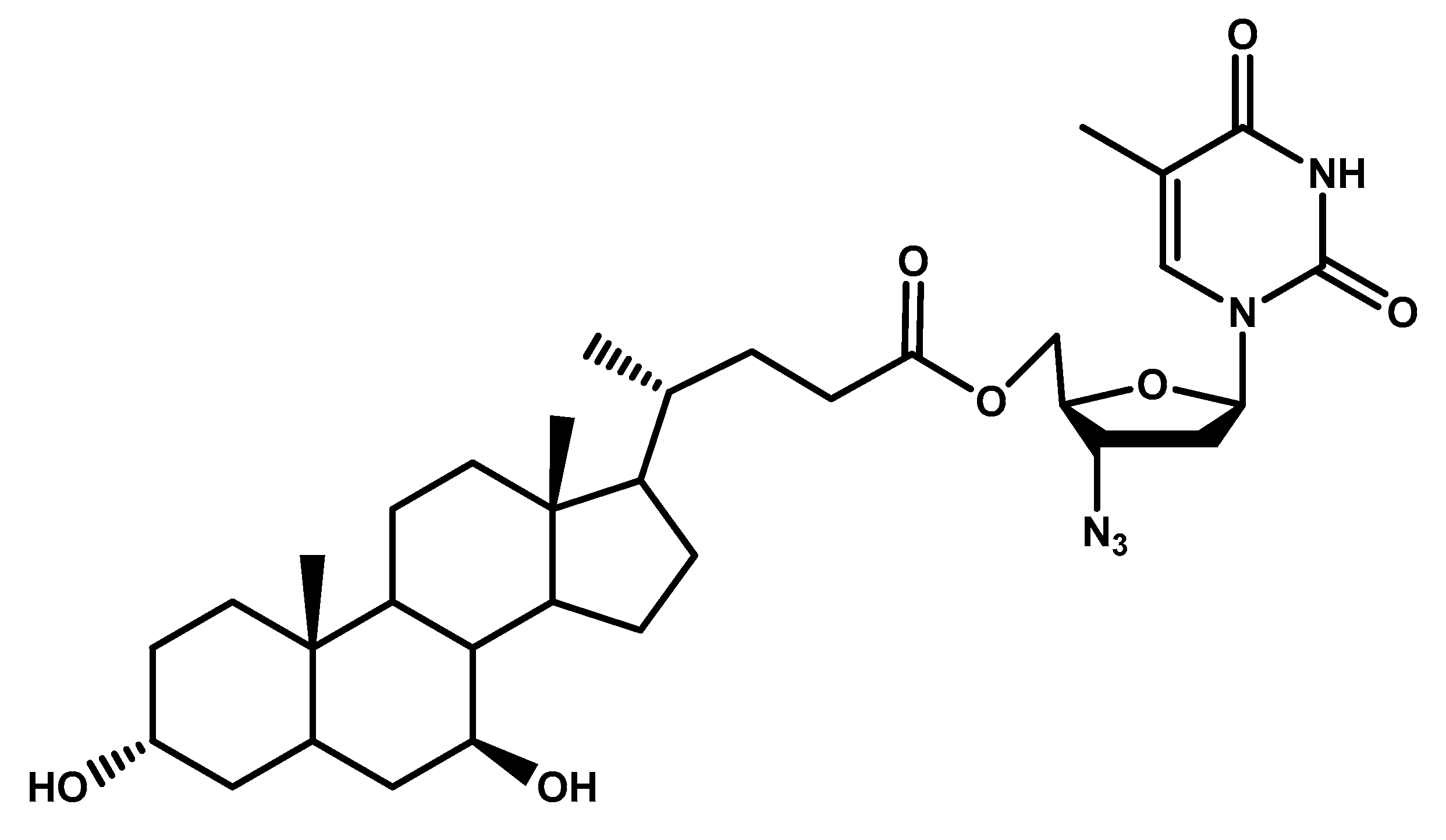
- Dalpiaz, A.; Paganetto, G.; Pavan, B.; Fogagnolo, M.; Medici, A.; Beggiato, S.; Perrone, D. Zidovudine and ursodeoxycholic acid conjugation: Design of a new prodrug potentially able to bypass the active efflux transport systems of the central nervous system. Mol. Pharm. 2012, 9, 957–968. [Google Scholar] [CrossRef] [PubMed]
- Gynther, M.; Laine, K.; Ropponen, J.; Leppänen, J.; Mannila, A.; Nevalainen, T.; Savolainen, J.; Järvinen, T.; Rautio, J. Large neutral amino acid transporter enables brain drug delivery via prodrugs. J. Med. Chem. 2008, 51, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, G.; La Russa, M.; Bruno, V.; Arenare, L.; Ippolito, R.; Copani, A.; Bonina, F.; Nicoletti, F. Systemically administered d-glucose conjugates of 7-chlorokynurenic acid are centrally available and exert anticonvulsant activity in rodents. Brain Res. 2000, 860, 149–156. [Google Scholar] [CrossRef]
- Namanja, H.A.; Emmert, D.; Davis, D.A.; Campos, C.; Miller, D.S.; Hrycyna, C.A.; Chmielewski, J. Toward eradicating hiv reservoirs in the brain: Inhibiting p-glycoprotein at the blood–brain barrier with prodrug abacavir dimers. J. Am. Chem. Soc. 2011, 134, 2976–2980. