Characterization of the Complete Chloroplast Genomes of Buddleja colvilei and B. sessilifolia: Implications for the Taxonomy of Buddleja L.
Abstract
:1. Introduction
2. Results and Discussion
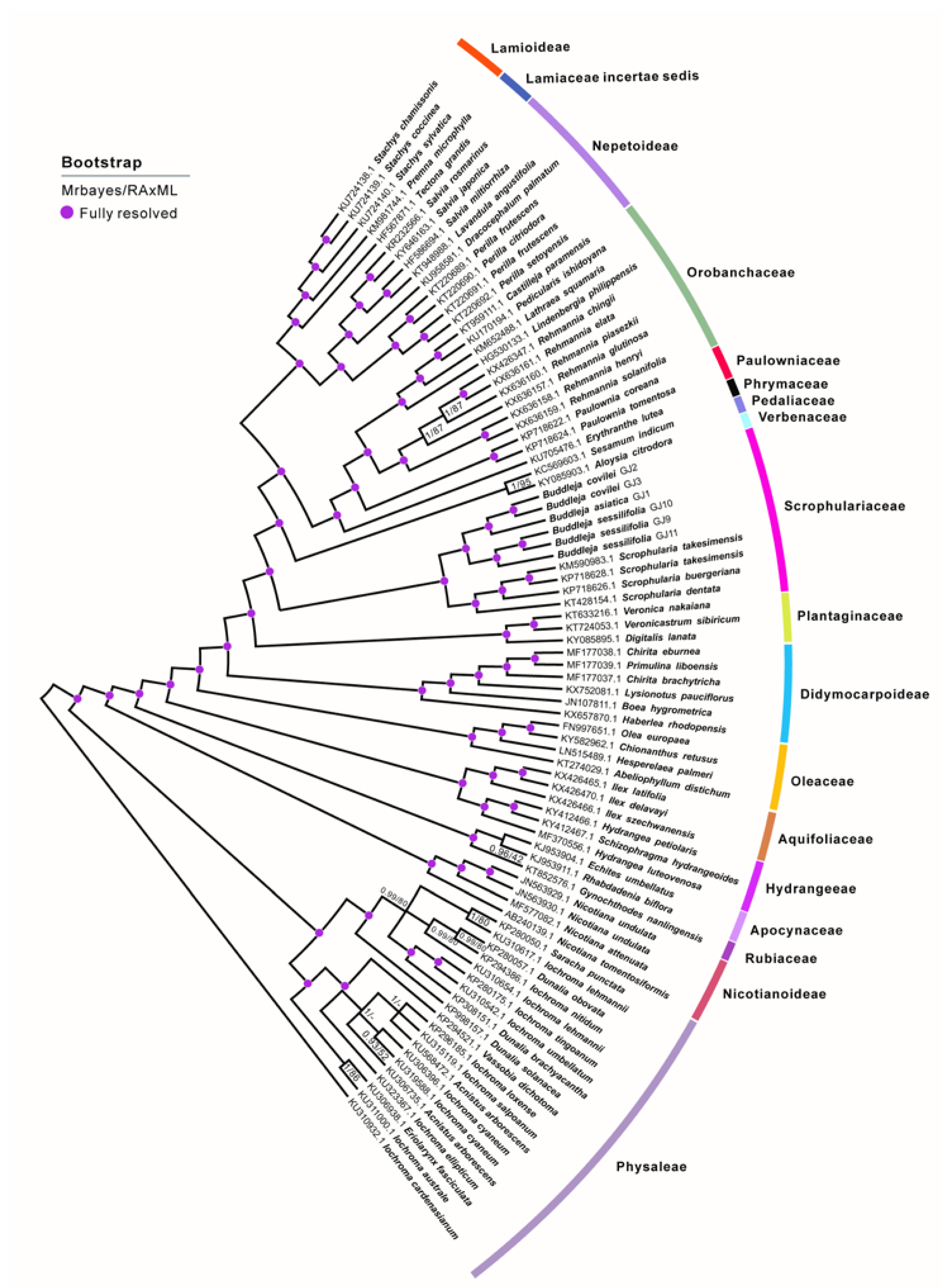
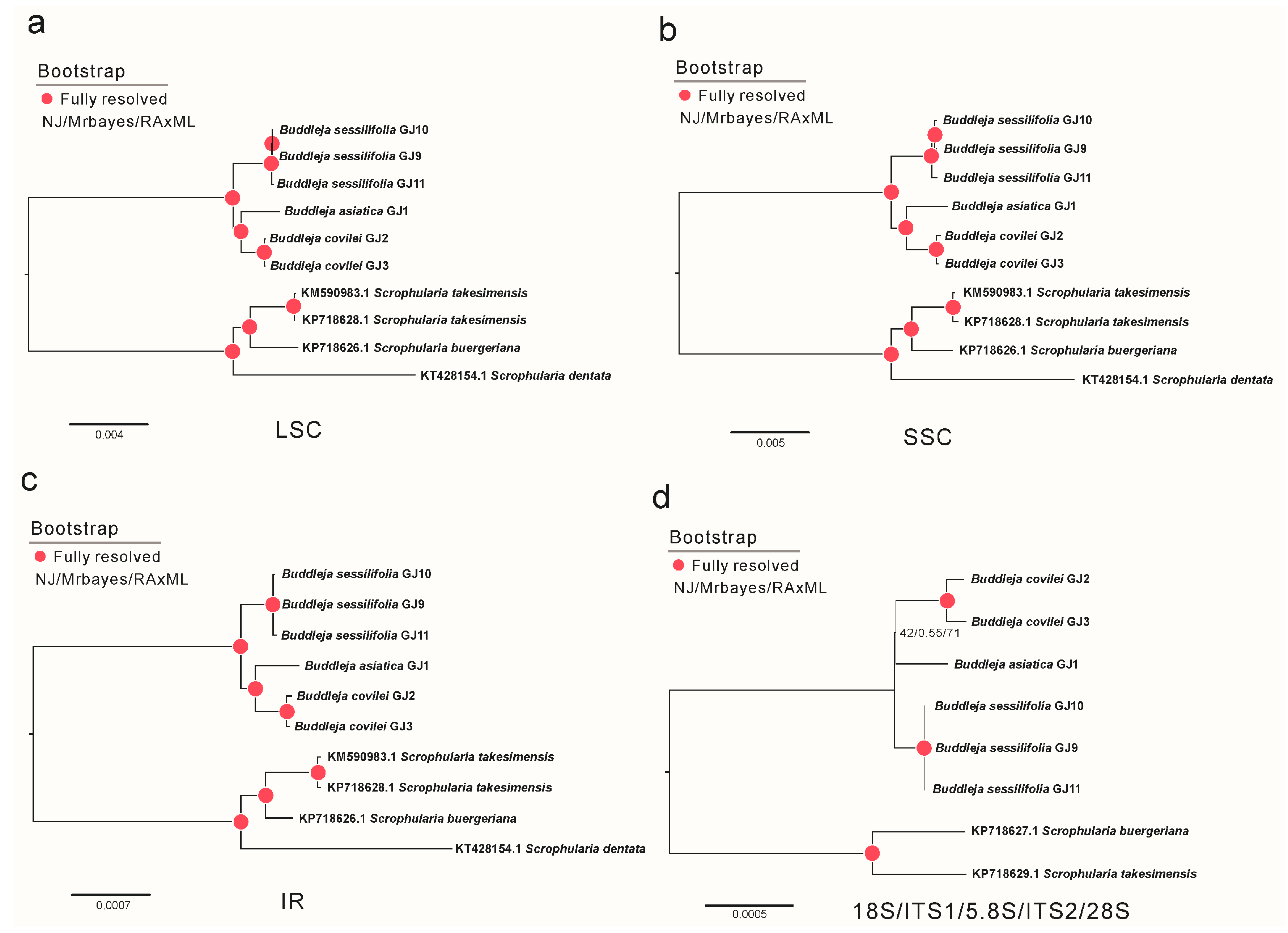
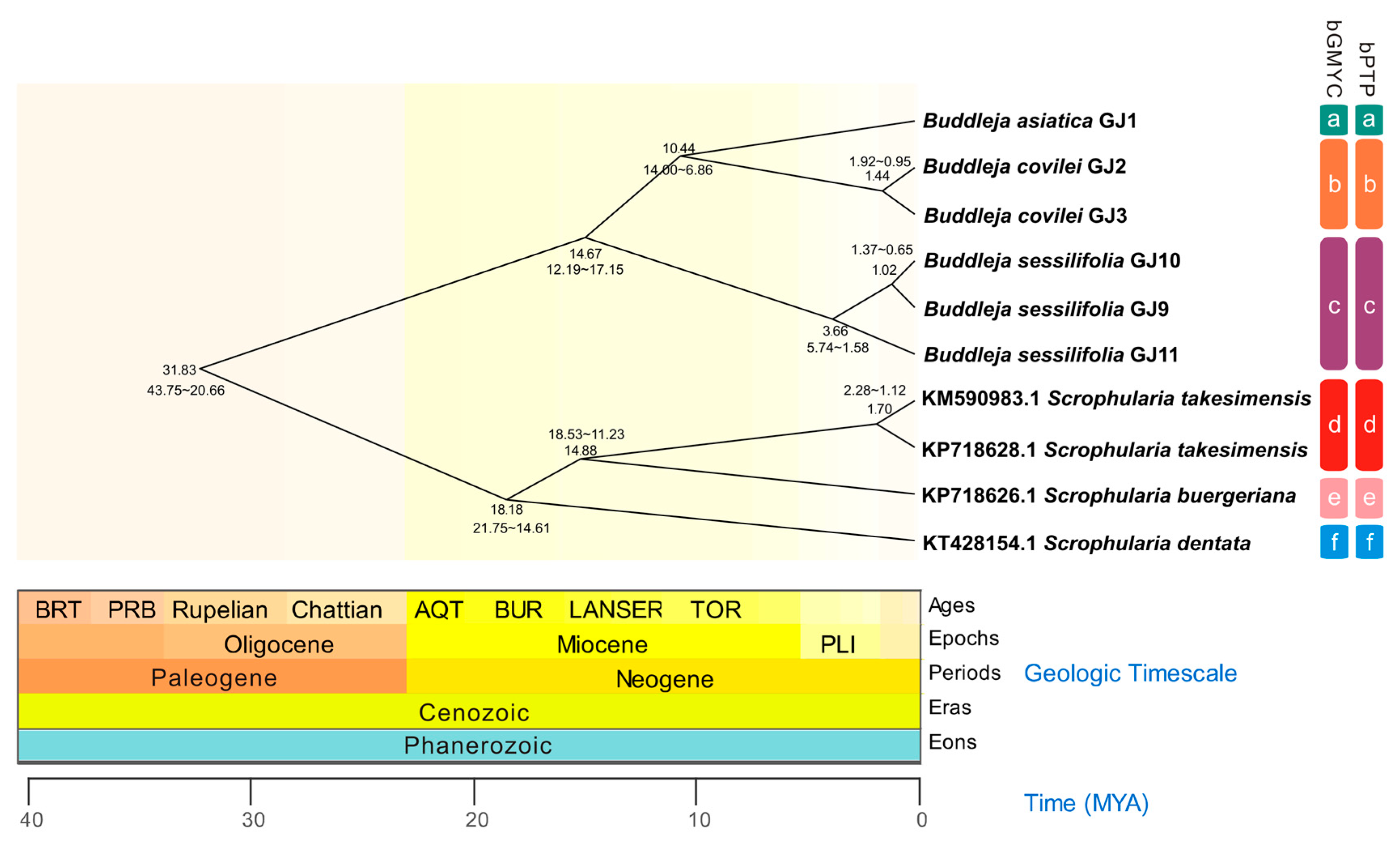
2.1. Phylogenetic Analysis and Species Delimitation
2.2. Genome Organization, Features and the Comparisons between B. colvilei and B. sessilifolia cp Genomes
2.3. Morphological Study and Taxonomic Treatment
3. Materials and Methods
3.1. DNA Extraction and Sequencing
3.2. Phylogenetic Analysis and Species Delimitation
3.2.1. Complete cp Genome Based Phylogenetic Analysis
3.2.2. Cp Data Partition
3.2.3. 18S-ITS1-5.8S-ITS2-28S
3.2.4. Model Selection
3.2.5. Maximum Likelihood
3.2.6. Bayesian Analyses
3.2.7. NJ Method
3.2.8. Species Delimitation
3.3. Chloroplast Genome Annotation and Comparisons
3.4. Morphological Study and Taxonomy Treatment
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Norman, E.M. Buddlejaceae. Flora Neotropica Monograph; The New York Botanical Garden Press: New York, NY, USA, 2000; p. 38, ISBN-10 0893274372, ISBN-13 978-0893274375. [Google Scholar]
- Chen, G.; Gong, W.C.; Ge, J.; Dunn, B.L.; Sun, W.B. Floral scents of typical Buddleja species with different pollination syndromes. Biochem. Syst. Ecol. 2012, 44, 173–178. [Google Scholar] [CrossRef]
- Chen, G.; Gong, W.C.; Ge, J.; Dunn, B.L.; Sun, W.B. Inflorescence scent, color, and nectar properties of “butterfly bush” (Buddleja davidii) in its native range. Flora 2014, 209, 172–178. [Google Scholar] [CrossRef]
- Stuart, D.D. Buddlejas (Royal Horticultural Society Plant Collector Guide); Timber Press: Portland, OR, USA, 2006; pp. 13–25. ISBN 9780881926880. [Google Scholar]
- Wikipedia. Available online: https://en.wikipedia.org/wiki/Buddleja_colvilei (accessed on 24 February 2018).
