The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells
Abstract
1. Introduction
- (i)
- the expression of antigen presenting molecules from the class II major histocompatibility complex (MHC II) and of co-stimulatory molecules (CD80, CD86);
- (ii)
- the secretion of pro-inflammatory cytokines (TNFα, IL1β, IL12…), chemokines (CCL2, CCL5…), nitric oxide (NO), and reactive oxygen species (ROS, such as superoxide anions). All are necessary to eradicate the aggressive agent.
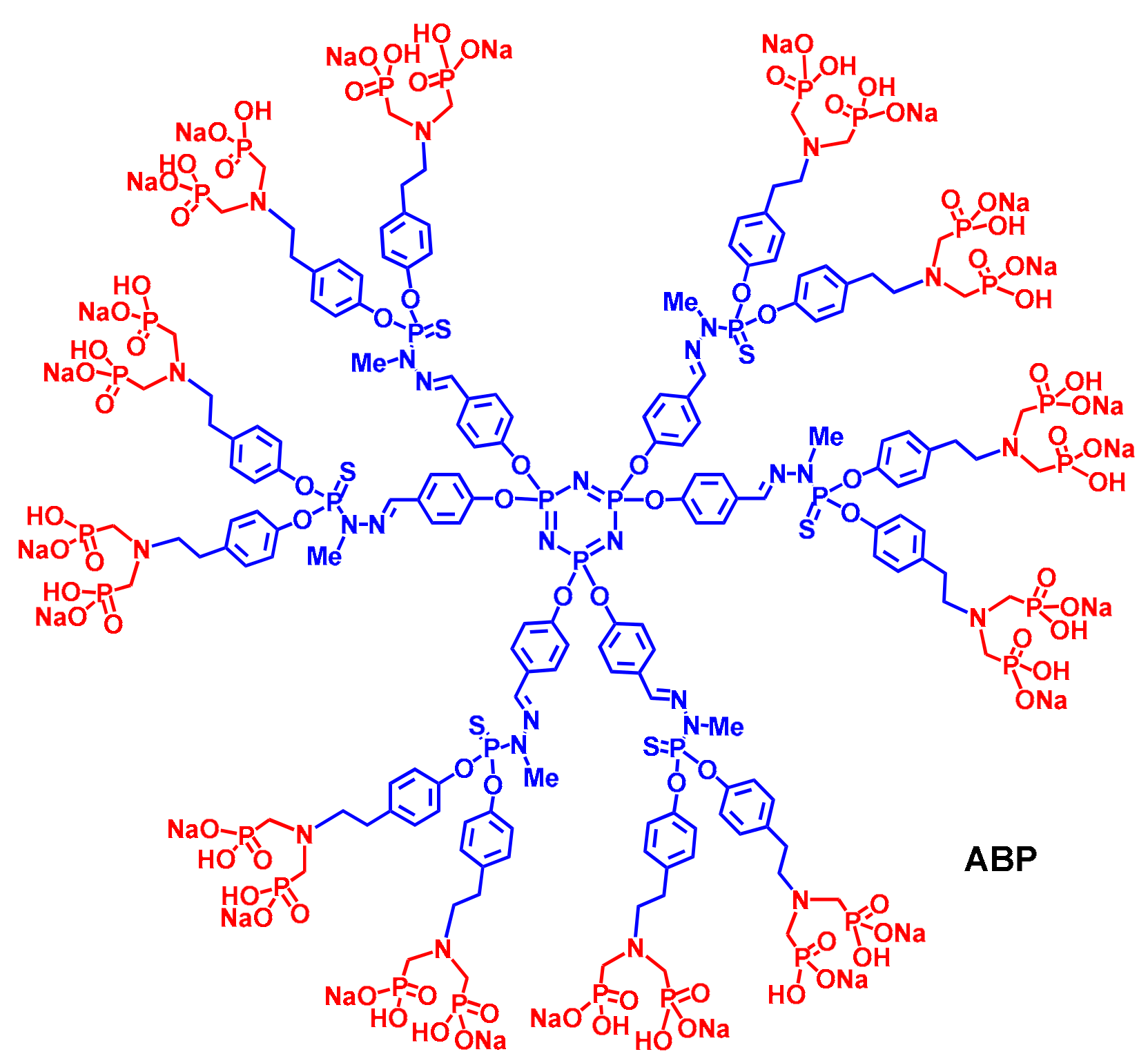
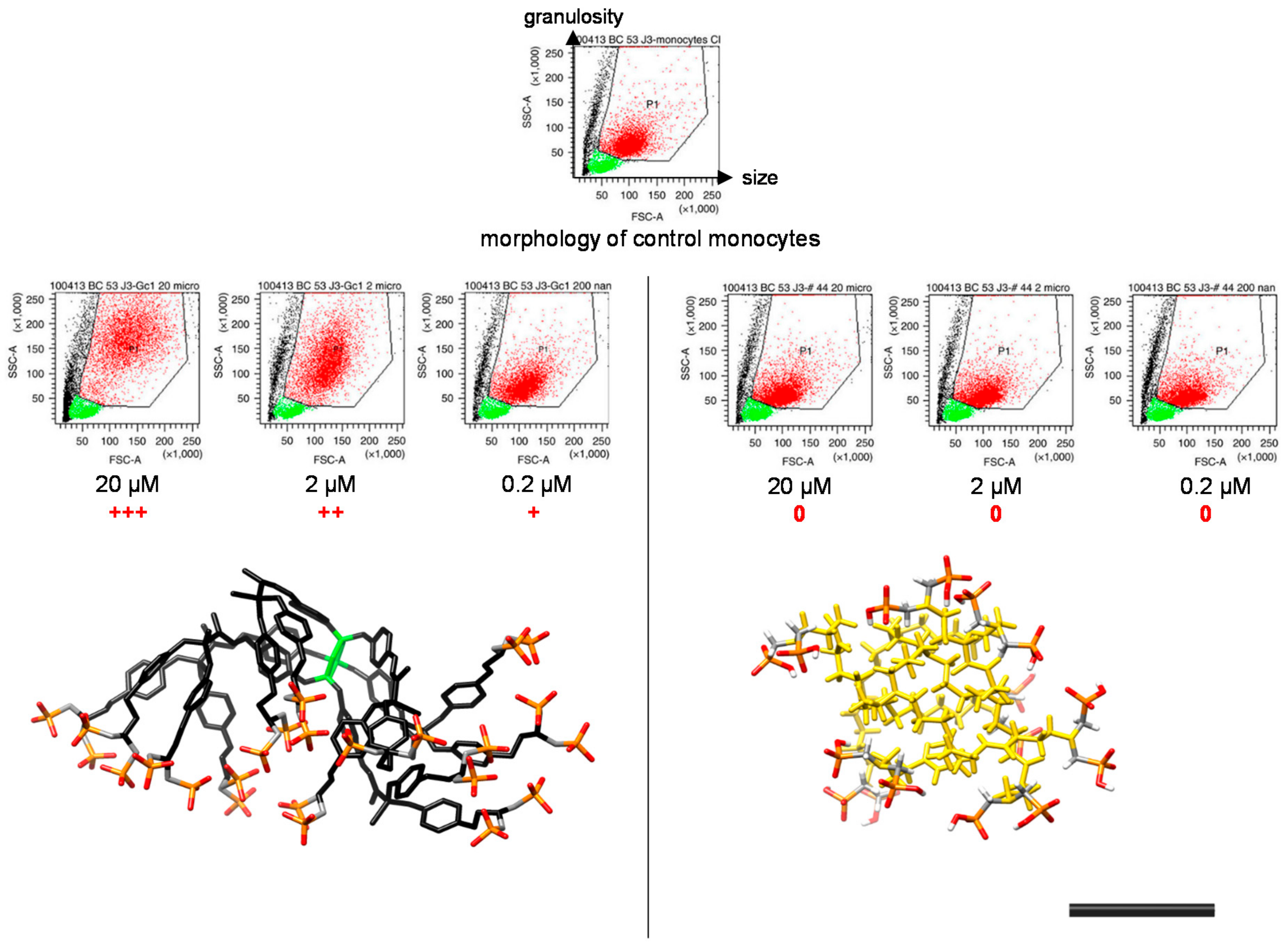
2. The ABP Dendrimer, Birth of a “Lead” Molecule
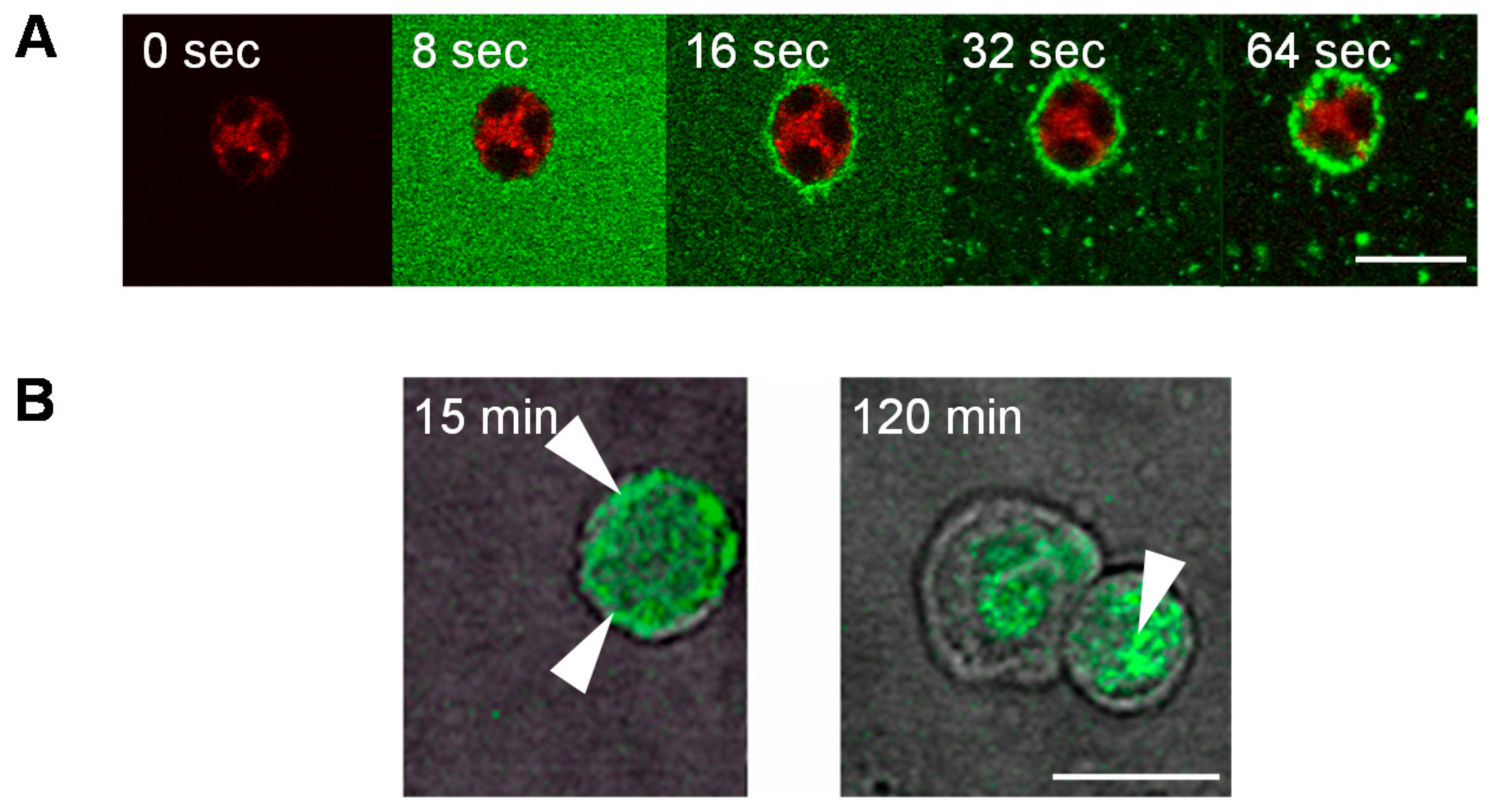
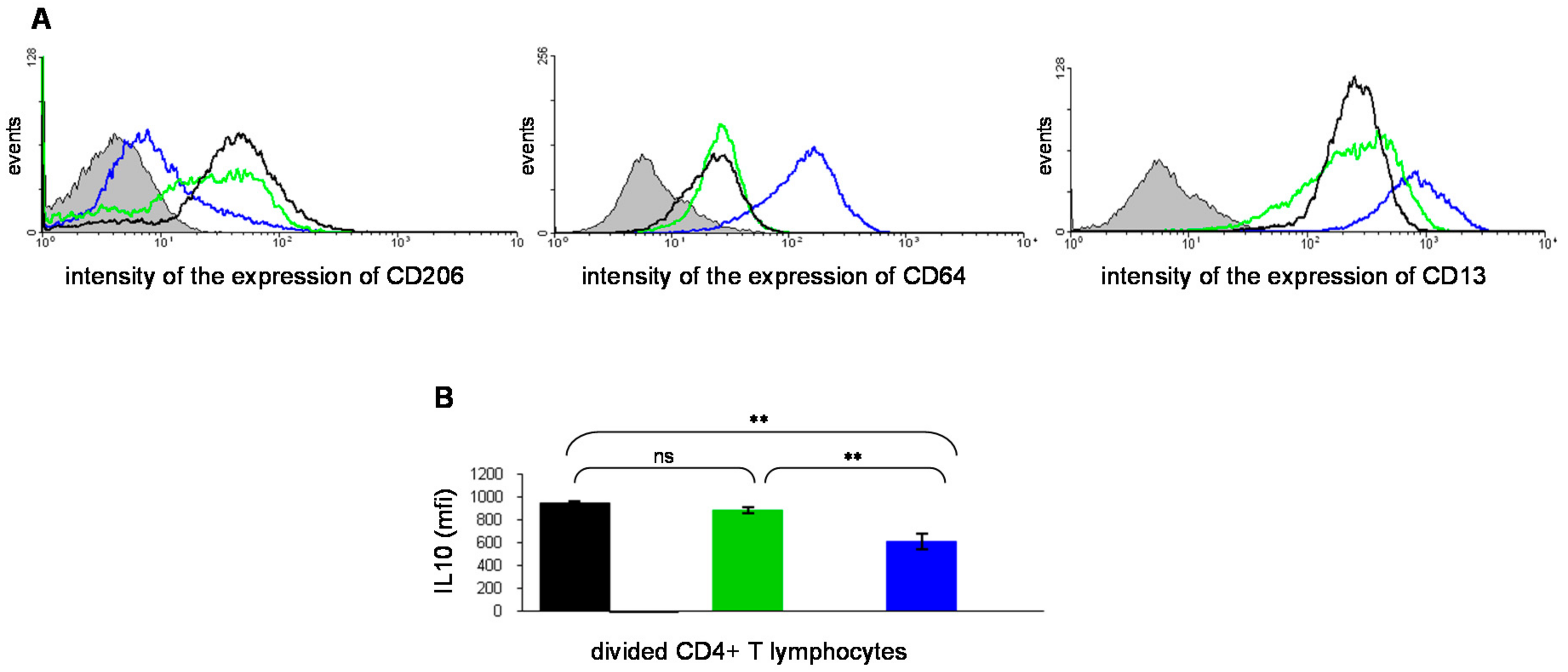
3. The ABP Dendrimer, an Immuno-Modulatory New Chemical Entity
4. Proof of Efficacy of the ABP Dendrimer in Animal Models of Inflammatory Diseases
4.1. Experimental Arthritis, Mouse Model of Rheumatoid Arthritis (RA)
4.2. Endotoxin-Induced Uveitis (EUI), Rat Model of Anterior Uveitis
4.3. Experimental Auto-Immune Encephalomyelitis (EAE), Mouse Model of Multiple Sclerosis (MS)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murray, P.J. Immune regulation by monocytes. Semin. Immunol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Perretti, M.; Leroy, X.; Bland, E.J.; Montero-Melendez, T. Resolution pharmacology: Opportunities for therapeutic innovation in inflammation. Trends Pharmacol. Sci. 2015, 36, 737–755. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.A.; Sousa, L.P.; Pinho, V.; Perretti, M.; Teixeira, M.M. Resolution of inflammation: What controls its onset? Front. Immunol. 2016, 7, 160. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Gomez, A.; Perretti, M.; Soehnlein, O. Resolution of inflammation: An integrated view. EMBO Mol. Med. 2013, 5, 661–674. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; Vieira, P.; Fiorentino, D.F.; Trounstine, M.L.; Khan, T.A.; Mosmann, T.R. Homology of cytokine synthesis inhibitory factor (IL-10) to the Epstein-Barr virus gene BCRFI. Science 1990, 248, 1230–1234. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yan, W.; Zheng, H.; Du, Q.; Zhang, L.; Ban, Y.; Li, N.; Wei, F. Regulation of IL-10 and IL-12 production and function in macrophages and dendritic cells. F1000Research 2015, 4, 1465. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-10 production by effector T cells: Th1 cells show self-control. J. Exp. Med. 2007, 204, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Geginat, J.; Larghi, P.; Paroni, M.; Nizzoli, G.; Penatti, A.; Pagani, M.; Gagliani, N.; Meroni, P.; Abrignani, S.; Flavell, R.A. The light and the dark sides of interleukin-10 in immune-mediated diseases and cancer. Cytokine Growth Factor Rev. 2016, 30, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Llorente, L.; Richaud-Patin, Y.; Garcia-Padilla, C.; Claret, E.; Jakez-Ocampo, J.; Cardiel, M.H.; Alcocer-Varela, J.; Grangeot-Keros, L.; Alarcon-Segovia, D.; Wijdenes, J.; et al. Clinical and biologic effects of anti-interleukin-10 monoclonal antibody administration in systemic lupus erythematosus. Arthritis Rheum. 2000, 43, 1790–1800. [Google Scholar] [CrossRef]
- Gatto, M.; Saccon, F.; Zen, M.; Bettio, S.; Iaccarino, L.; Punzi, L.; Doria, A. Success and failure of biological treatments in systemic lupus erythematosus: A critical review. J. Autoimmun. 2016, 74, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Doria, A.; Cervera, R.; Gatto, M.; Chehab, G.; Schneider, M. The new targeted therapy in systemic lupus erythematosus: Is the glass half-full or half-empty? Autoimmun. Rev. 2017, 16, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Papadopoulos, K.P.; Autio, K.A.; Ott, P.A.; Patel, M.R.; Wong, D.J.; Falchook, G.S.; Pant, S.; Whiteside, M.; Rasco, D.R.; et al. Safety, antitumor activity, and immune activation of pegylated recombinant human interleukin-10 (AM0010) in patients with advanced solid tumors. J. Clin. Oncol. 2016, 34, 3562–3569. [Google Scholar] [CrossRef] [PubMed]
- Zanin-Zhorov, A.; Weiss, J.M.; Trzeciak, A.; Chen, W.; Zhang, J.; Nyuydzefe, M.S.; Arencibia, C.; Polimera, S.; Schueller, O.; Fuentes-Duculan, J.; et al. Selective oral ROCK2 inhibitor reduces clinical scores in patients with psoriasis vulgaris and normalizes skin pathology via concurrent regulation of IL-17 and IL-10. J. Immunol. 2017, 198, 3809–3814. [Google Scholar] [CrossRef] [PubMed]