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, J.; Hrycyna, C. Research spotlight: Tools for eradicating hiv in the brain: Prodrug dimeric inhibitors of p-gp. Ther. Deliv. 2012, 3, 689–692. [Google Scholar] [CrossRef] [PubMed]


| Strategy Tested | Agent | Notes | Future Outlook | Ref. |
|---|---|---|---|---|
| Human insulin receptor monoclonal antibody | None | High affinity and high transcytosis. | Further investigation and optimization. | [33,34] |
| Human insulin receptor monoclonal antibody | Iduronidase & IGG fusion protein | Clinical trials for Hurler syndrome in children. | Further results from trial. | [38] |
| Bispecific antibody | TfR and BACE1 binding sites | Application of dual action antibodies. | Research of binding site combinations. | [42] |
| Peptide vectorization | Doxorubicin with d-penetrin or SynB1 | 6-fold increase in doxorubicin permeation. | d-penetrin or SybB1 can both be used as BBB targeting entities for other drugs. | [53] |
| Transferrin decorated carbon dots | Doxorubicin | Greater efficacy vs free doxorubicin in 4 pediatric cell lines. | Drugs other than doxorubicin can be tested. | [70] |
| Single walled carbon nanotubes | Levodopa | Sustained release properties with low toxicity. | Application as drug carriers in BBB penetration. | [74] |
| Single walled carbon nanotubes coated with PEG and functionalized with lactoferrin | Dopamine | PEG coating increases stability of the NPs while lactoferrin produces favorable striatum accumulation | Testing in mice is promising. Further toxicity and kinetics studies before human application is tested. | [75] |
| Carboxyfullerene NPs | None | Interaction and inhibition with inflammatory factors maintains BBB integrity. | Fullerenes are unexplored with vast potential should solubility be overcome. | [78] |
| PEG-poly(lactic co-glycolic acid) NPs surface modified with lactoferrin | None | Administration intranasally for CNS delivery. Low toxicity and enhanced cellular uptake. | Lactoferrin modification of NPs for CNS delivery. | [79] |
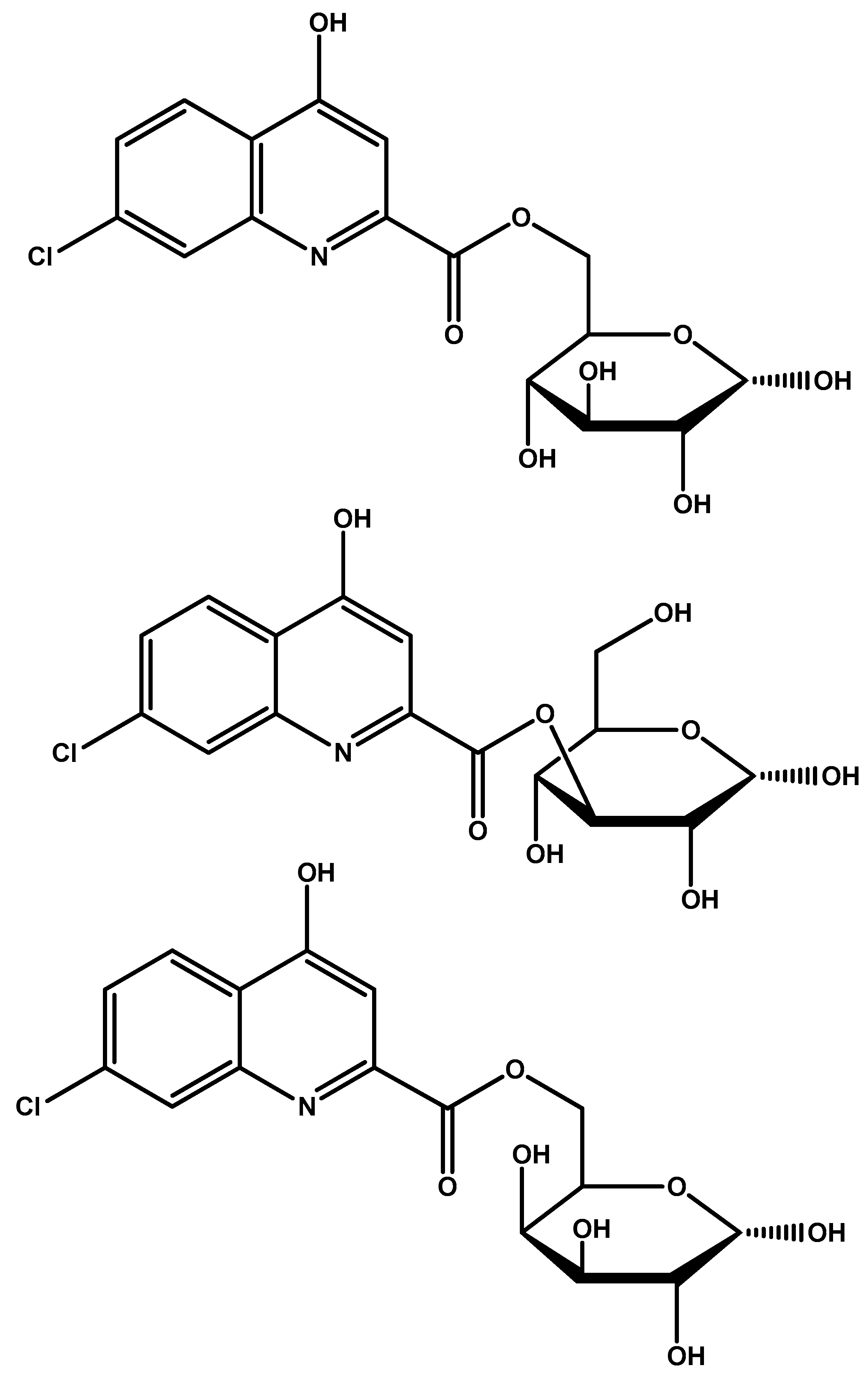
| Glycosylated derivatives of l-DOPA prodrugs | l-DOPA | Carbamate derivatives are more stable than ester ones. Glycosylated derivatives at C-6 is better than C-3. | Glycosylation at C-6 provides better inhibition and uptake through GLUT-1 | [88,89,90,91] |
