Serum Protein Electrophoretic Pattern in Neonatal Calves Treated with Clinoptilolite
Abstract
:1. Introduction
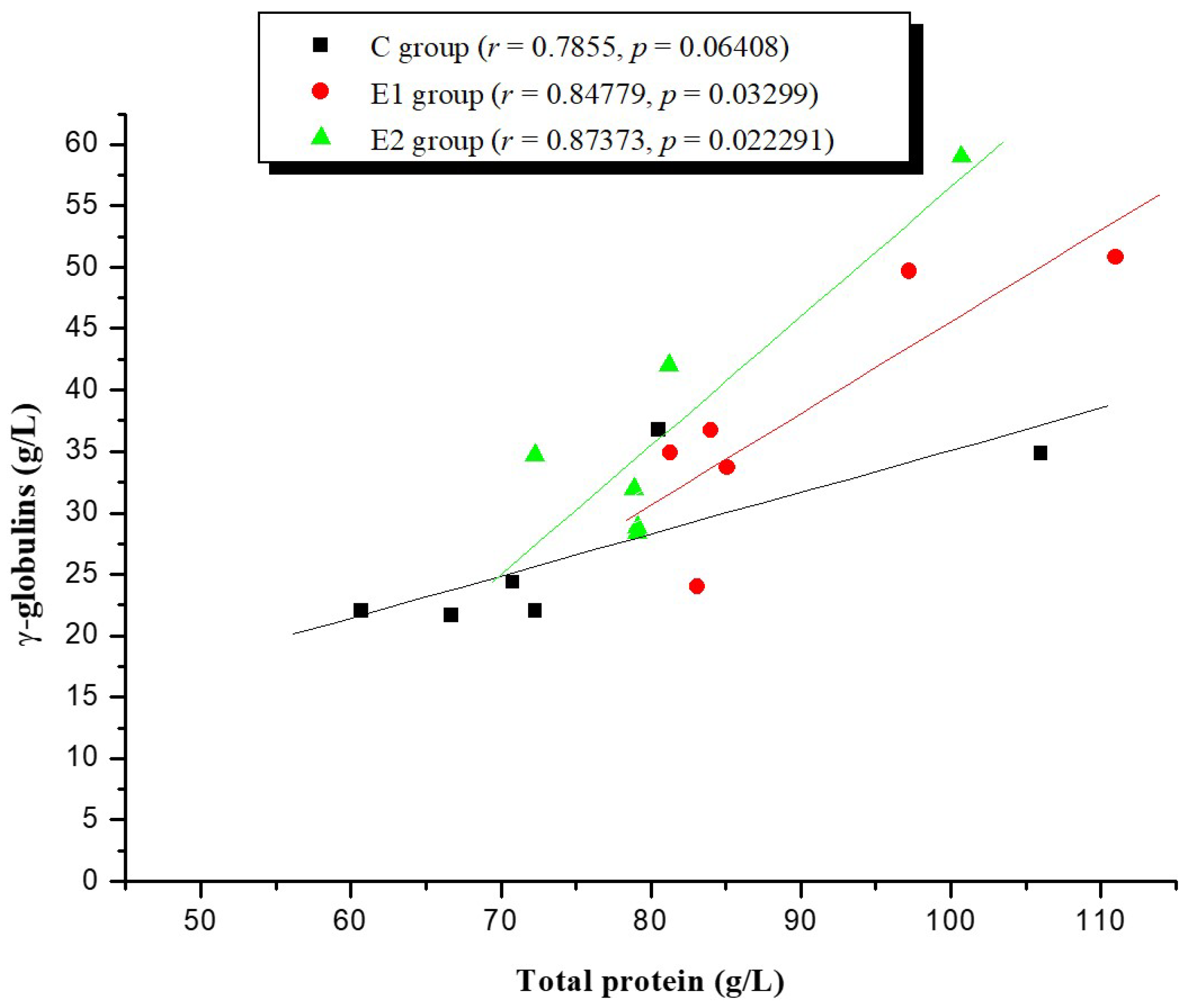
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals Experiment
4.2. Clinoptilolite
4.3. Quantitative Analysis of Total Protein
4.4. Electrophoresis Analysis of Serum Protein Fractions
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Davis, C.L.; Drackley, J.K. The Development, Nutrition and Management of the Young Calf; Iowa State University Press: Iowa City, IA, USA, 1998; pp. 180–205. ISBN 9780813829807. [Google Scholar]
- Tizard, I.R. Immunity in the fetus and newborn. In Veterinary Immunology. An Introduction, 4th ed.; W.B. Saunders College Publishing: Philadelphia, PA, USA, 1992; pp. 248–260. ISBN 0-7216-4686-7. [Google Scholar]
- Blattler, U.; Hammon, H.M.; Morel, C.; Philipona, C.; Rauprich, A.; Rome, V.; Le Huerou-Luron, I.; Guilloteau, P.; Blum, J.W. Feeding colostrum, its composition and feeding duration variably modify proliferation and morphology of the intestine and digestive enzyme activities of neonatal calves. J. Nutr. 2001, 131, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Chigerwe, M.; Tyler, J.W.; Summers, M.K.; Middleton, J.R.; Schultz, L.G.; Nagy, D.W. Evaluation of factors affecting serum IgG concentrations in bottle-fed calves. J. Am. Vet. Med. Assoc. 2009, 234, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Mohri, M.; Seifi, H.A.; Daraei, F. Effects of short-term supplementation of clinoptilolite in colostrum and milk on hematology, serum proteins, performance, and health in neonatal dairy calves. Food Chem. Toxicol. 2008, 46, 2112–2117. [Google Scholar] [CrossRef] [PubMed]
- Weaver, D.M.; Tyler, J.W.; Van Metre, D.C.; Hostetler, D.E.; Barrington, G.M. Passive transfer of colostral immunoglobulins in calves-review. J. Vet. Intern. Med. 2000, 14, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Fratrić, N.; Stojić, V.; Janković, D.; Šamanc, H.; Gvozdić, D. The effect of a clinoptilolit based mineral adsorber on concentrations of immunoglobulin G in the serum of newborn calves fed different amounts of colostrum. Acta Vet. 2005, 55, 11–21. [Google Scholar]
- Fratrić, N.; Stojić, V.; Rajcić, S.; Radojičić, B. The effect of mineral adsorbent in calf diet colostrum on the levels of serum immunoglobulin G, protein and glucose. Acta Vet. 2007, 57, 169–180. [Google Scholar]
- Gvozdić, D.; Stojić, V.; Fratrić, N.; Pesut, O.; Jovanović, I.; Kirovski, D.; Šamanc, H.; Vujanać, I. Eficiency of immunoglobulin absorption in newborn calves receiving oral clinoptilolite treatement. Sci. Pap. Vet. Med. Timis. 2007, XL, 234–242. [Google Scholar]