- Chernykh, E.R.; Shevela, E.Y.; Starostina, N.M.; Morozov, S.A.; Davydova, M.N.; Menyaeva, E.V.; Ostanin, A.A. Safety and therapeutic potential of M2 macrophages in stroke treatment. Cell Transplant. 2016, 25, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Belmant, C.; Sicard, H.; Poupot, R.; Bonneville, M.; Fournié, J.J. Y2K+1 state-of-the-art on non-peptide phosphoantigens, a novel category of immunostimulatory molecules. Microbes Infect. 2001, 3, 645–654. [Google Scholar] [CrossRef]
- Martinet, L.; Poupot, R.; Fournié, J.J. Pitfalls on the roadmap to gammadelta T cell-based cancer immunotherapies. Immunol. Lett. 2009, 124, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Fruchon, S.; Fournié, J.J.; Poupot, M.; Poupot, R. Anti-inflammatory properties of dendrimers per se. Sci. World J. 2011, 11, 1367–1382. [Google Scholar] [CrossRef] [PubMed]
- Poupot, M.; Griffe, L.; Marchand, P.; Maraval, A.; Rolland, O.; Martinet, L.; L’Faqihi-Olive, F.E.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; et al. Design of phosphorylated dendritic architectures to promote human monocyte activation. FASEB J. 2006, 20, 2339–2351. [Google Scholar] [CrossRef] [PubMed]
- Mackaness, G.B. The immunological basis of acquired cellular resistance. J. Exp. Med. 1964, 120, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: A marker of alternative immunologic macrophage activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Poupot, M.; Martinet, L.; Turrin, C.O.; Majoral, J.P.; Fournié, J.J.; Caminade, A.M.; Poupot, R. Anti-inflammatory and immuno-suppressive activation of human monocytes by a bio-active dendrimer. J. Leukoc. Biol. 2009, 85, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Marchand, P.; Griffe, L.; Poupot, M.; Turrin, C.O.; Bacquet, G.; Fournié, J.J.; Majoral, J.P.; Poupot, R.; Caminade, A.M. Dendrimers ended by non-symmetrical azadiphosphonate groups: Synthesis and immunological properties. Bioorg. Med. Chem. Lett. 2009, 19, 3963–3966. [Google Scholar] [CrossRef] [PubMed]
