Anti-Helicobacter, Antitubercular and Cytotoxic Activities of Scalaranes from the Red Sea Sponge Hyrtios erectus †
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of Compounds 1–15
2.2. Structural Elucidation of Compounds 1–15
2.3. Biological Activities of the Isolated Compounds 1–15
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Biological Materials
3.3. Purification of Compounds 1–15
3.4. Biological Activity of Compounds 1–15
3.4.1. Anti-Helicobacter pylori Activity Assessment
3.4.2. Antitubercular Activity Assessment
3.4.3. Cytotoxic Activity Assessment
3.4.3.1. Cell Culture
3.4.3.2. Trypan-Blue Exclusion Assay
3.4.3.3. Cytotoxic Assessment
3.4.3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mehbub, M.F.; Perkins, M.V.; Zhang, W.; Franco, C.M.M. New marine natural products from sponges (Porifera) of the order Dictyoceratida (2001 to 2012); a promising source for drug discovery, exploration and future prospects. Biotechnol. Adv. 2016, 34, 473–491. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.S.; El-Halawany, A.M.; Alahdal, A.M.; Hassanean, H.A.; Ahmed, S.A. A new bioactive metabolite isolated from the Red Sea marine sponge Hyrtios erectus. Molecules 2016, 21, 82. [Google Scholar] [CrossRef] [PubMed]
- Elhady, S.S.; Al-Abd, A.M.; El-Halawany, A.M.; Alahdal, A.M.; Hassanean, H.A.; Ahmed, S.A. Antiproliferative scalarane-based metabolites from the Red Sea sponge Hyrtios erectus. Mar. Drugs 2016, 14, 130. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Yamaki, R.K.; Kelly, M.; Scheuer, P.J. Salmahyrtisol A, a novel cytotoxic sesterterpene from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2002, 65, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Tan, R.; Cichacz, Z.A. Antineoplastic agents. 542. isolation and structure of sesterstatin 6 from the Indian Ocean sponge Hyrtios erecta. J. Nat. Prod. 2005, 68, 1253–1255. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Shaala, L.A.; Emara, S. Antimycobacterial scalarane-based sesterterpenes from the Red Sea sponge Hyrtios erecta. J. Nat. Prod. 2005, 68, 1782–1784. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Deng, Z.; Pei, Y.; Fu, H.; Li, J.; Proksch, P.; Lin, W. Sesterterpenoids from the marine sponge Hyrtios erectus. J. Nat. Prod. 2004, 67, 921–924. [Google Scholar] [CrossRef] [PubMed]