- Step, D.S.; Litherland, N.B.; Burciaga-Roble, O.; Breshears, M.; Krehbiel, C.R.; Confer, A.W.; Fulton, R.W.; Morgan, G.L.; Thornsberry, M.; Fassig, S.M. Clinical observations, biochemical data and postmortem and histopathologic findings in young dairy calves fed zeolite clinoptilolite binder combined with milk replacer. Am. J. Vet. Res. 2008, 69, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Mumpton, F.A. La roca magica: Uses of natural zeolites in agriculture and industry. Proc. Natl. Acad. Sci. USA 1999, 96, 3463–3470. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.; Pavelic, K. Antiviral properties of clinoptilolite. Microporous Mesoporous Mater. 2005, 79, 165–169. [Google Scholar] [CrossRef]
- Ivković, S.; Deutsch, U.; Silberbach, A.; Walraph, E.; Mannel, M. Dietary supplementation with the tribomechanically activated zeolite clinoptilolite in immunodeficiency: Effects on the immune system. Adv. Ther. 2004, 21, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Pavelic, K.; Hadzija, M.; Bedrica, L.; Pavelic, J.; Dikic, I.; Katic, M.; Kralj, M.; Bosnar, M.H.; Kapitanovic, S.; Poljak-Blazi, M.; et al. Natural zeolite clinoptilolite: New adjuvant in anticancer therapy. J. Mol. Med. 2001, 78, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Sverko, V.; Sobocanec, S.; Balog, T.; Colic, M.; Marotti, T. Natural micronised clinoptilolite mixtures with Urtica dioica L extract as possible antioxidants. Food Technol. Biotechnol. 2004, 42, 189–192. [Google Scholar]
- Papaioannou, D.; Katsoulos, P.D.; Panousis, N.; Karatzias, H. The role of natural and synthetic zeolites as feed additives on the prevention and/or the treatament of certain farm animal diseases: A review. Microporous Mesoporous Mater. 2005, 84, 161–170. [Google Scholar] [CrossRef]
- Thilsing-Hansen, T.; Jorgensen, R.J.; Enemark, J.M.D.; Iarsen, T. The effect of zeolite A supplementation in the dry period of periparturient calcium, phosphorus and magnesium homeostasis. J. Dairy Sci. 2002, 85, 1855–1862. [Google Scholar] [CrossRef]
- Piccione, G.; Alberghina, D.; Marafioti, S.; Giannetto, C.; Casella, S.; Assenza, A.; Fazio, F. Electrophoretic serum protein fraction profile during the different physiological phases in Comisana ewes. Reprod. Domest. Anim. 2012, 47, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Piccione, G.; Casella, S.; Giannetto, C.; Panzera, M.; Pennisi, P.; Alberghina, D. Influence of short-term storage on electrophoretic profile of bovine serum proteins. J. Appl. Anim. Res. 2014, 42, 123–125. [Google Scholar] [CrossRef]
- Tothova, C.; Nagy, O.; Seidel, H.; Kovac, G. The effect of storage on the protein electrophoretic pattern in bovine serum. Iran. J. Vet. Sci. Technol. 2010, 2, 77–84. [Google Scholar]
- Tothova, C.; Nagy, O.; Seidel, H.; Kovac, G. Serum protein electrophoretic pattern in clinically healthy calves and cows determined by agarose gel electrophoresis. Comp. Clin. Pathol. 2013, 22, 15–20. [Google Scholar] [CrossRef]