- Li, P.T.; Leeuwenberg, A.J.M. Myrsinaceae through Loganiaceae. In Flora of China; Wu, C.Y., Raven, P.H., Eds.; Science Press: Beijing, China, 1996; Volume 15, p. 332. ISBN 7030079922. [Google Scholar]
- Leeuwenberg, A.J.M. The Loganiaceae of Africa XVIII Buddleja L. II Revision of the African and Asiatic Species; Mededelingen Landbouwhogeschool: Wageningen, The Netherlands, 1979; pp. 97, 103–105. ISBN 582.935.4(5). [Google Scholar]
- The Biodiversity Red List of China: Higher Plant. Available online: http://www.zhb.gov.cn/gkml/hbb/bgg/201309/ t20130912_260061.htm (accessed on 24 February 2018).
- Mace, G.M. The role of taxonomy in species conservation. Philos. Trans. R. Soc. B 2004, 359, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.Y. Loganiaceae. In Flora Yunnanica; Kunming Institute of Botany, Chinese Academy of Sciences, Eds.; Science Press: Beijing, China, 1983; Volume 3, pp. 465–467. ISBN 7900392033. [Google Scholar]
- Li, P.T. Study on the genus Buddleja L. of China. Acta Botanica Yunnanica 1982, 4, 227–274. [Google Scholar]
- Li, P.T. Notes on the Chinese Euphorbiaceae and Loganiaceae. J. South Chin. Agric. Univ. 1988, 9, 49–53. [Google Scholar]
- Zhang, M.Z.; Qiu, L.Q. Loganiaceae. In Flora Reipublicae Popularis Sinicae; Editorial Board of the Flora Republicae Popularis Sinicae, Ed.; Science Press: Beijing, China, 1992; Volume 61, p. 282. ISBN 9787030271662. [Google Scholar]
- Moore, R. Cyto-taxonomic notes on Buddleja. Am. J. Bot. 1960, 47, 511–517. [Google Scholar] [CrossRef]
- Chen, G.; Sun, W.B.; Sun, H. Ploidy variation in Buddleja L. (Buddlejaceae) in the Sino- Himalayan region and its biogeographical implications. Bot. J. Linn. Soc. 2007, 154, 305–312. [Google Scholar] [CrossRef]
- Bierzychudek, P. Patterns in plant parthenogenesis. Experientia 1985, 41, 1255–1264. [Google Scholar] [CrossRef]
- Kirchheimer, B.; Schinkel, C.F.; Dellinger, A.S.; Klatt, S.; Moser, D.; Winkler, M.; Lenoir, J.; Caccianiaga, M.; Guisan, A.; Nieto-Lugilde, D.; et al. A matter of scale: Apparent niche differentiation on diploid and tetraploid plants may depend on extent and grain of analysis. J. Biogeogr. 2016, 43, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, C.C.F.; Kirchheimer, B.; Dellinger, A.S.; Klatt, S.; Winkler, M.; Dullinger, S.; Hörandl, E. Correlations of polyploidy and apomixis with elevation and associated environmental gradients in an alpine plant. AoB Plants 2016, 8, plw064. [Google Scholar] [CrossRef] [PubMed]
- Birky, C.J. The inheritance of genes in mitochondria and chloroplasts: Laws, mechanisms, and models. Annu. Rev. Genet. 2001, 35, 125–148. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, T.; Baslam, M.; Inomata, T.; Oikawa, K.; Itoh, K.; Ohnishi, T.; Iton, K.; Ohnishi, T.; Kinoshita, T.; Mitsui, T. Optimized method of extracting rice chloroplast DNA for High-Quality plastome resequencing and de novo assembly. Front. Plant Sci. 2018, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Soltis, P.S.; Soltis, D.E. The role of genetic and genomic attributes in the success of polyploids. Proc. Natl. Acad. Sci. USA 2000, 97, 7051–7057. [Google Scholar] [CrossRef] [PubMed]
- Kress, W.J.; Wurdack, K.J.; Zimmer, E.A.; Weigt, L.A.; Janzen, D.H. Use of DNA Barcodes to Identify Flowering Plants. Proc. Natl. Acad. Sci. USA 2005, 102, 8369–8374. [Google Scholar] [CrossRef] [PubMed]
- Celiński, K.; Kijak, H.; Wojnicka-Półtorak, A.; Buczkowska-Chmielewska, K.; Sokołowska, J.; Chudzińska, E. Effectiveness of the DNA Barcoding Approach for Closely Related Conifers Discrimination: A Case Study of the Pinus Mugo Complex. C. R. Biol. 2017, 340, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Park, I.; Kim, W.J.; Yeo, S.M.; Choi, G.; Kang, Y.M.; Piao, R.; Moon, B.C. The Complete Chloroplast Genome Sequences of Fritillaria ussuriensis Maxim. and Fritillaria cirrhosa D. Don, and Comparative Analysis with Other Fritillaria Species. Molecules 2017, 22, 982. [Google Scholar] [CrossRef] [PubMed]
- Chau, J.H.; O’Leary, N.; Sun, W.B.; Olmstead, R.G. Phylogenetic relationships in tribe Buddlejeae (Scrophulariaceae) based on multiple nuclear and plastid markers. Bot. J. Linn. Soc. 2017, 184, 137–166. [Google Scholar] [CrossRef]
- Olmstead, R.G.; de Pamphilis, C.W.; Wolfe, A.D.; Young, N.D.; Elisons, W.J.; Reeves, P.A. Disintegration of the Scrophulariaceae. Am. J. Bot. 2001, 88, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Angiosperm Phylogeny Group. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG II. Bot. J. Linn. Soc. 2003, 141, 399–436. [Google Scholar] [CrossRef]
- Ding, L.; Spicer, R.A.; Yang, J.; Xu, Q.; Cai, F.L.; Li, S.; Lai, Q.Z.; Wang, H.Q.; Spicer, T.E.V.; Yue, Y.H.; et al. Quantifying the Rise of the Himalaya Orogen and Implications for the South Asian Monsoon. Geology 2017, 45, 215–218. [Google Scholar] [CrossRef]
- Sun, B.N.; Wu, J.Y.; Liu, Y.S.; Ding, S.T.; Li, X.C.; Xie, S.P.; Yan, D.F.; Lin, Z.C. Reconstructing Neogene Vegetation and Climates to Infer Tectonic Uplift in Western Yunnan, China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 304, 328–336. [Google Scholar] [CrossRef]
- Raman, G.; Park, V.; Kwak, M.; Lee, B.; Park, S. Characterization of the complete chloroplast genome of Arabis stellari and comparisons with related species. PLoS ONE 2017, 12, e0183197. [Google Scholar] [CrossRef] [PubMed]