- Miyaoka, H.; Nishijima, S.; Mitome, H.; Yamada, Y. Three new scalarane sesterterpenoids from the Okinawan sponge Hyrtios erectus. J. Nat. Prod. 2000, 63, 1369–1372. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Okamoto, T.; Hayashi, K.; Yokoyama, N.; Sasaki, T.; Kitagawa, I. Marine natural products. XXXII. Absolute configurations of C-4 of the manoalide family, biologically active sesterterpenes from the marine sponge Hyrtios erecta. Chem. Pharm. Bull. 1994, 42, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Bourguet-Kondracki, M.L.; Martin, M.T.; Debitus, C.; Guyot, M. 12-epi-heteronemin: New sesterterpene from the marine sponge Hyrtios erecta. Tetrahedron Lett. 1994, 35, 109–110. [Google Scholar] [CrossRef]
- Iguchi, K.; Shimada, Y.; Yamada, Y. Hyrtiosal, a new sesterterpenoid with a novel carbon skeleton from the Okinawan marine sponge Hyrtios erectus. J. Org. Chem. 1992, 57, 522–524. [Google Scholar] [CrossRef]
- Crews, P.; Bescansa, P.; Bakus, G.J. A non-peroxide norsesterterpene from a marine sponge Hyrtios erecta. Experientia 1985, 41, 690–691. [Google Scholar] [CrossRef] [PubMed]
- Ryu, G.; Matsunaga, S.; Fusetani, N. Three new cytotoxic sesterterpenes from the marine sponge Hyrtios cf. erectus. J. Nat. Prod. 1996, 59, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Crews, P.; Bescansa, P. Sesterterpenes from a common marine sponge, Hyrtios erecta. J. Nat. Prod. 1986, 49, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, N.; Sato, A.; Hata, T.; Sato, N.; Sasagawa, K.; Kobayashi, T. Cytotoxic scalarane sesterterpenes from a sponge, Hyrtios erecta. J. Nat. Prod. 1998, 61, 468–473. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, G.; Koeng, G.M.; Wright, A.D.; Kaminsky, R. A new bioactive sesterterpene and antiplasmodial alkaloids from the marine sponge Hyrtios cf. erecta. J. Nat. Prod. 2000, 63, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.; Cavaliere, P.; Ungur, N.; D’Souza, L.; Parameswaram, P.S.; Cimino, G. New scalaranes from the nudibranch Glossodoris atromarginata and its sponge prey. J. Nat. Prod. 1999, 62, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Pettit, G.R.; Cichaz, Z.A.; Tan, R.; Herald, D.L.; Melody, N.; Hoard, M.S.; Doubek, D.L.; Hooper, J.N.A. Antineoplastic agents. 385. The isolation and structure of a scalarane-type sesterterpene from the Indian Ocean porifera Hyrtios erecta. Collect. Czech. Chem. Commun. 1998, 63, 1671–1677. [Google Scholar] [CrossRef]