- Zarcula, S.; Cernescu, H.; Mircu, C.; Tulcan, C.; Morvay, A.; Baul, S.; Popovici, D. Influence of Breed, Parity and Food Intake on Chemical Composition of First Colostrum in Cow. Sci. Pap. Anim. Sci. Biotechnol. 2010, 43, 154–158. [Google Scholar]
- Bgatova, N.P. Influence of long-term administration of naturally occuring sorbents on the ultrastructure of small intestine enterocyte in rats. Bull. Exp. Biol. Med. 1998, 6, 626–629. [Google Scholar] [CrossRef]
- Pârvu, G. Supravegherea Nutriţional-Metabolică a Animalelor; Editura Ceres: Bucureşti, Romania, 1992; pp. 233–248. [Google Scholar]
- Suh, G.H.; Hur, T.Y.; Son, D.S.; Choe, C.Y.; Jung, Y.H.; Ahn, B.S.; Lee, C.Y.; Lee, C.G. Differences in the serum immunoglobulin concentration between dairy and beef calves from birth to 14 days of age. J. Vet. Sci. 2003, 4, 257–260. [Google Scholar] [PubMed]
- Piccione, G.; Casella, S.; Giannetto, C.; Vazzana, I.; Niutta, P.; Giudice, E. Influence of age on profile of serum proteins in the calf. Acta Vet. 2009, 59, 413–422. [Google Scholar]
- Sadeghi, A.; Shawrang, P. Effects of natural zeolite clinoptilolite on passive immunity and diarrhea in newborn calves. Livest. Sci. 2008, 113, 307–310. [Google Scholar] [CrossRef]
- Quigley, J. Passive Immunity in Newborn Calves. Adv. Dairy Technol. 2002, 14, 273–292. [Google Scholar]
- Zarcula, S.; Tulcan, C.; Šamanc, H.; Kirovski, D.; Cernescu, H.; Mircu, C. Clinical observations in calves fed colostrum supplimented with clinoptilolite. Sci. Pap. Vet. Med. Timis. 2010, XLIII, 64–69. [Google Scholar]
- Šamanc, H.; Kirovski, D.; Adamović, M.; Vujanać, I.; Fratrić, N.; Prodanović, R. Effects of natural zeolite on body weight, weight gain, hematology and biochemical blood parameters in calves. Vet. Glas. 2008, 62, 153–166. [Google Scholar] [CrossRef]
- Mumpton, F.A.; Fishman, P.H. The application of natural zeolites in animal science and aquaculture. J. Anim. Sci. 1977, 45, 1188–1203. [Google Scholar] [CrossRef]
- Rodriguez-Fuentes, G.; Barrios, M.A.; Iraizoz, A.; Perdomo, I.; Cedre, B. Enterex: Anti-diarrheic drug based on purified natural clinoptilolite. Zeolites 1997, 19, 441–448. [Google Scholar] [CrossRef]
- Papaioannou, D.S.; Kyriakis, C.S.; Alexopoulos, C.; Tzika, E.D.; Polizopoulou, Z.S.; Kyriakis, S.C. A field study on the effect on the dietary use of a clinoptilolite-rich tuff, alone or in combination with certain antimicrobials, on the health status and performance of weaned, growing and finishing pigs. Res. Vet. Sci. 2004, 76, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Stojić, V.; Gvozdić, D.; Nikolić, J.; Šamanc, H.; Jovanović, I.; Tomašević-Čanović, M.; Vujanac, I. The serum levels of insulin and IGF-1 in newborn piglets treated with clinoptilolite. Acta Vet. 2003, 53, 219–228. [Google Scholar]
- Stojić, V.; Gvozdić, D.; Šamanc, H.; Jovanović, I.; Fratrić, N. Thyroid gland hormones in newborn calves treated with clinoptilolite receiving different amounts of colostrum. Acta Vet. 2005, 55, 3–10. [Google Scholar]