- Ni, L.; Zhao, Z.; Dorje, G.; Ma, M. The Complete Chloroplast Genome of Ye-Xing-Ba (Scrophularia dentata; Scrophulariaceae), an Alpine Tibetan Herb. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiang, B.; Li, X.; Qian, J.; Wang, L.; Ma, L.; Tian, X.; Wang, Y. The Complete Chloroplast Genome Sequence of the Medicinal Plant Swertia mussotii Using the PacBio RS II Platform. Molecules 2016, 21, 1029. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, L.; Zhao, W.; Xu, J.; Li, Y.Y.; Zhang, X.Y.; Shen, X.F.; Wu, M.L.; Hou, X.G. Complete Chloroplast Genome Sequence and Phylogenetic Analysis of Paeonia ostii. Molecules 2018, 23, 246. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Xu, C.; Li, C.; Sun, J.; Zuo, Y.; Shi, S.; Cheng, T.; Guo, J.; Zhou, S. Ycf1, the most promising plastid DNA barcode of land plants. Sci. Rep. 2015, 5, 8348. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.Y.; Ji, Y.H. Comparative and Phylogenetic Analyses of the Complete Chloroplast Genomes of Three Arcto-Tertiary Relicts: Camptotheca acuminata, Davidia involucrata, and Nyssa sinensis. Front. Plant Sci. 2017, 8, 1536. [Google Scholar] [CrossRef] [PubMed]
- Goulding, S.E.; Olmstead, R.G.; Morden, C.W.; Wolfe, K.H. Ebb and flow of the chloroplast inverted repeat. Mol. Gen. Genet. 1996, 252, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.B.; Palmer, J.D. The chloroplast genome arrangement of Lobelia thuliniana (Lobeliaceae): Expansion of the inverted repeat in an ancestor of the Campanulales. Plant Syst. Evol. 1999, 214, 49–64. [Google Scholar] [CrossRef]
- Zhu, A.; Guo, W.; Gupta, S.; Fan, W.; Mower, J.P. Evolutionary Dynamics of the Plastid Inverted Repeat: The Effects of Expansion, Contraction, and Loss on Substitution Rates. New Phytol. 2015, 209, 1747–1756. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.D.; Osorio, B.; Thompson, W.F. Evolutionary Significance of Inversions in Legume Chloroplast DNAs. Curr. Genet. 1988, 14, 65–74. [Google Scholar] [CrossRef]
- Boudreau, E.; Otis, C.; Turmel, M. Conserved Gene Clusters in the Highly Rearranged Chloroplast Genomes of Chlamydomonas moewusii and Chlamydomonas reinhardtii. Plant Mol. Biol. 1994, 24, 585–602. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.L.; Jansen, R.K.; Chumley, T.; Kim, K.J. Gene Relocations within Chloroplast Genomes of Jasminum and Menodora (Oleaceae) Are Due to Multiple, Overlapping Inversions. Mol. Biol. Evol. 2007, 24, 1161–1180. [Google Scholar] [CrossRef] [PubMed]
- Calie, P.J.; Hughes, K.W. The Consensus Land Plant Chloroplast Gene Order Is Present, with Two Alterations, in the Moss Physcomitrella patens. Mol. Gen. Genet. 1987, 208, 335–341. [Google Scholar] [CrossRef]
- Downie, S.R.; Olmstead, R.G.; Zurawski, G.; Soltis, D.E.; Soltis, P.S.; Watson, J.C.; Palmer, J.D. Six Independent Losses of the Chloroplast DNA rpl2 Intron in Dicotyledons: Molecular and Phylogenetic Implications. Evolution 1991, 45, 1245–1259. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.D.; Doyle, J.J.; Kajita, T.; Nemoto, T.; Ohashi, H. The Chloroplast rpl2 Intron and ORF184 as Phylogenetic Markers in the Legume Tribe Desmodieae. Syst. Bot. 1997, 22, 133–138. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wicke, S.; Wang, H.; Jin, J.J.; Chen, S.Y.; Zhang, S.D.; Li, D.Z.; Yi, T.S. Plastid Genome Evolution in the Early-Diverging Legume Subfamily Cercidoideae (Fabaceae). Front. Plant Sci. 2018, 9, 403–4012. [Google Scholar] [CrossRef] [PubMed]
- Li, F.W.; Kuo, L.Y.; Pryer, K.M.; Rothfels, C.J. Genes Translocated into the Plastid Inverted Repeat Show Decelerated Substitution Rates and Elevated GC Content. Genome Biol. Evol. 2016, 8, 2452–2458. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Cai, X.H. Pigments from floral nectar guide of Buddleja species. Unpublished work. 2018. [Google Scholar]
- Miller, R.; Owens, S.J.; Rørslett, B. Plants and colour: Flowers and pollination. Opt. Laser Technol. 2011, 43, 282–294. [Google Scholar] [CrossRef]
- Chen, G.; Gong, W.C.; Ge, J.; Niu, Y.; Zhang, X.; Dunn, B.L.; Sun, W.B. Comparison of floral properties and breeding system in dimorphic Buddleja delavayi (Scrophulariaceae). J. Syst. Evol. 2015, 53, 196–202. [Google Scholar] [CrossRef]
- Murray, M.G.; Thompson, W.F. Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res. 1980, 8, 4321–4326. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.B.; Li, D.Z.; Li, H.T. Highly effective sequencing whole chloroplast genomes of Angiosperms by nine novel universal primer pairs. Mol. Ecol. Resour. 2014, 14, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.K.; Jain, M. NGS QC Toolkit: A Toolkit for Quality Control of Next Generation Sequencing Data. PLoS ONE 2012, 7, e30619. [Google Scholar] [CrossRef] [PubMed]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Dierckxsens, N.; Patrick, M.; Guillaume, S. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2016, 45, e18. [Google Scholar] [CrossRef]
- Bi, G.Q.; Mao, Y.X.; Xing, Q.K.; Cao, M. HomBlocks: A multiple-alignment construction pipeline for organelle phylogenomics based on locally collinear block searching. Genomics 2018, 110, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Gblocks, v. 0.91 b. 2. Available online: http://molevol.cmima.csic.es/castresana/Gblocks/Gblocks_documentation.html (accessed on 24 February 2018).
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Daron, M.S. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [PubMed]
- FigTree v1. 3.1: Tree Figure Drawing Tool. Available online: http://beastbioedacuk/Tracer (accessed on 30 January 2018).