- Rolland, O.; Turrin, C.O.; Bacquet, G.; Poupot, R.; Poupot, M.; Caminade, A.M.; Majoral, J.P. Efficient synthesis of phosphorus-containing dendrimers capped with isosteric functions of amino-bis(methylene) phosphonic acids. Tetrahedron Lett. 2009, 50, 2078–2082. [Google Scholar] [CrossRef]
- Ledall, J.; Fruchon, S.; Garzoni, M.; Pavan, G.M.; Caminade, A.M.; Turrin, C.O.; Blanzat, M.; Poupot, R. Interaction studies reveal specific recognition of an anti-inflammatory polyphosphorhydrazone dendrimer by human monocytes. Nanoscale 2015, 7, 17672–17684. [Google Scholar] [CrossRef] [PubMed]
- Rolland, O.; Griffe, L.; Poupot, M.; Maraval, A.; Ouali, A.; Coppel, Y.; Fournié, J.J.; Bacquet, G.; Turrin, C.O.; Caminade, A.M.; et al. Tailored control and optimization of the number of phosphonic acid termini on phosphorus-containing dendrimers for the ex-vivo activation of human monocytes. Chem. Eur. J. 2008, 14, 4836–4850. [Google Scholar] [CrossRef] [PubMed]
- Ielasi, F.; Ledall, J.; Anes, A.P.; Fruchon, S.; Caminade, A.M.; Poupot, R.; Turrin, C.O.; Blanzat, M. Influence of PPH dendrimers’ surface functions on the activation of human monocytes: A study of their interactions with pure lipid model systems. Phys. Chem. Chem. Phys. 2016, 18, 21871–21880. [Google Scholar] [CrossRef] [PubMed]
- Caminade, A.M.; Fruchon, S.; Turrin, C.O.; Poupot, M.; Ouali, A.; Maraval, A.; Garzoni, M.; Maly, M.; Furer, V.; Kovalenko, V.; et al. The key role of the scaffold on the efficiency of dendrimer nanodrugs. Nat. Commun. 2015, 6, 7722. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Garzoni, M.; Bochicchio, D.; Caminade, A.M.; Couderc, F.; Ong-Meang, V.; Davignon, J.L.; Turrin, C.O.; Pavan, G.M.; Poupot, R. Three-dimensional directionality is a pivotal structural feature for the bioactivity of azabisphosphonate-capped poly(phosphorhydrazone) nanodrug dendrimers. Biomacromolecules 2018, 19, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Griffe, L.; Poupot, M.; Marchand, P.; Maraval, A.; Turrin, C.O.; Rolland, O.; Métivier, P.; Bacquet, G.; Fournié, J.J.; Caminade, A.M.; et al. Multiplication of human Natural Killer cells by nanosized phosphonate-capped dendrimers. Angew. Chem. Int. Edit. 2007, 46, 2523–2526. [Google Scholar] [CrossRef] [PubMed]