- Pettit, G.R.; Tan, R.; Melody, N.; Cichacz, Z.A.; Herald, D.L.; Hoard, M.S.; Pettit, R.K.; Chapuis, J.-C. Antineoplastic agents. 397: Isolation and structure of sesterstatins 4 and 5 from Hyrtios erecta (the Republic of Maldives). Bioorg. Med. Chem. Lett. 1998, 8, 2093–2098. [Google Scholar] [CrossRef]
- Pettit, G.R.; Cichacz, Z.A.; Tan, R.; Hoard, M.S.; Melody, N.; Pettit, R.K. Antineoplastic agents. 386. isolation of sesterstatins 1-3 from the marine sponge Hyrtios erecta. J. Nat. Prod. 1998, 61, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Shigemori, H.; Ishibashi, M.; Mizobe, F.; Kawashima, A.; Nakaike, S.; Kobayashi, J.I. New sesterterpenes with nerve growth factor synthesis-stimulating activity from the Okinawan marine sponge Hyrtios sp. Chem. Pharm. Bull. 1993, 41, 2190–2191. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A.; Singab, A.N.B.; van Soest, R.W.M.; Fusetani, N. Hyrtiosenolides A and B, two new sesquiterpene γ-methoxybutenolides and a new sterol from a Red Sea sponge Hyrtios species. J. Nat. Prod. 2004, 67, 1736–1739. [Google Scholar] [CrossRef] [PubMed]
- Piña, I.C.; Sanders, M.L.; Crews, P. Puupehenone congeners from an Indo-Pacific Hyrtios sponge. J. Nat. Prod. 2003, 66, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Salmoun, M.; Devijver, C.; Daloze, D.; Braekman, J.C.; Gomez, R.; de Kluijver, M.; Van Soest, R.W.M. New sesquiterpene/quinones from two sponges of the genus Hyrtios. J. Nat. Prod. 2000, 63, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.E.; Tahir, A.; Andersen, R.J. A new acyclic diketotriterpenoid isolated from the Indonesian marine sponge Hyrtios erectus. J. Nat. Prod. 1999, 62, 653–654. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Aoki, S.; Sakai, H.; Kawazoe, K.; Kihara, N.; Sasaki, T.; Kitagawa, I. Altohyrtin A, a potent anti-tumor macrolide from the Okinawan marine sponge Hyrtios altum. Tetrahedron Lett. 1993, 34, 2795–2798. [Google Scholar] [CrossRef]