- Hassanpour, H.; Zare-Maivan, H.; Sonboli, A.; Kazempour-Osaloo, S.; Wagner, F.; Tomasello, S.; Oberprieler, C. Phylogenetic Species Delimitation Unravels a New Species in the Genus Sclerorhachis (Rech.F.) Rech.F. (Compositae, Anthemideae). Plant Syst. Evol. 2018, 304, 185–203. [Google Scholar] [CrossRef]
- Reid, N.M.; Carstens, B.C. Phylogenetic estimation error can decrease the accuracy of species delimitation: A Bayesian implementation of the general mixed Yule-coalescent model. BMC Evol. Biol. 2012, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A.; Suchard, M.; Xie, D.; Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 2012, 29, 1969–1973. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.B.; Marin, J.; Suleski, M.; Paymer, M.; Kumar, S. Tree of Life Reveals Clock-Like Speciation and Diversification. Mol. Biol. Evol. 2015, 32, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pérez, M.L.; López, J.; Fernández-Mazuecos, M.; Rodríguez-Riaño, T.; Vargas, P.; Ortega-Olivencia, A. The role of birds and insects in pollination shifts of Scrophularia (Scrophulariaceae). Mol. Phylogenet. Evol. 2013, 69, 239–254. [Google Scholar] [CrossRef] [PubMed]
- Wyman, S.K.; Jansen, R.K.; Boore, J.L. Automatic annotation of organellar genomes with DOGMA. Bioinformatics 2004, 20, 3252–3255. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.I.; Cronk, Q.C.B. Plann: A command-line application for annotating plastome sequences. Appl. Plant Sci. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Lohse, M.; Drechsel, O.; Kahlau, S.; Bock, R. OrganellarGenomeDRAW—A suite of tools for generating physical maps of plastid and mitochondrial genomes and visualizing expression data sets. Nucleic Acids Res. 2013, 41, W575–W581. [Google Scholar] [CrossRef] [PubMed]
