Wound Stress, an Unheeded Factor for Echinacoside Accumulation in Cistanche deserticola Y. C. Ma
Abstract
1. Introduction
2. Results and Discussion
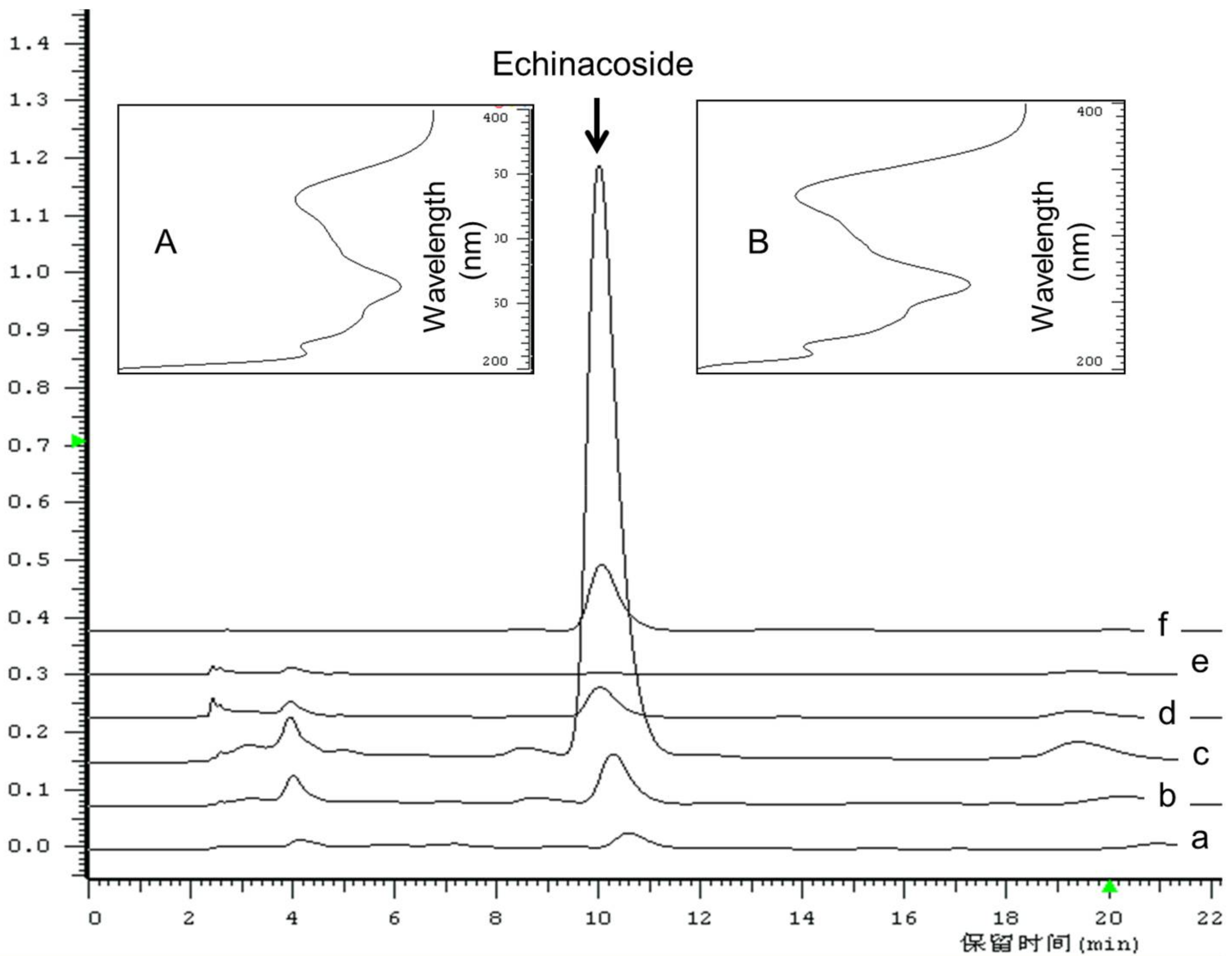
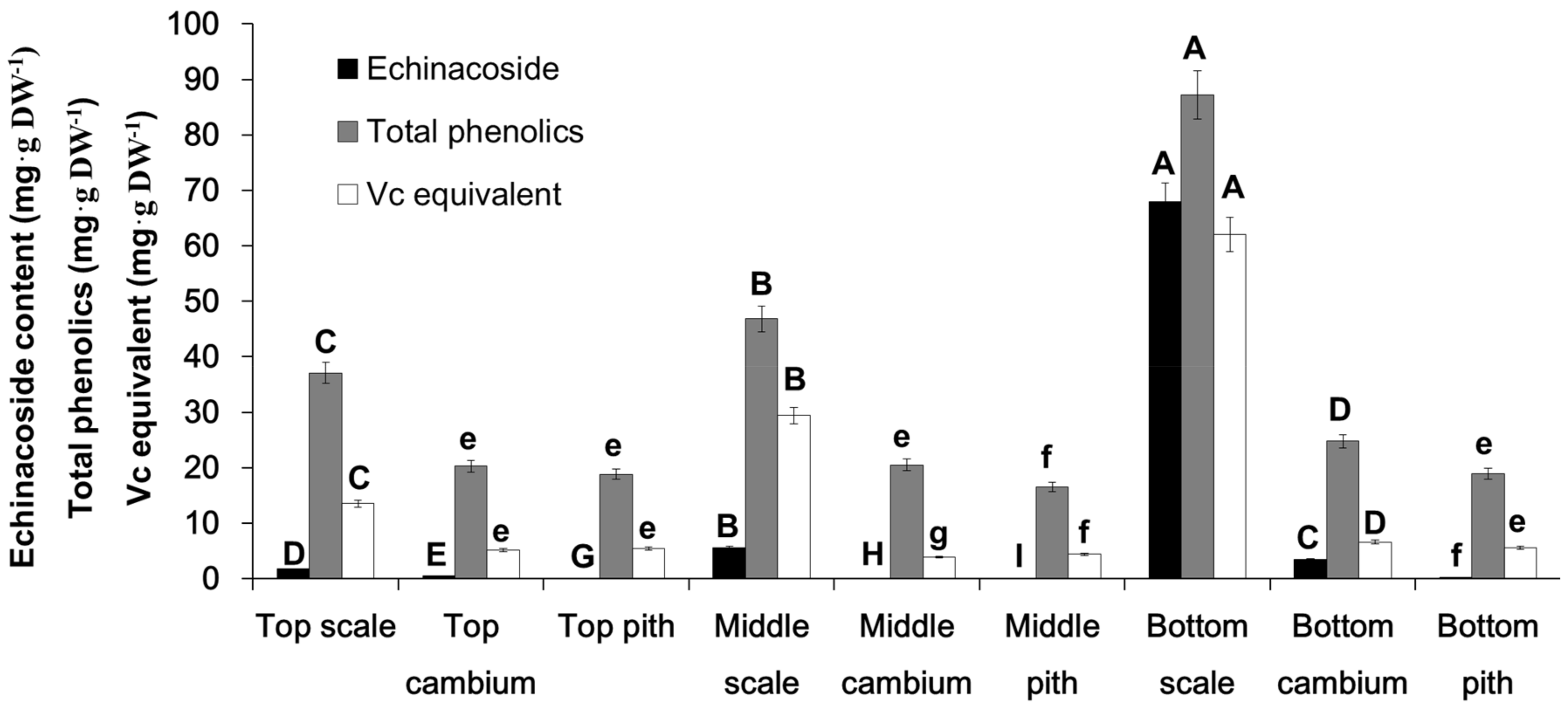
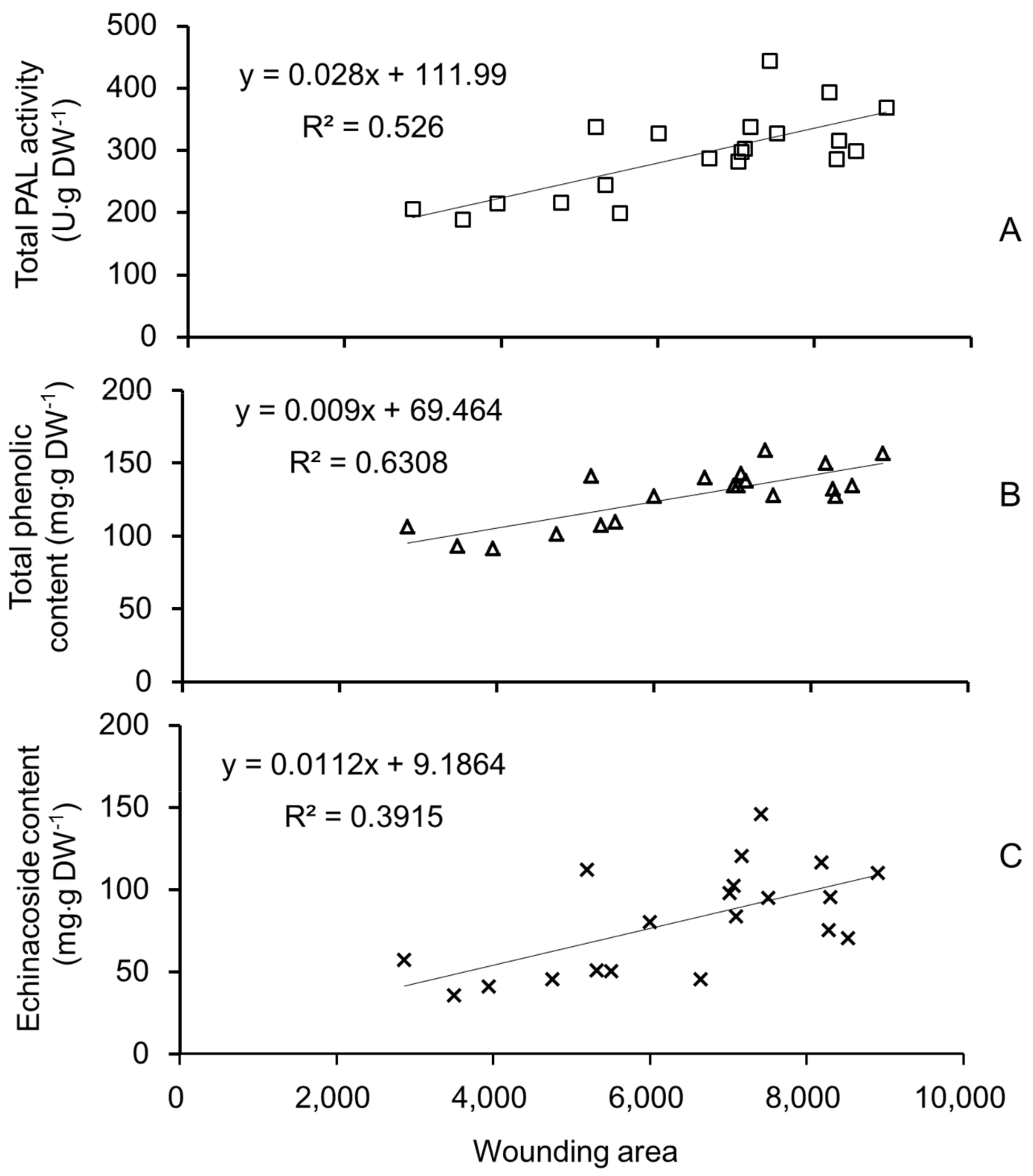
2.1. Content Determination of Echinacoside and Total Phenolic Compounds
2.2. DPPH Scavenging Capacity of Methanol Extract from Different Tissues
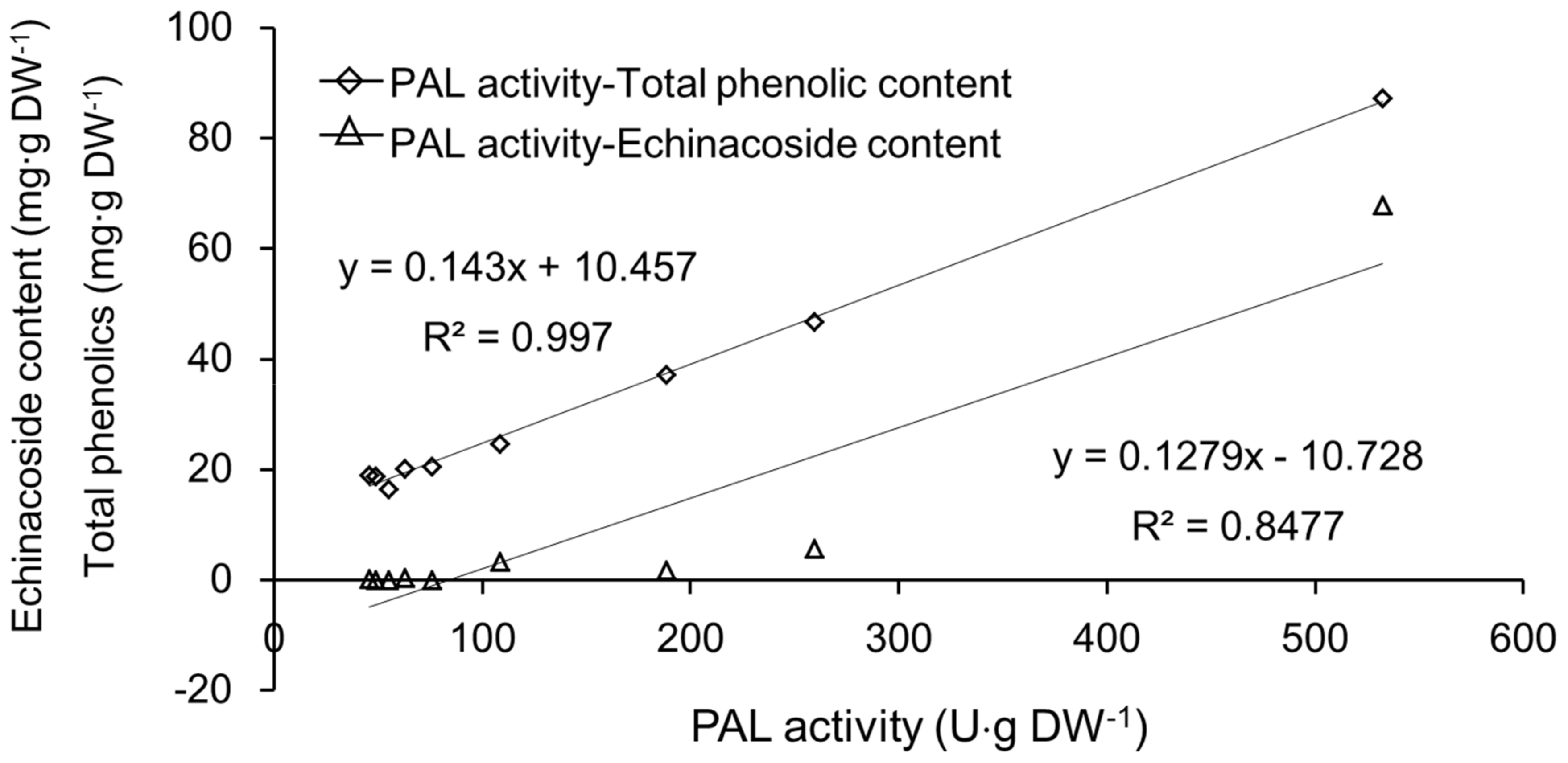
2.3. PAL Activity in Different Tissues from Different Parts
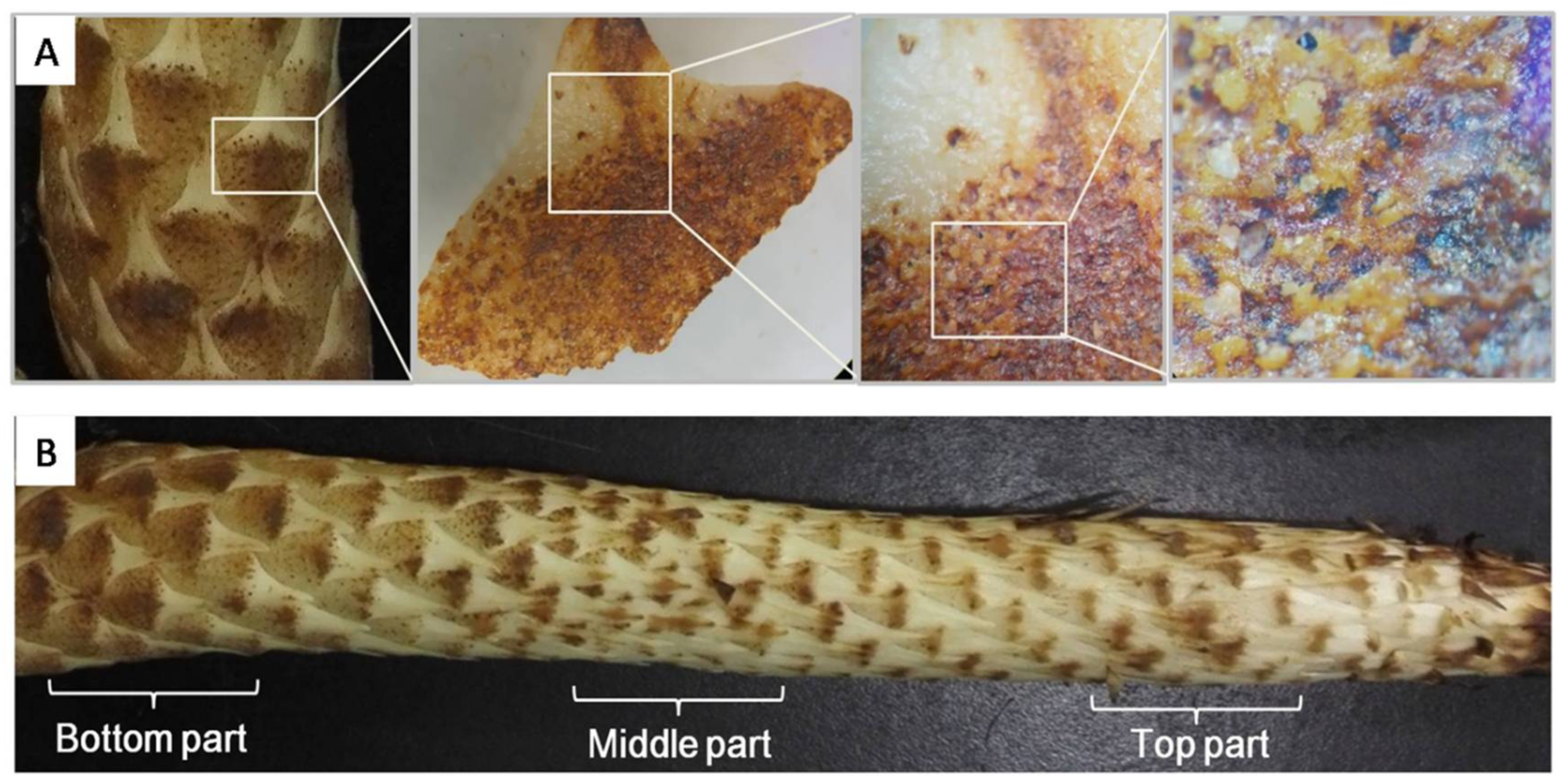
2.4. Microscopic Observation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Sample Collection and Partition
3.3. Extract Preparation
3.4. Content Determination of Echinacoside Using RP-HPLC
3.5. Total Phenolic Compound Determination Using the Folin–Ciocalteu Method
3.6. DPPH Scavenging Activity Assay
3.7. PAL Activity Assay
3.8. Stereomicroscopic Visualization
3.9. Wounding Area Quantification