- Kobayashi, M.; Aoki, S.; Sakai, H.; Kihara, N.; Sasaki, T.; Kitagawa, I. Altohyrtins B and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolide congeners of altohyrtin A, from the Okinawan marine sponge Hyrtios altum. Chem. Pharm. Bull. 1993, 41, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Aoki, S.; Kitagawa, I. Absolute stereostructures of altohyrtin A and its congeners, potent cytotoxic macrolides from the Okinawan marine sponge Hyrtios altum. Tetrahedron Lett. 1994, 35, 1243–1246. [Google Scholar] [CrossRef]
- Kobayashi, M.; Aoki, S.; Gato, K.; Kitagawa, I. Marine natural products. XXXVIII. Absolute stereostructures of altohyrtins A, B, and C and 5-desacetylaltohyrtin A, potent cytotoxic macrolides, from the Okinawan marine sponge Hyrtios altum. Chem. Pharm. Bull. 1996, 44, 2142–2149. [Google Scholar] [CrossRef]
- Koch, P.; Djerassi, C.; Lakshmi, V.; Schmitz, F.J. Identification of sterols with oxygenated side chain in a sponge of the species Hyrtios. Helv. Chim. Acta 1983, 66, 2431–2436. [Google Scholar] [CrossRef]
- Kobayashi, J.; Murayama, T.; Ishibashi, M.; Kosuge, S.; Takamatsu, M.; Ohizumi, Y.; Kobayashi, H.; Ohta, T.; Nozoe, S.; Sasaki, T. Hyrtiosins A and B, new indole alkaloids from the Okinawan marine sponge Hyrtios erecta. Tetrahedron 1990, 46, 7699–7702. [Google Scholar] [CrossRef]
- Kobayashi, M.; Aoki, S.; Gato, K.; Matsunami, K.; Kurosu, M.; Kitagawa, I. Marine natural products. XXXIV. Trisindoline, a new antibiotic indole trimer, produced by a bacterium of Vibrio sp. separated from the marine sponge Hyrtios altum. Chem. Pharm. Bull. 1994, 42, 2449–2451. [Google Scholar] [CrossRef] [PubMed]
- Bourguet-Kondracki, M.L.; Martin, M.T.; Guyot, M. A new β-carboline alkaloid isolated from the marine sponge Hyrtios erecta. Tetrahedron Lett. 1996, 37, 3457–3460. [Google Scholar] [CrossRef]
- Varoglu, M.; Corbett, T.H.; Valeriote, F.A.; Crews, P. Asperazine, a selective cytotoxic alkaloid from a sponge-derived culture of Aspergillus niger. J. Org. Chem. 1997, 62, 7078–7079. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.T.A. Hyrtioerectines A-C, cytotoxic alkaloids from the Red Sea sponge Hyrtios erectus. J. Nat. Prod. 2005, 68, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Sauleau, P.; Martin, M.-T.; Dau, M.-E.T.H.; Youssef, D.T.A.; Bourguet-Kondracki, M.-L. Hyrtiazepine, an azepino-indole-type alkaloid from the Red Sea marine sponge Hyrtios erectus. J. Nat. Prod. 2006, 69, 1676–1679. [Google Scholar] [CrossRef] [PubMed]