- Mayor, C.; Brudno, M.; Schwartz, J.R.; Poliakov, A.; Rubin, E.M.; Frazer, K.A.; Pachter, L.S.; Dubchak, I. VISTA: Visualizing global DNA sequence alignments of arbitrary length. Bioinformatics 2000, 16, 1046–1047. [Google Scholar] [CrossRef] [PubMed]
- Darzentas, N. Circoletto: Visualizing Sequence Similarity with Circos. Bioinformatics 2010, 26, 2620–2621. [Google Scholar] [CrossRef] [PubMed]
- Okonechnikov, K.; Golosova, O.; Fursov, M. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.C.E.; Mau, B.; Blattner, F.R.; Perna, N.T. Mauve: Multiple Alignment of Conserved Senomic Sequence with Rearrangements. Genome Res. 2000, 14, 1394–1403. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |

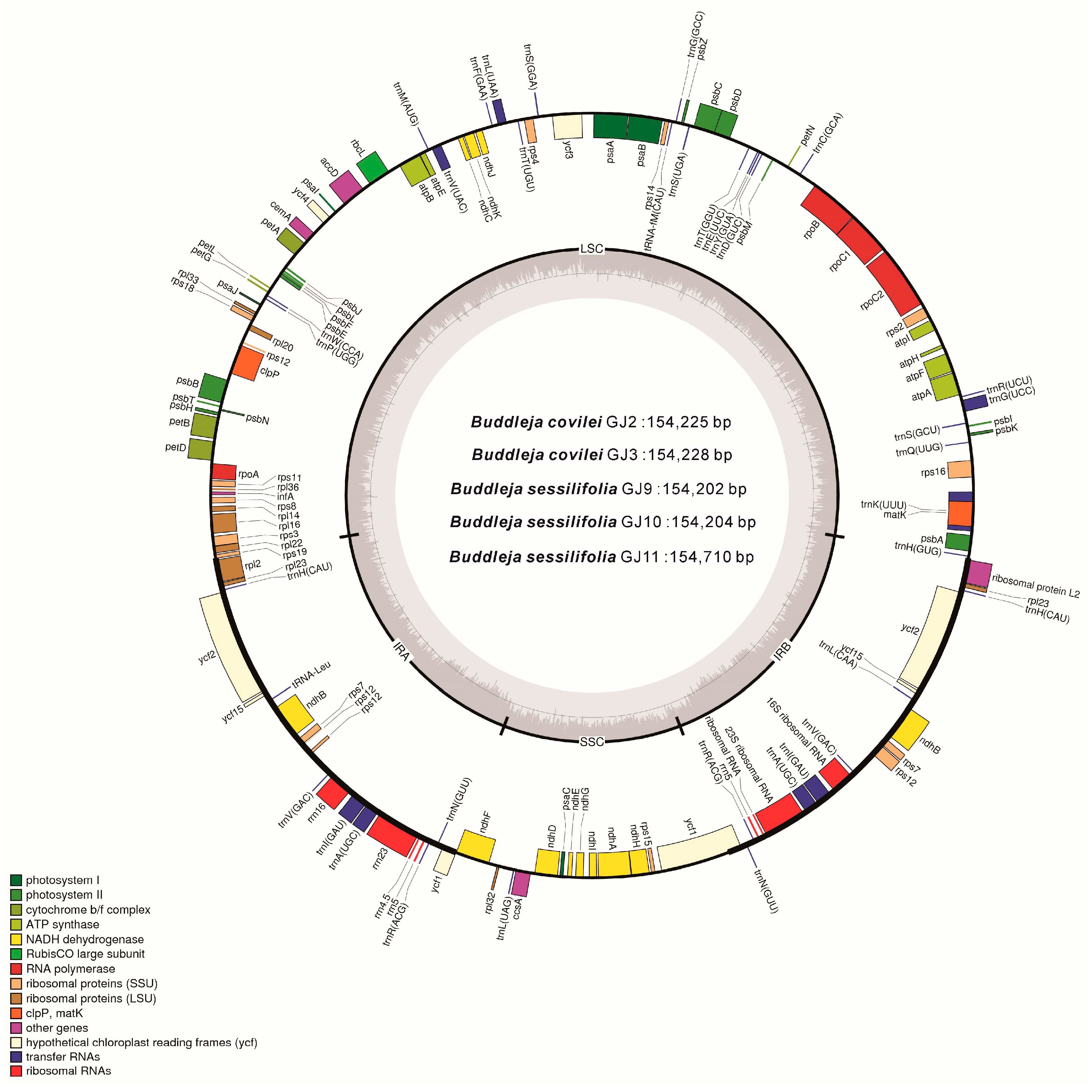
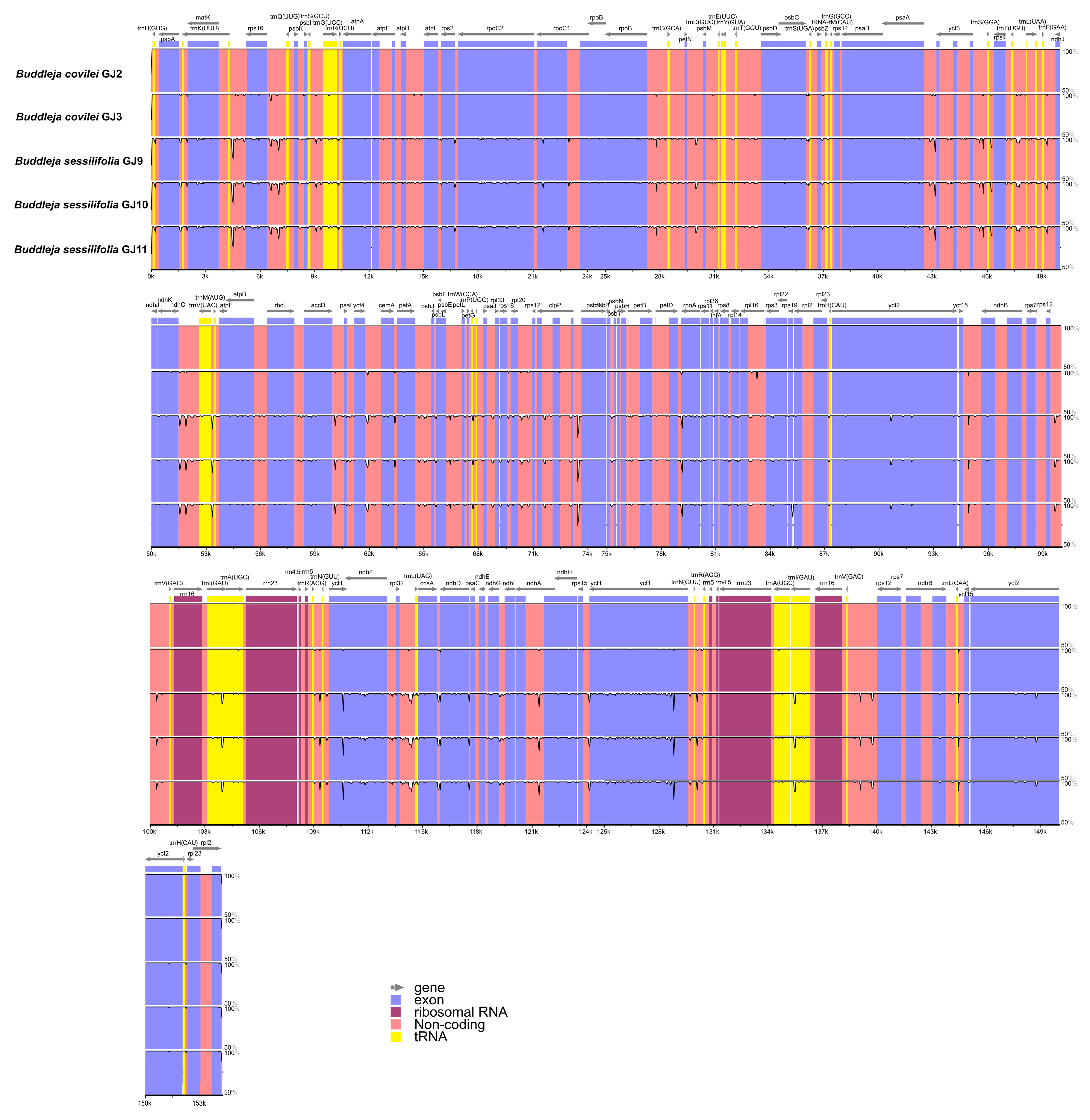
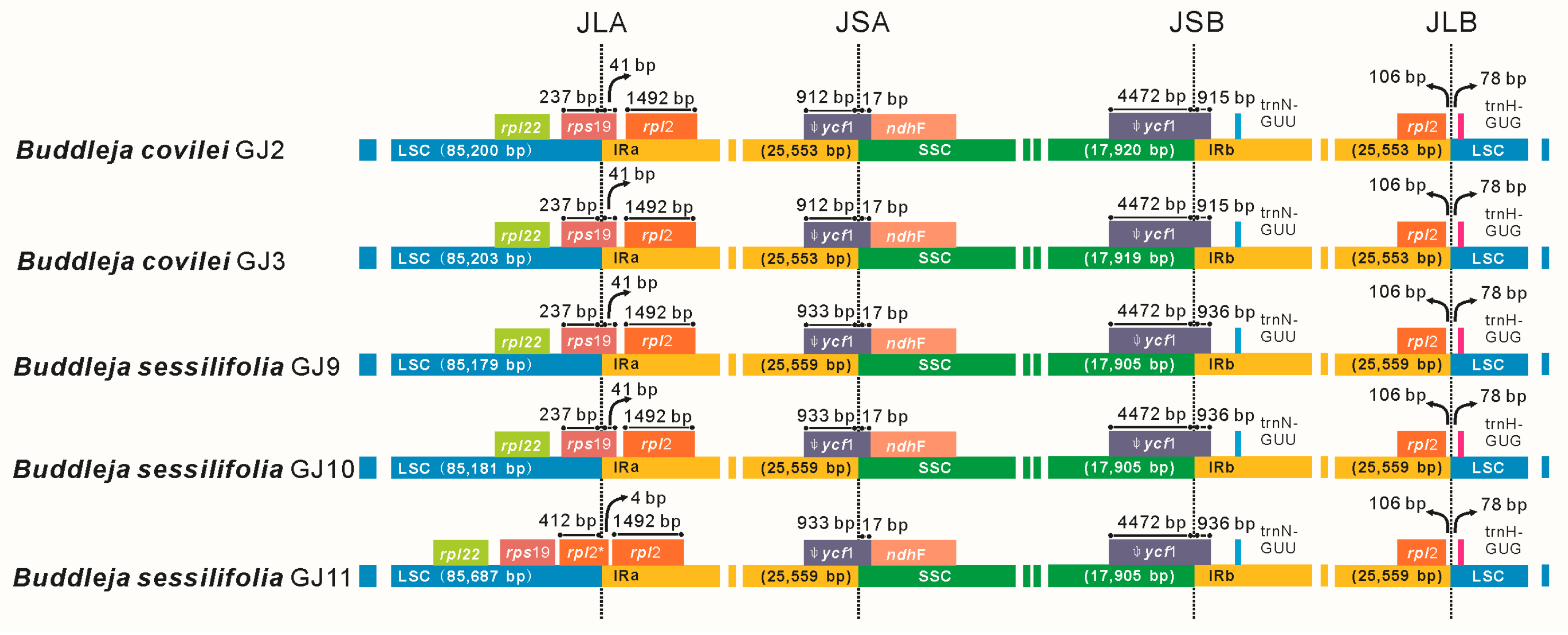
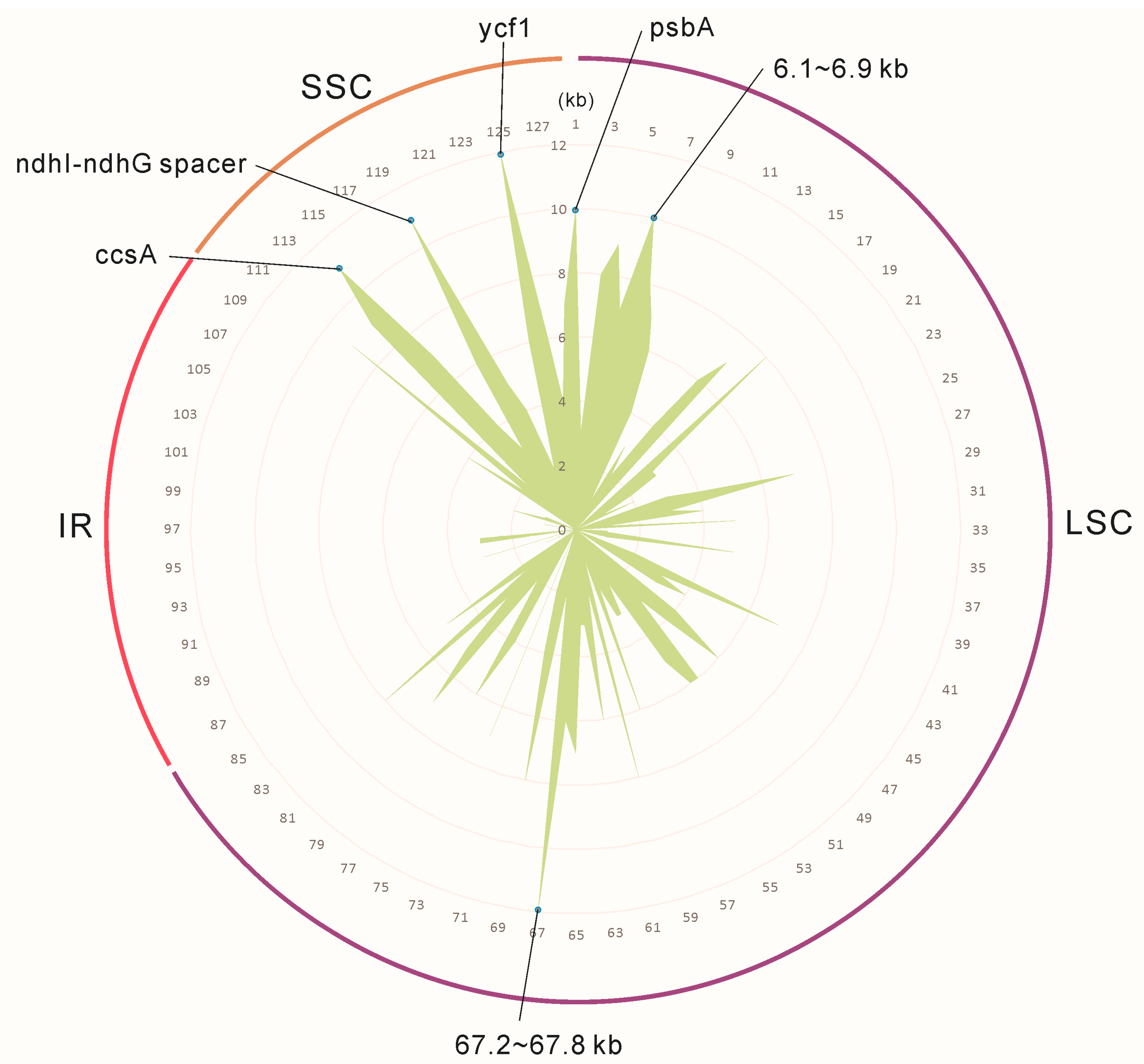
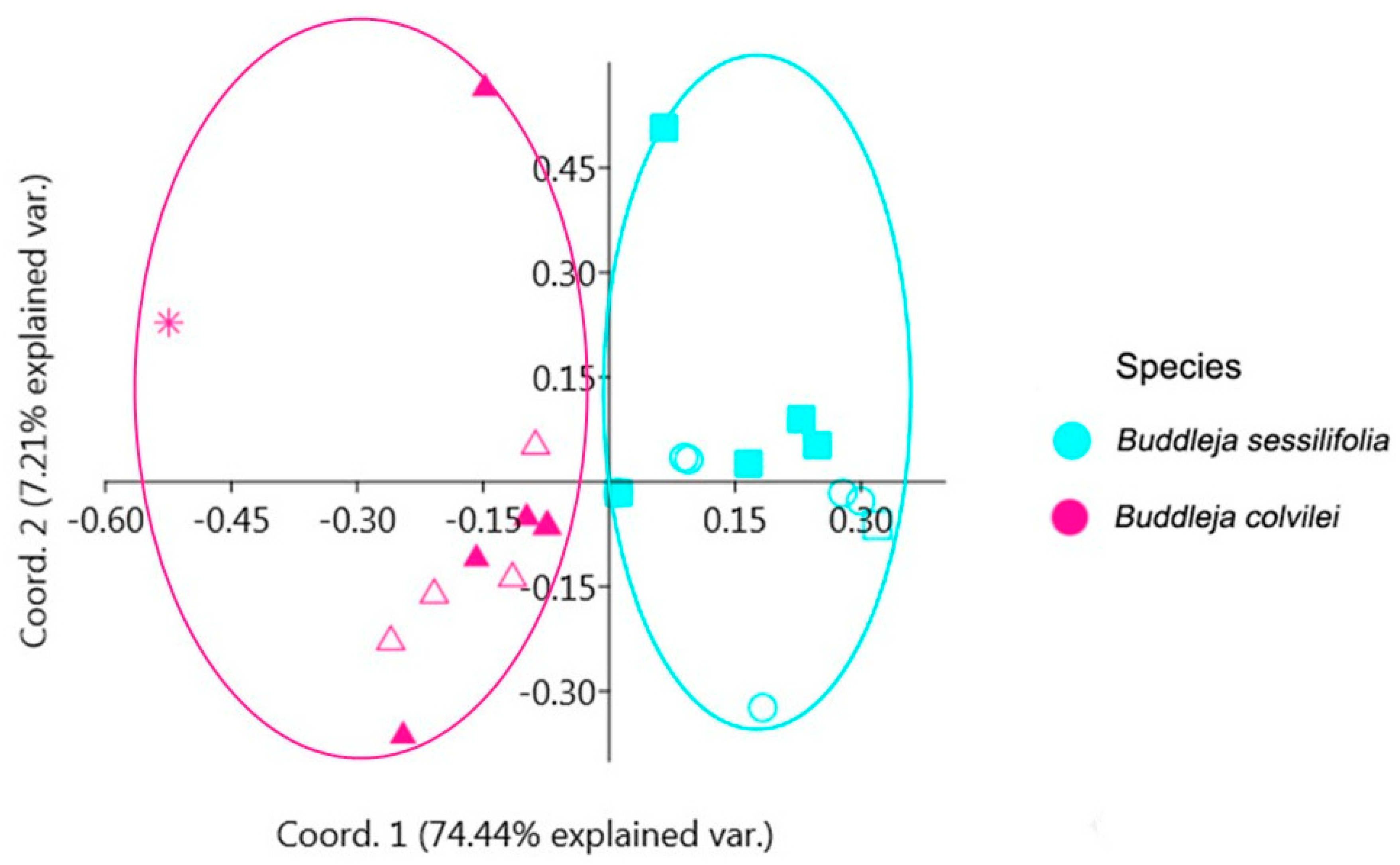
| Species | B. colvilei | B. sessilifolia | ||||
|---|---|---|---|---|---|---|
| GJ2 | GJ3 | GJ9 | GJ10 | GJ11 | ||
| Size (bp) | Total CP genome | 154,225 | 154,228 | 154,202 | 154,204 | 154,710 |
| LSC region | 85,200 | 85,203 | 85,179 | 85,181 | 85,687 | |
| SSC region | 17,920 | 17,919 | 17,905 | 17,905 | 17,905 | |
| IR region | 25,553 | 25,553 | 25,559 | 25,559 | 25,559 | |
| GC content (%) | Total CP genome | 38.07 | 38.07 | 38.10 | 38.10 | 38.11 |
| LSC region | 36.19 | 36.19 | 36.21 | 36.21 | 36.25 | |
| SSC region | 32.30 | 32.30 | 32.42 | 32.42 | 32.39 | |
| IR region | 43.23 | 43.23 | 43.23 | 43.23 | 43.23 | |
| Category | Function | Group of Genes Gene Names | |||||
|---|---|---|---|---|---|---|---|
| RNA Genes | Ribosomal RNA Genes | rrn4.5# | rrn5# | rrn16# | rrn23# | ||
| Transfer RNA genes | trnH-GUG | trnK-UUU * | trnQ-UUG | trnS-GCU | trnG-UCC * | trnR-UCU | |