- Rezvani, K.; Rouce, R.; Liu, E.; Schpall, E. Engineering Natural Killer cells for cancer immunotherapy. Mol. Ther. 2017, 25, 1769–1781. [Google Scholar] [CrossRef] [PubMed]
- Poupot, M.; Turrin, C.O.; Caminade, A.M.; Fournié, J.J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against Multiple Myeloma. Nanomedicine 2016, 12, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Poupot, M.; Fournié, J.J.; Poupot, R. Trogocytosis and killing of IL4-polarized monocytes by autologous NK cells. J. Leukoc. Biol. 2008, 84, 1298–1305. [Google Scholar] [CrossRef] [PubMed]
- Pont, F.; Familiades, J.; Déjean, S.; Fruchon, S.; Cendron, D.; Poupot, M.; Poupot, R.; L’Faqihi-Olive, F.; Prade, N.; Ycart, B.; et al. The gene expression profile of phosphoantigen-specific human γδ T lymphocytes is a blend of αβ T cell and NK cell signatures. Eur. J. Immunol. 2012, 42, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.O.; Fournié, J.J.; Majoral, J.P.; Caminade, A.M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4+ T cell proliferation enhances ex-vivo expansion of NK cells from PBMCs for immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Degboé, Y.; Fruchon, S.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Poupot, R.; Cantagrel, A.; Davignon, J.L. Modulation of pro-inflammatory activation of monocytes and dendritic cells by aza-bis-phosphonate dendrimer as an experimental therapeutic agent. Arthritis Res. Ther. 2014, 16, R98. [Google Scholar] [CrossRef] [PubMed]
- Horai, R.; Saijo, S.; Tanioka, H.; Nakae, S.; Sudo, K.; Okahara, A.; Ikuse, T.; Asano, M.; Iwakura, Y. Development of chronic inflammatory arthropathy resembling rheumatoid arthritis in interleukin 1 receptor antagonist-deficient mice. J. Exp. Med. 2000, 191, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Saijo, S.; Horai, R.; Sudo, K.; Mori, S.; Iwakura, Y. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc. Natl. Acad. Sci. USA 2003, 100, 5986–5990. [Google Scholar] [CrossRef] [PubMed]