- Salmoun, M.; Devijver, C.; Daloze, D.; Braekman, J.-C.; Van Soest, R.W.M. 5-Hydroxytryptamine-derived alkaloids from two marine sponges of the genus Hyrtios. J. Nat. Prod. 2002, 65, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Aoki, S.; Ye, Y.; Higuchi, K.; Takashima, A.; Tanaka, Y.; Kitagawa, I.; Kobayashi, M. Novel neuronal nitric oxide synthase (nNOS) selective inhibitors, aplysinopsin-type indole alkaloids, from marine sponge Hyrtios erecta. Chem. Pharm. Bull. 2001, 49, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Kazlauskas, R.; Murphy, P.; Quinn, R.; Wells, R. Heteronemin, a new scalarin type sesterterpene from the sponge Heteronema erecta. Tetrahedron Lett. 1976, 17, 2631–2634. [Google Scholar] [CrossRef]
- El Sayed, K.A.; Bartyzel, P.; Shen, X.; Perry, T.L.; Zjawiony, J.K.; Hamann, M.T. Marine natural products as antituberculosis agents. Tetrahedron 2000, 56, 949–953. [Google Scholar] [CrossRef]
- Nasu, S.S.; Yeung, B.K.S.; Hamann, M.T.; Scheuer, P.J.; Kelly-Borges, M.; Goins, K. Puupehenone-related metabolites from two Hawaiian sponges, Hyrtios spp. J. Org. Chem. 1995, 60, 7290–7292. [Google Scholar] [CrossRef]
- Evidente, A.; Kornienko, A.; Lefranc, F.; Cimmino, A.; Dasari, R.; Evidente, M.; Mathieu, V.; Kiss, R. Sesterterpenoids with anticancer activity. Curr. Med. Chem. 2015, 22, 3502–3522. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.P.; Thompson, J.E.; Faulkner, D.J. Sesterterpenes from Spongia idia. J. Org. Chem. 1980, 45, 4976–4979. [Google Scholar] [CrossRef]
- Terem, B.; Scheuer, P.J. Scalaradial derivatives from the nudibranch Chromodoris youngbleuthi and the sponge Spongia oceania. Tetrahedron 1986, 42, 4409–4412. [Google Scholar] [CrossRef]
- Hochlowski, J.E.; Faulkner, D.J.; Bass, L.S.; Clardy, J. Metabolites of the dorid nudibranch Chromodoris sedna. J. Org. Chem. 1983, 48, 1738–1740. [Google Scholar] [CrossRef]
- Bergquist, P.R.; Cambie, R.C.; Kernan, M.R. Scalarane sesterterpenes from Collospongia auris, a new thorectid sponge. Biochem. Syst. Ecol. 1990, 18, 349–357. [Google Scholar] [CrossRef]