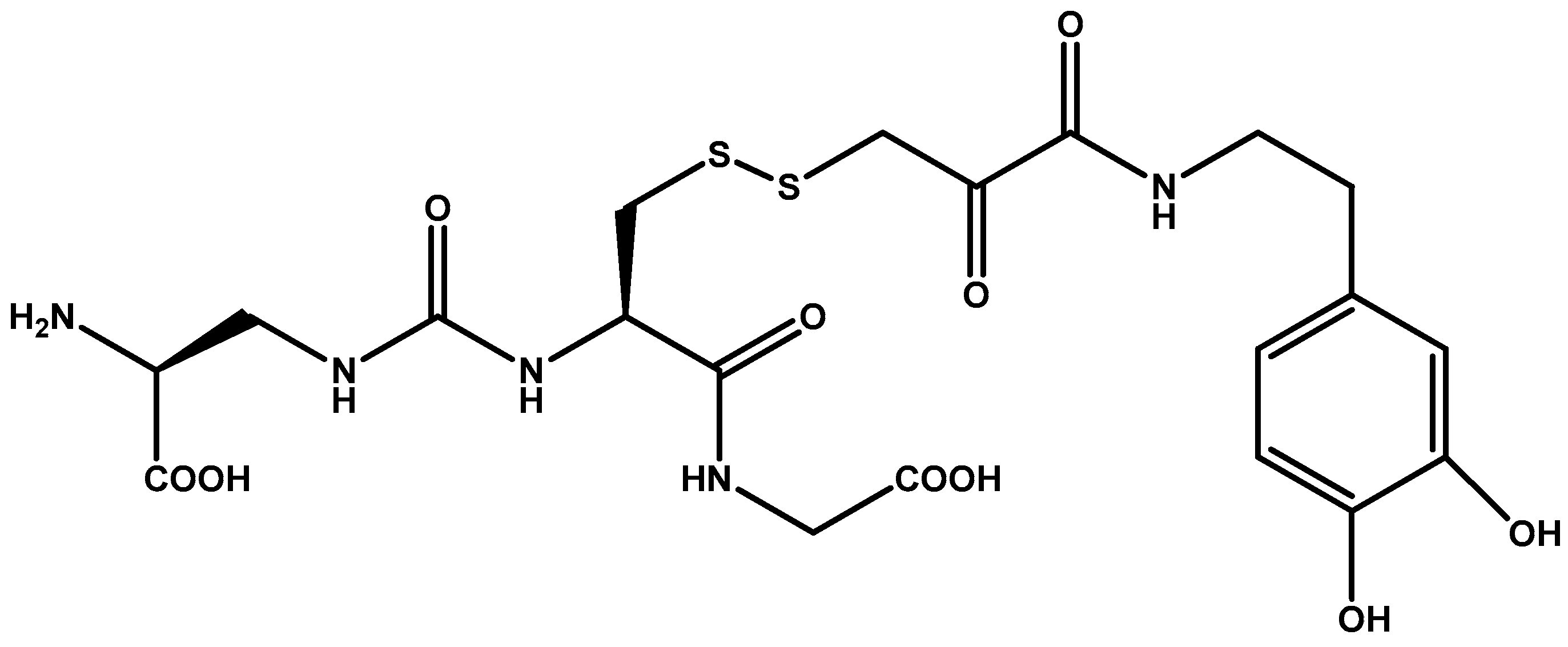
| Dimer prodrug | Abacavir | Sulphide and ester linkages between two P-gp substrates increases BBB penetration. | Two identical or different P-gp substrates allows for dual-drug delivery. | [96] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeiadeh, I.; Najjar, A.; Karaman, R. Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review. Molecules 2018, 23, 1289. https://doi.org/10.3390/molecules23061289
Zeiadeh I, Najjar A, Karaman R. Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review. Molecules. 2018; 23(6):1289. https://doi.org/10.3390/molecules23061289
Chicago/Turabian StyleZeiadeh, Isra’, Anas Najjar, and Rafik Karaman. 2018. "Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review" Molecules 23, no. 6: 1289. https://doi.org/10.3390/molecules23061289
APA StyleZeiadeh, I., Najjar, A., & Karaman, R. (2018). Strategies for Enhancing the Permeation of CNS-Active Drugs through the Blood-Brain Barrier: A Review. Molecules, 23(6), 1289. https://doi.org/10.3390/molecules23061289