3.10. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ding, Y.; Zhang, K.M.; Cang, X.X.; Sun, H.; Xiao, W.; Zhu, J.B. Advance in chemical constituents and biological activity of genus Cistanche. J. Dalian Polytech. Univer. 2016, 35, 395–402. [Google Scholar]
- Committee of Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; Medical Science Press: Beijing, China, 2015; pp. 135–136. [Google Scholar]
- Sheng, G.Q.; Pu, X.P.; Lei, L.; Tu, P.; Li, C. Tubuloside B from Cistanche salsa rescue the PC12 neuronal cells from methylphenylpyridinium ion induced apoptosis and oxidatives stress. Planta Med. 2002, 68, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Pu, X.P.; Song, Z.H.; Li, Y.Y.; Tu, P. Acteoside from Cistanche salsa inhibits apoptosis by 1, 2-methyl phenylpyridinium ion in cerebellar granule neurons. Planta Med. 2003, 69, 652–666. [Google Scholar] [CrossRef] [PubMed]
- Geng, X.C.; Song, L.W.; Pu, X.P. Neuroprotective Effects of Phenylethanoid Glycosides from Cistanche salsa against 1-Methyl-4-phenyl-1,2,3,6-tetrahydro-pyridine Dopaminergic Toxicity in C57 Mice. Biol. Pharm. Bull. 2004, 27, 797–801. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tian, X.F.; Pu, X.P. Phenylethanoid glycosides from Cistanche salsa inhibit apoptosis induced by 1-methyl-4-phenylpyridinium ion in neurons. J. Ethnopharmacol. 2005, 97, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Z.; Cheng, X.Y. Enhancement of phenylethanoid glycosides biosynthesis in cell cultures of Cistanchedeserticola by osmotic stress. Plant Cell Rep. 2008, 27, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Wang, X.D.; Zhao, B.; Wang, Y.C. Enhanced production of phenylethanoid glycosides by precursor feeding to cell culture of Cistanche deserticola. Process Biochem. 2005, 40, 3480–3484. [Google Scholar] [CrossRef]
- Liu, J.Y.; Guo, Z.G.; Zeng, Z.L. Improved accumulation of phenylethanoid glycosides by precursor feeding to suspension culture of Cistanche salsa. Biochem. Eng. J. 2007, 33, 88–93. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Guo, B.; Zhou, H.Y.; Ni, W.; Liu, C.Z. Repeated elicitation enhances phenylethanoid glycosides accumulation in cell suspension cultures of Cistanche deserticola. J. Biochem. Eng. 2005, 24, 203–207. [Google Scholar] [CrossRef]
- Chen, Q.L.; Wu, Z.B.; Guo, Y.H. Cultivation Techniques of Cistanche deserticola and its Host Haloxylon ammodendron. Mod. Chin. Med. 2015, 17, 359–368. [Google Scholar]
- Dixon, R.A.; Paiva, N.L. Stress-lnduced Phenylpropanoid Metabolism. Plant Cell 1995, 7, 1085–1097. [Google Scholar] [CrossRef] [PubMed]
- Min, W.H.; Yeo, H.Y.; Jun, Y.K.; Seong, H.K. Fungal and Plant Phenylalanine Ammonia-lyase. Microbiology 2011, 39, 257–265. [Google Scholar]
- Cynthia, L.C.; Antoine, P.; Patrick, F.D. Effects of PHENYLALANINE AMMONIA LYASE (PAL) knockdown on cell wall composition, biomass digestibility, and biotic and abiotic stress responses in Brachy podium. J. Exp. Bot. 2015, 66, 4317–4335. [Google Scholar]