- Braekman, J.C.; Daloze, D.; Kaisin, M.; Moussiaux, B. Ichthyotoxic sesterterpenoids from the neo guinean sponge Carteriospongia foliascens. Tetrahedron 1985, 41, 4603–4614. [Google Scholar] [CrossRef]
- Kikuchi, H.; Tsukitani, Y.; Shimizu, I.; Kobayashi, M.; Kitagawa, I. Marine Natural Products. XI. An antiinflammatory scalarane-type bishomosesterterpene, foliaspongin, from the Okinawan marine sponge Phyllospongia foliascens (PALLAS). Chem. Pharm. Bull. 1983, 31, 552–556. [Google Scholar] [CrossRef]
- Nakagawa, M.; Hamamoto, Y.; Ishihama, M.; Hamasaki, S.; Endo, M. Pharmacologically active homosesterterpenes from Palauan sponges. Tetrahedron Lett. 1987, 28, 431–434. [Google Scholar] [CrossRef]
- Kazlauskas, R.; Murphy, P.; Wells, R. Five new C26 tetracyclic terpenes from a sponge (Lendenfeldia sp.). Aust. J. Chem. 1982, 35, 51–59. [Google Scholar] [CrossRef]
- A Gonzalez, M. Scalarane sesterterpenoids. Curr. Bioact. Compd. 2010, 6, 178–206. [Google Scholar] [CrossRef]
- Alahdal, A.M.; Shaala, L.A.; Noor, A.O.; Elfaky, M.A.; Elhady, S.S.; Almohammadi, A.; Bagalagel, A.; Lashkar, M.O.; Almasri, D.M.; Youssef, D. Evaluation of the antiproliferative and cytotoxic activities of marine invertebrates-derived fungi. Pak. J. Pharm. Sci. 2017, 30, 1001–1006. [Google Scholar] [PubMed]
- Cimino, G.; De Stefano, S.; Minale, L.; Trivellone, E. 12-epi-Scalarin and 12-epi-deoxoscalarin, sesterterpenes from the sponge Spongia nitens. J. Chem. Soc. Perkin Trans. 1977, 1, 1587–1593. [Google Scholar] [CrossRef]
- Mahidol, C.; Prawat, H.; Sangpetsiripan, S.; Ruchirawat, S. Bioactive scalaranes from the Thai sponge Hyrtios gumminae. J. Nat. Prod. 2009, 72, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, S.; Miura, S.; Van Soest, R.W.; Ohta, T. Three new cytotoxic sesterterpenes from a marine sponge Spongia sp. J. Nat. Prod. 2003, 66, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Fattorusso, E.; Magno, S.; Santacroce, C.; Sica, D. Scalarin, a new pentacyclic C-25 terpenoid from the sponge Cacospongia scalaris. Tetrahedron 1972, 28, 5993–5997. [Google Scholar] [CrossRef]