| tmC-GCA | trnD-GUC | trnY-GUA | trnE-UUC | trnT-GGU | trnS-UGA | ||
| trnG-GCC | trnfM-AUG | trnS-GGA | trnT-UGU | trnL-UAA * | trnF-GAA | ||
| trnV-UAC * | trnM-AUG | trnW-CCA | trnP-UGG | trnH-CAU # | trnL-UUG # | ||
| trnV-GAC # | trnI-GAU *# | trnA-UGC *# | trnR-AGC # | trnN-GUU # | trnL-UAG | ||
| Protein genes | Subunits of Photosystem I | psaA | psaB | psaC | psaI | psaJ | |
| ycf3 ** | ycf4 | ||||||
| Subunits of Photosystem II | psbA | psbB | psbC | psbD | psbE | psbF | |
| psbH | psbI | psbJ | psbK | psbL | psbM | ||
| psbN | psbT | psbZ | |||||
| Subunits of cytochrome | petA | petB *# | petD * | petG | petL | petN | |
| Subunits of ATP synthase | atpA | atpB | atpE | atpF * | atpH | atpI | |
| Large subunit of RuBisCO | rbcL | ||||||
| Subunits of NADH dehydrogenase | ndhA * | ndhB *# | ndhC | ndhD | ndhE | ndhF | |
| ndhG | ndhH | ndhI | ndhJ | ndhK | |||
| ATP-dependent protease subunit P | clpP ** | ||||||
| Chloroplast envelope membrane protein | cemA | ||||||
| Translation initiation factor | infA | ||||||
| Ribosomal proteins | Small subunit of ribosome (SSU) | rps2 | rps3 | rps4 | rps7 | rps8 | rps11 |
| rps12 **# | rps14 | rps15 | rps16 * | rps18 | rps19 | ||
| Transcription | Large subunit of ribosome (LSU) | rpl2 *# | rpl14 | rpl16 * | rpl20 | rpl22 | rpl23# |
| rpl32 | rpl33 | rpl36 | |||||
| DNA-dependent RNA polymerase | rpoA | rpoB | rpoC1 * | rpoC2 | |||
| Other genes | Maturase | matK | |||||
| Subunit of acetyl-CoA | accD | ||||||
| C-type cytochrome synthesis gene | ccsA | ||||||
| Component of TIC complex | ycf1 Ψ # | ||||||
| Hypothetical proteins | ycf2# | ||||||
| ycf15 Ψ # | |||||||
| Characteristic | B. sessilifolia | B. colvilei |
|---|---|---|
| Plant | Subshrubs, ca. 1 m high | Shrubs or small trees, 2–6 (−11 m) high |