- Saimaru, H.; Orihara, Y. Biosynthesis of acteoside in cultured cells of Olea europaea. J. Nat. Med. 2010, 64, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Chen, T.; Yao, F.W.; Li, C.P.; Tang, Q.L.; Sun, M.; Sun, G.Y.; Hu, S.N.; Yu, J.; et al. RNA-Seq Based De Novo Transcriptome Assembly and Gene Discovery of Cistanche deserticola Fleshy Stem. PLoS ONE 2015, 10, e0125722. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.S.; Jia, J.M.; Hur, Y.J.; Chung, Y.S.; Lee, J.H.; Yun, D.J.; Chung, W.S.; Yi, G.H.; Kim, T.H.; Kim, D.H. Molecular characterization of phenylalanine ammonia lyase gene from Cistanche deserticola. Mol. Biol. Rep. 2011, 38, 3741–3750. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.X.; Zhang, X.H.; Cai, J.Z. Study on secondary metabolic organ of echinacoside in herbs of Cistanche tubulosa. China J. Chin. Mat. Med. 2007, 32, 2591–2594. [Google Scholar]
- Xu, Z.C.; Peters, R.J.; Weirather, J.; Luo, H.M.; Liao, B.S.; Zhang, X.; Zhu, Y.J.; Ji, A.J.; Zhang, B.; Hu, S.N.; et al. Full-length transcriptome sequences and splice variants obtained by a combination of sequencing platforms applied to different root tissues of