- Bonacorsi, C.; Raddi, M.S.G.; Carlos, I.Z.; Sannomiya, M.; Vilegas, W. Anti-helicobacter pylori activity and immunostimulatory effect of extracts from Byrsonima crassa Nied. (Malpighiaceae). BMC Complement. Altern. Med. 2009, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Franzblau, S.G.; Witzig, R.S.; McLaughlin, J.C.; Torres, P.; Madico, G.; Hernandez, A.; Degnan, M.T.; Cook, M.B.; Quenzer, V.K.; Ferguson, R.M.; et al. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 1998, 36, 362–366. [Google Scholar] [PubMed]
- Collins, L.; Franzblau, S.G. Microplate alamar blue assay versus BACTEC 460 system for high-throughput screening of compounds against Mycobacterium tuberculosis and Mycobacterium avium. Antimicrob. Agents Chemother. 1997, 41, 1004–1009. [Google Scholar] [PubMed]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, A.M.; Al-Abd, A.M.; Lightfoot, D.A.; El-Shemy, H.A. Anti-cancer characteristics of mevinolin against three different solid tumor cell lines was not solely p53-dependent. J. Enzyme Inhib. Med. Chem. 2012, 27, 673–679. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |


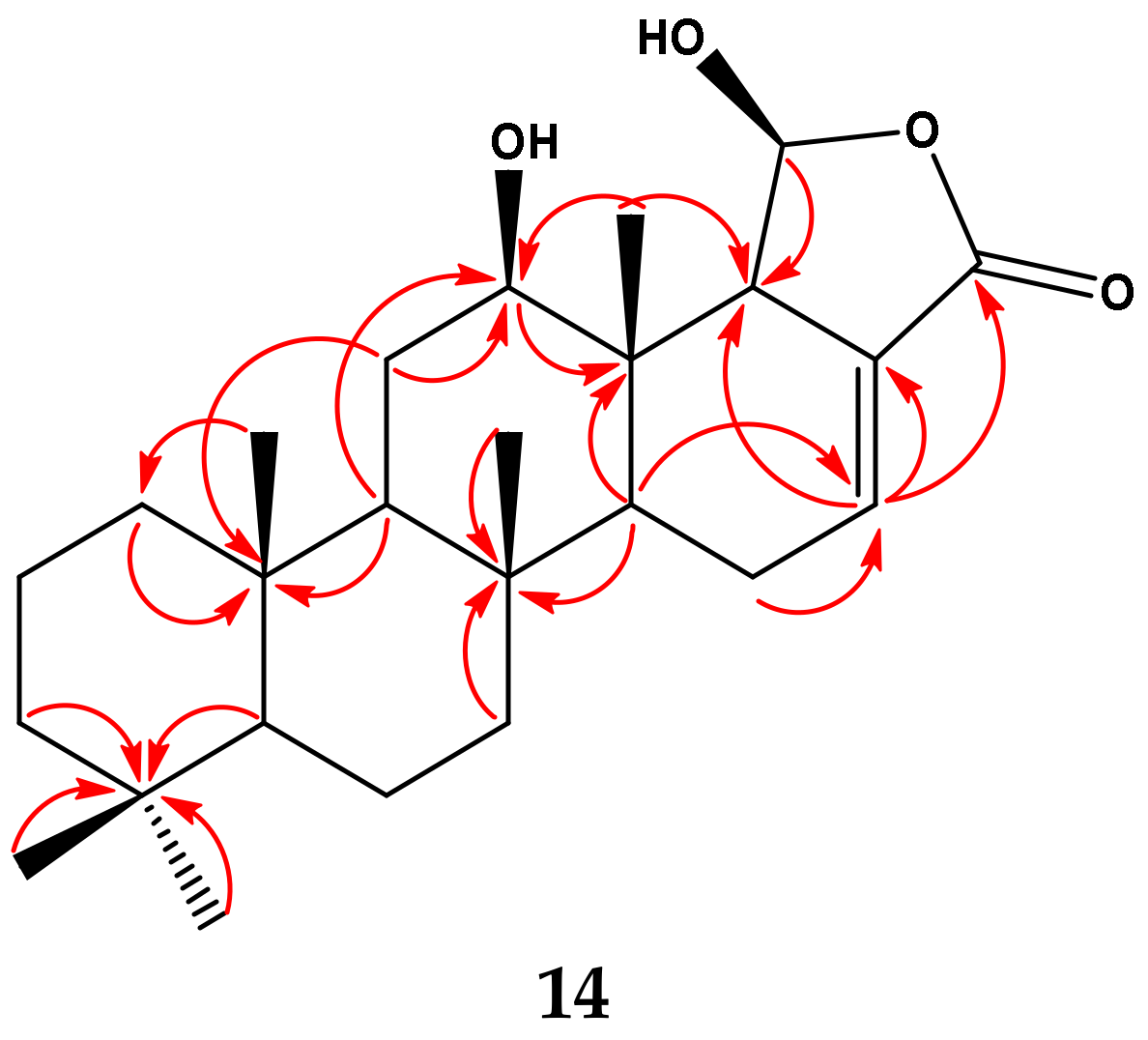
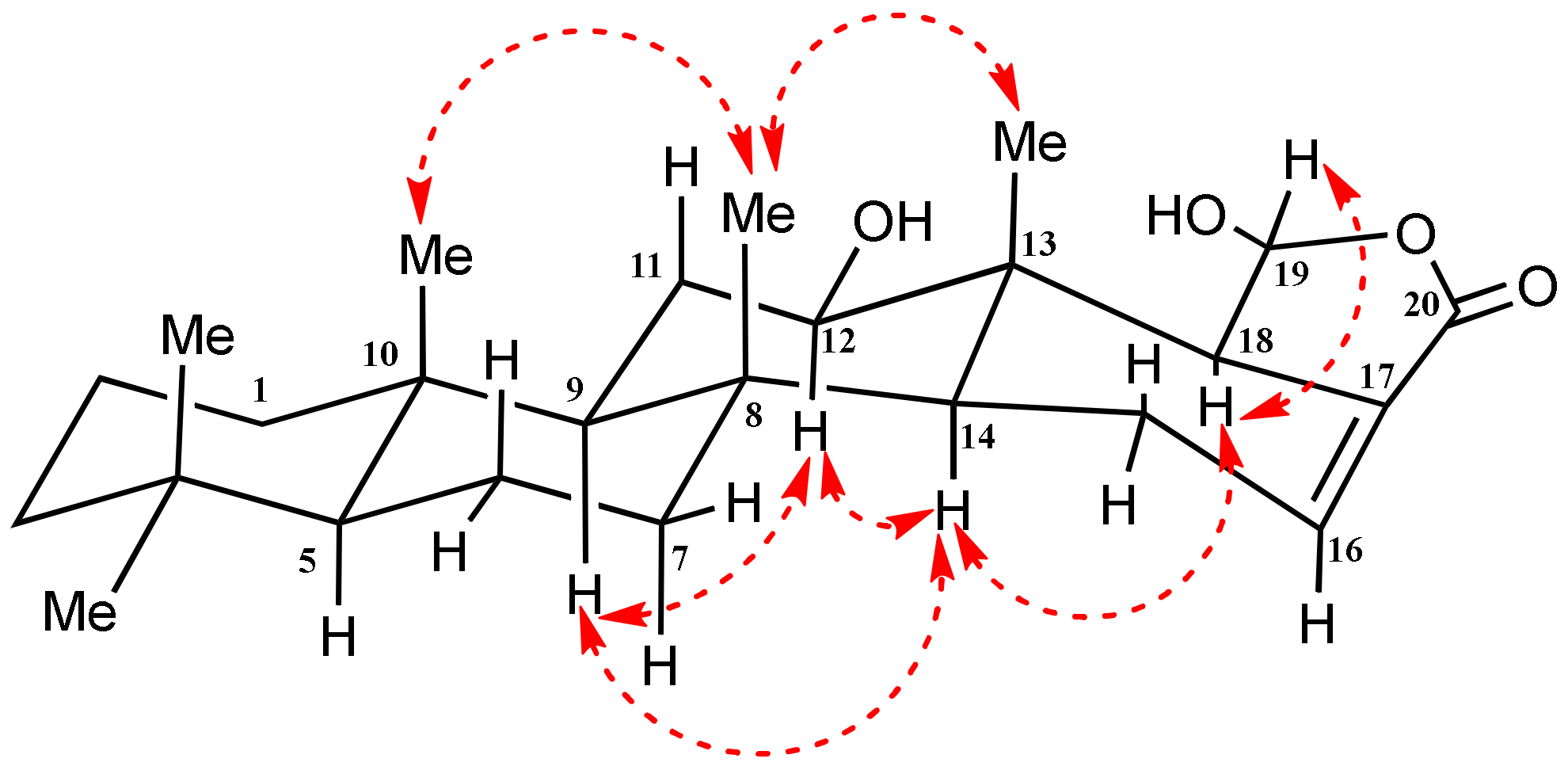
| Position | δC | δH (m, J in Hz) | HMBC (H→C) a |
|---|---|---|---|
| 1 | 39.8, CH2 | 1.73, 0.83 (m) | C-10 |
| 2 | 18.5, CH2 | 1.63, 1.46 (m) | C-4, C-10 |
| 3 | 42.0, CH2 | 1.39, 1.15 (m) | C-4 |
| 4 | 33.2, C | - | - |
| 5 | 56.4, CH | 0.83 (m) | C-4 |
| 6 | 18.0, CH2 | 1.58, 1.42 (m) | |
| 7 | 41.4, CH2 | 1.74, 0.95 (m) | C-8 |
| 8 | 37.4, C | - | - |
| 9 | 58.8, CH | 0.93 (m) | C-10, C-12 |
| 10 | 37.4, C | - | - |
| 11 | 26.1, CH2 | 1.79, 1.50 (m) | C-10, C-12 |
| 12 | 80.4, CH | 3.57 (dd, 11.05, 4.25) | C-9, C-11, C-18, C-25 |
| 12-OH | 4.19 (s) | ||
| 13 | 39.9, C | - | - |
| 14 | 52.7, CH | 1.25 (m) | C-8, C-9, C-13, C-16, C-18 |
| 15 | 23.5, CH2 | 2.17, 2.37 (m) | C-16 |
| 16 | 136.3, CH | 6.87 (dd, 6.80, 3.40) | C-14, C-15, C-18, C-20 |
| 17 | 127.3, C | - | - |
| 18 | 58.7, CH | 2.54 (m) | C-12, C-13, C-25 |
| 19 | 98.5, CH | 5.74 (d, 5.10) | C-18 |
| 20 | 166.9, C | - | - |
| 21 | 21.3, CH3 | 0.81 (s) | C-4 |
| 22 | 33.2, CH3 | 0.83 (s) | C-4 |
| 23 | 16.5, CH3 | 0.85 (s) | C-1, C-5, C-9, C-10 |
| 24 | 16.7, CH3 | 0.93 (s) | C-7, C-8, C-9, C-14 |
| 25 | 9.1, CH3 | 0.86 (s) | C-12, C-13, C-14, C-18 |
| Compound | Anti-H. pylori (MIC) | Anti-TB (MIC) | Cytotoxic (IC50 ± SEM) | ||