| Branchlets | Quadrangular, glabrous | Subterete, with scattered stellate and glandular hairs |
| Petiole | Almost none | 4–10 mm long |
| Calyx | 3–5.5 mm long, smooth and glabrous on both sides | 6–8 mm long, outside densely stellate-tomentose with glandular hairs and more or less scattered stellate hairs, inside with glandular hairs. |
| Corolla | White, pink to pink purple, yellow inside, 7.5–14 mm long, glabrous outside, pilose inside above the middle | Purple to wine red, white inside, 23–30 mm long, scattered glandular and stellate hairs on both sides |
| Indumentum of ovary and capsules | Glabrous | Densely stellate tomentose |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, J.; Cai, L.; Bi, G.-Q.; Chen, G.; Sun, W. Characterization of the Complete Chloroplast Genomes of Buddleja colvilei and B. sessilifolia: Implications for the Taxonomy of Buddleja L. Molecules 2018, 23, 1248. https://doi.org/10.3390/molecules23061248
Ge J, Cai L, Bi G-Q, Chen G, Sun W. Characterization of the Complete Chloroplast Genomes of Buddleja colvilei and B. sessilifolia: Implications for the Taxonomy of Buddleja L. Molecules. 2018; 23(6):1248. https://doi.org/10.3390/molecules23061248
Chicago/Turabian StyleGe, Jia, Lei Cai, Gui-Qi Bi, Gao Chen, and Weibang Sun. 2018. "Characterization of the Complete Chloroplast Genomes of Buddleja colvilei and B. sessilifolia: Implications for the Taxonomy of Buddleja L." Molecules 23, no. 6: 1248. https://doi.org/10.3390/molecules23061248
APA StyleGe, J., Cai, L., Bi, G.-Q., Chen, G., & Sun, W. (2018). Characterization of the Complete Chloroplast Genomes of Buddleja colvilei and B. sessilifolia: Implications for the Taxonomy of Buddleja L. Molecules, 23(6), 1248. https://doi.org/10.3390/molecules23061248