- Horai, R.; Nakajima, A.; Habiro, K.; Kotani, M.; Nakae, S.; Matsuki, T.; Nambu, A.; Saijo, S.; Kotaki, H.; Sudo, K.; et al. TNF-alpha is crucial for the development of auto-immune arthritis in IL-1 receptor antagonist deficient mice. J. Clin. Invest. 2004, 114, 1603–1611. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Nigon, D.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; et al. A phosphorus-based dendrimer targets inflammation and osteoclastogenesis in experimental arthritis. Sci. Transl. Med. 2011, 3, 81ra35. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.L.; Hayder, M.; Baron, M.; Boyer, J.F.; Constantin, A.; Apparailly, F.; Poupot, R.; Cantagrel, A. Targeting monocytes/macrophages in the treatment of rheumatoid arthritis. Rheumatology 2013, 52, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Hayder, M.; Poupot, M.; Baron, M.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P.; Eisenberg, R.A.; Fournié, J.J.; Cantagrel, A.; Poupot, R.; et al. Frequency and route of administration in the treatment of experimental arthritis by phosphorus-based dendrimer. Ann. Rheum. Dis. 2012, 71 (Suppl. 1), A8. [Google Scholar] [CrossRef]
- Kyburz, D.; Corr, M. The KRN mouse model of inflammatory arthritis. Springer Semin. Immunopathol. 2003, 25, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Bosch, X. Dendrimers to treat rheumatoid arthritis. ACS Nano 2011, 5, 6779–6785. [Google Scholar] [CrossRef] [PubMed]
- Leah, E. Dendrimer drug mends monocytes. Nat. Rev. Rheumatol. 2011, 7, 376. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, J.T.; McDevitt, H.O.; Guss, R.B.; Egbert, R. Endotoxin-induced uveitis in rats as a model for human disease. Nature 1980, 268, 611–613. [Google Scholar] [CrossRef]
- Chen, F.T.; Liu, Y.C.; Yang, C.M.; Yang, C.H. Anti-inflammatory effect of the proteasome inhibitor Bortezomib on endotoxin-induced uveitis in rats. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3682–3694. [Google Scholar] [CrossRef] [PubMed]