|---|---|---|---|---|---|
| MCF-7 | HCT-116 | HepG2 | |||
| 1 | 4.39 | 0.54 | 24.6 ± 2.3 | 25.5 ± 3.3 | 19.8 ± 1.4 |
| 2 | NA | 16.00 | 1.2 ± 0.1 | 0.4 ± 0.1 | 1.1 ± 0.1 |
| 3 | 10.10 | 5.05 | NA | NA | NA |
| 4 | 9.11 | 1.12 | 25.9 ± 1.9 | 17.5 ± 1.3 | 24.7 ± 4.8 |
| 5 | 146.02 | 9.13 | 22.0 ± 0.4 | 15.2 ± 2.0 | 15.3 ± 1.1 |
| 6 | 8.78 | 4.39 | - | - | - |
| 7 | 20.23 | 5.05 | 1.6 ± 0.1 | 1.4 ± 0.05 | 1.6 ± 0.5 |
| 8 | 80.95 | 10.12 | 32.7 ± 3.2 | 34.5 ± 7.5 | 23.5 ± 1.8 |
| 9 | 77.73 | 19.42 | NA | NA | NA |
| 10 | 8.47 | 4.23 | 12.4 ± 0.9 | 2.9 ± 0.7 | 5.5 ± 0.9 |
| 11 | 263.71 | 16.47 | 55.6 ± 2.1 | 17.8 ± 1.9 | 20.1 ± 1.5 |
| 12 | 32.97 | 8.24 | 37.3 ± 5.5 | 22.8 ± 2.2 | 34.9 ± 6.1 |
| 13 | 16.03 | 8.02 | 54.2 ± 3.3 | 26.5 ± 1.1 | 26.6 ± 3.8 |
| 14 | 81.38 | 20.33 | NA | NA | NA |
| 15 | 33.97 | 16.97 | 34.9 ± 4.9 | 48.6 ± 7.2 | 27.3 ± 3.3 |
| isoniazid | ---- | 0.87 | ---- | ---- | ---- |
| clarithromycin | 1.31 | ---- | ---- | ---- | ---- |
| doxorubicin | ---- | ---- | 0.41 ± 0.1 | 0.11 ± 0.04 | 0.85 ± 0.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alahdal, A.M.; Asfour, H.Z.; Ahmed, S.A.; Noor, A.O.; Al-Abd, A.M.; Elfaky, M.A.; Elhady, S.S. Anti-Helicobacter, Antitubercular and Cytotoxic Activities of Scalaranes from the Red Sea Sponge Hyrtios erectus. Molecules 2018, 23, 978. https://doi.org/10.3390/molecules23040978
Alahdal AM, Asfour HZ, Ahmed SA, Noor AO, Al-Abd AM, Elfaky MA, Elhady SS. Anti-Helicobacter, Antitubercular and Cytotoxic Activities of Scalaranes from the Red Sea Sponge Hyrtios erectus. Molecules. 2018; 23(4):978. https://doi.org/10.3390/molecules23040978
Chicago/Turabian StyleAlahdal, Abdulrahman M., Hani Z. Asfour, Safwat A. Ahmed, Ahmad O. Noor, Ahmed M. Al-Abd, Mahmoud A. Elfaky, and Sameh S. Elhady. 2018. "Anti-Helicobacter, Antitubercular and Cytotoxic Activities of Scalaranes from the Red Sea Sponge Hyrtios erectus" Molecules 23, no. 4: 978. https://doi.org/10.3390/molecules23040978
APA StyleAlahdal, A. M., Asfour, H. Z., Ahmed, S. A., Noor, A. O., Al-Abd, A. M., Elfaky, M. A., & Elhady, S. S. (2018). Anti-Helicobacter, Antitubercular and Cytotoxic Activities of Scalaranes from the Red Sea Sponge Hyrtios erectus. Molecules, 23(4), 978. https://doi.org/10.3390/molecules23040978