Sample Availability: Not available. |

| Parameters | Group | Parturition | 6 h | 16 h | 30 h |
|---|---|---|---|---|---|
| Total protein (g/L) | C | 42.65 ± 6.26 | 55.40 ± 5.28 | 74.03 ± 3.89 *,** | 76.17 ± 6.54 *,** |
| E1 | 46.40 ± 1.32 | 72.23 ± 7.12 * | 84.35 ± 4.50 *,** | 90.28 ± 4.75 *,** | |
| E2 | 38.28 ± 5.65 | 54.73 ± 10.73 | 66.40 ± 12.53 * | 81.88 ± 3.96 *,** | |
| Albumin (g/L) | C | 32.86 ± 4.67 | 27.69 ± 3.74 | 32.47 ± 3.28 | 36.43 ± 3.57 |
| E1 | 33.36 ± 1.32 | 34.72 ± 8.52 | 32.88 ± 4.33 | 34.86 ± 1.97 | |
| E2 | 28.69 ± 4.68 | 25.94 ± 5.02 | 23.42 ± 3.83 | 29.16 ± 3.15 | |
| α1-globulin (g/L) | C | 1.40 ± 0.21 | 1.54 ± 0.33 | 2.42 ± 0.40 | 2.71 ± 0.48 |
| E1 | 2.54 ± 0.61 | 2.06 ± 0.84 | 4.23 ± 1.78 | 4.04 ± 0.56 | |
| E2 | 0.94 ± 0.18 | 1.12 ± 0.28 | 0.83 ± 0.19 | 2.32 ± 0.60 * | |
| α2-globulin (g/L) | C | 2.43 ± 0.57 | 3.82 ± 1.31 | 4.23 ± 1.69 | 2.24 ± 0.36 |
| E1 | 4.15 ± 2.01 | 8.10 ± 1.85 | 4.48 ± 0.57 | 2.98 ± 0.19 | |
| E2 | 4.01 ± 0.32 | 5.72 ± 1.12 | 5.33 ± 1.67 | 3.66 ± 0.96 | |
| β-globulin (g/L) | C | 4.41 ± 1.34 | 6.89 ± 1.58 | 7.03 ± 1.17 | 7.83 ± 0.85 * |
| E1 | 5.13 ± 1.20 | 8.56 ± 1.27 | 8.71 ± 0.40 | 10.06 ± 0.74 * | |
| E2 | 4.08 ± 0.83 | 8.14 ± 1.24 * | 8.71 ± 2.49 | 9.28 ± 0.52 * | |
| γ-globulin (g/L) | C | 1.50 ± 0.48 | 15.86 ± 4.06 * | 27.83 ± 3.85 *,** | 26.95 ± 2.84 *,** |
| E1 | 0.68 ± 0.26 | 18.68 ± 3.56 * | 34.03 ± 3.32 *,** | 38.30 ± 4.19 *,**,a | |
| E2 | 0.57 ± 0.18 | 13.81 ± 4.87 * | 28.01 ± 7.48 *,** | 37.47 ± 4.77 *,** | |
| A/G | C | 3.90 ± 0.67 | 1.16 ± 0.24 * | 0.85 ± 0.14 *,** | 0.92 ± 0.06 *,** |
| E1 | 3.04 ± 0.50 | 1.02 ± 0.30 * | 0.66 ± 0.12 *,** | 0.65 ± 0.08 *,**,a | |
| E2 | 2.88 ± 0.27 | 0.92 ± 0.08 * | 0.60 ± 0.06 *,** | 0.59 ± 0.10 *,**,a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, S.; Kirovski, D.; Mircu, C.; Hutu, I.; Otavă, G.; Paul, C.; Boldura, O.M.; Tulcan, C. Serum Protein Electrophoretic Pattern in Neonatal Calves Treated with Clinoptilolite. Molecules 2018, 23, 1278. https://doi.org/10.3390/molecules23061278
Marc S, Kirovski D, Mircu C, Hutu I, Otavă G, Paul C, Boldura OM, Tulcan C. Serum Protein Electrophoretic Pattern in Neonatal Calves Treated with Clinoptilolite. Molecules. 2018; 23(6):1278. https://doi.org/10.3390/molecules23061278
Chicago/Turabian StyleMarc, Simona, Danijela Kirovski, Călin Mircu, Ioan Hutu, Gabriel Otavă, Cristina Paul, Oana Maria Boldura, and Camelia Tulcan. 2018. "Serum Protein Electrophoretic Pattern in Neonatal Calves Treated with Clinoptilolite" Molecules 23, no. 6: 1278. https://doi.org/10.3390/molecules23061278
APA StyleMarc, S., Kirovski, D., Mircu, C., Hutu, I., Otavă, G., Paul, C., Boldura, O. M., & Tulcan, C. (2018). Serum Protein Electrophoretic Pattern in Neonatal Calves Treated with Clinoptilolite. Molecules, 23(6), 1278. https://doi.org/10.3390/molecules23061278