- El Zaoui, I.; Touchard, E.; Berdugo, M.; Abadie, C.; Kowalczuk, L.; Deloche, C.; Zhao, M.; Naud, M.C.; Combette, J.M.; Behar-Cohen, F. Subconjunctival injection of XG-102, a c-Jun N-terminal kinase inhibitor peptide, in the treatment of endotoxin-induced uveitis in rats. J. Ocul. Pharmacol. Ther. 2015, 31, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Caminade, A.M.; Abadie, C.; Davignon, J.L.; Combette, J.M.; Turrin, C.O.; Poupot, R. An azabisphosphonate-capped poly(phosphorhydrazone) dendrimer for the treatment of endotoxin-induced uveitis. Molecules 2013, 18, 9305–9316. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nun, A.; Kaushansky, N.; Kawakami, N.; Krishnamoorthy, G.; Berer, K.; Liblau, R.; Hohlfeld, R.; Wekerle, H. From classic to spontaneous and humanized models of multiple sclerosis: Impact on understanding pathogenesis and drug development. J. Autoimmun. 2014, 54, 33–50. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, J.H.; Lucchinetti, C.; Rodriguez, M.; Weinshenker, B.G. Multiple sclerosis. N. Engl. J. Med. 2000, 343, 938–952. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Dornmair, K.; Meinl, E.; Wekerle, H. The search for the target antigens of multiple sclerosis, part 1: Autoreactive CD4+ T lymphocytes as pathogenic effectors and therapeutic targets. Lancet Neurol. 2016, 15, 198–209. [Google Scholar] [CrossRef]
- Hayder, M.; Varilh, M.; Turrin, C.O.; Saoudi, A.; Caminade, A.M.; Poupot, R.; Liblau, R.S. Phosphorus-based dendrimer ABP treats neuroinflammation by promoting IL-10-producing CD4+ T. cells. Biomacromolecules 2015, 16, 3425–3433. [Google Scholar] [CrossRef] [PubMed]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Svenson, S. The dendrimer paradox—High medical expectations but poor clinical translation. Chem. Soc. Rev. 2015, 44, 4131–4144. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Park, E.J.; Na, D.H. Recent progress in dendrimer-based nanomedicine development. Arch. Pharm. Res. 2018. [Google Scholar] [CrossRef] [PubMed]
- McGowan, I.; Gomez, K.; Bruder, K.; Febo, I.; Chen, B.A.; Richardson, B.A.; Husnik, M.; Livant, E.; Price, C.; Jacobson, C. MTN-004 Protocol Team. Phase 1 randomized trial of the vaginal safety and acceptability of SPL7013 (VivaGel®) in sexually active young women (MTN-004). AIDS 2011, 25, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/study/NCT00740584?term=SPL7013+and+HIV+Infections&rank=1 (accessed on 26 April 2018).