Salvia miltiorrhiza and tanshinone biosynthesis. Plant J. 2015, 82, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Gloria, L.G.; Mikal, S.; Marita, C. Wound-induced phenylalanine ammonia lyase activity: Factors affecting its induction and correlation with the quality of minimally processed lettuces. Postharvest Biol. Technol. 1996, 9, 223–233. [Google Scholar]
- Bernards, M.A.; Susag, L.M.; Bedgar, D.L.; Anterola, A.M.; Lewis, N.G. Induced phenylpropanoid metabolism during suberization and lignification: A comparative analysis. J. Plant Physiol. 2000, 157, 601–607. [Google Scholar] [CrossRef]
- Liu, Y.J. Pharmaceutical Statistics; China Medical Science Press: Beijing, China, 2013; p. 341. [Google Scholar]
- Yang, Z.Y.; Lu, D.Y.; Yao, S.; Ma, Z.G. Chemical fingerprint and quantitative analysis of Cistanchedeserticola by HPLC-DAD-ESI-MS. J. Food Drug Anal. 2013, 21, 50–57. [Google Scholar]
- Hu, G.S.; Xu, K.X.; Soon, J.J.; Doh, H.K. Relationship of Phenolic Compounds and Free-radical Scavenging Activity in Black and Red Rice Extract. Korean J. Breed Sci. 2010, 42, 129–138. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Wu, T.; Chang, Y.; Zhan, X.; Jia, J. Wound Stress, an Unheeded Factor for Echinacoside Accumulation in Cistanche deserticola Y. C. Ma. Molecules 2018, 23, 893. https://doi.org/10.3390/molecules23040893
Hu G, Wu T, Chang Y, Zhan X, Jia J. Wound Stress, an Unheeded Factor for Echinacoside Accumulation in Cistanche deserticola Y. C. Ma. Molecules. 2018; 23(4):893. https://doi.org/10.3390/molecules23040893
Chicago/Turabian StyleHu, Gaosheng, Tianran Wu, Yue Chang, Xinyi Zhan, and Jingming Jia. 2018. "Wound Stress, an Unheeded Factor for Echinacoside Accumulation in Cistanche deserticola Y. C. Ma" Molecules 23, no. 4: 893. https://doi.org/10.3390/molecules23040893
APA StyleHu, G., Wu, T., Chang, Y., Zhan, X., & Jia, J. (2018). Wound Stress, an Unheeded Factor for Echinacoside Accumulation in Cistanche deserticola Y. C. Ma. Molecules, 23(4), 893. https://doi.org/10.3390/molecules23040893