- Fruchon, S.; Poupot, R. Pro-inflammatory versus anti-inflammatory effects of dendrimers: The two faces of immuno-modulatory nanoparticles. Nanomaterials 2017, 7, 251. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Han, Y.; Liu, L.; Shen, W.; Zhang, H.; Wang, Y.; Cui, X.; Wang, Y.; Liu, G.; Qi, R. Protective effects and mechanisms of G5 PAMAM dendrimers against acute pancreatitis induced by caerulein in mice. Biomacromolecules 2015, 16, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Teo, I.; Toms, S.M.; Marteyn, B.; Barata, T.S.; Simpson, P.; Johnston, K.A.; Schnupf, P.; Puhar, A.; Bell, T.; Tang, C.; et al. Preventing acute gut wall damage in infectious diarrhoeas with glycosylated dendrimers. EMBO Mol. Med. 2012, 4, 866–881. [Google Scholar] [CrossRef] [PubMed]
- Fruchon, S.; Mouriot, S.; Thiollier, T.; Grandin, C.; Caminade, A.M.; Turrin, C.O.; Contamin, H.; Poupot, R. Repeated intravenous injections in non-human primates demonstrate preclinical safety of an anti-inflammatory phosphorus-based dendrimer. Nanotoxicology 2015, 9, 933–941. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Non-applicable. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fruchon, S.; Poupot, R. The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells. Molecules 2018, 23, 1272. https://doi.org/10.3390/molecules23061272
Fruchon S, Poupot R. The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells. Molecules. 2018; 23(6):1272. https://doi.org/10.3390/molecules23061272
Chicago/Turabian StyleFruchon, Séverine, and Rémy Poupot. 2018. "The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells" Molecules 23, no. 6: 1272. https://doi.org/10.3390/molecules23061272
APA StyleFruchon, S., & Poupot, R. (2018). The ABP Dendrimer, a Drug-Candidate against Inflammatory Diseases That Triggers the Activation of Interleukin-10 Producing Immune Cells. Molecules, 23(6), 1272. https://doi.org/10.3390/molecules23061272




