A Novel Colorimetric Fluorescent Probe for SO2 and Its Application in Living Cells Imaging
Abstract
:1. Introduction
2. Results
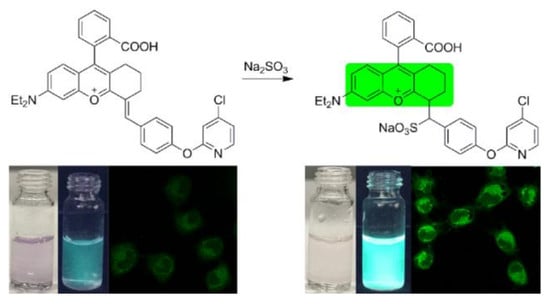
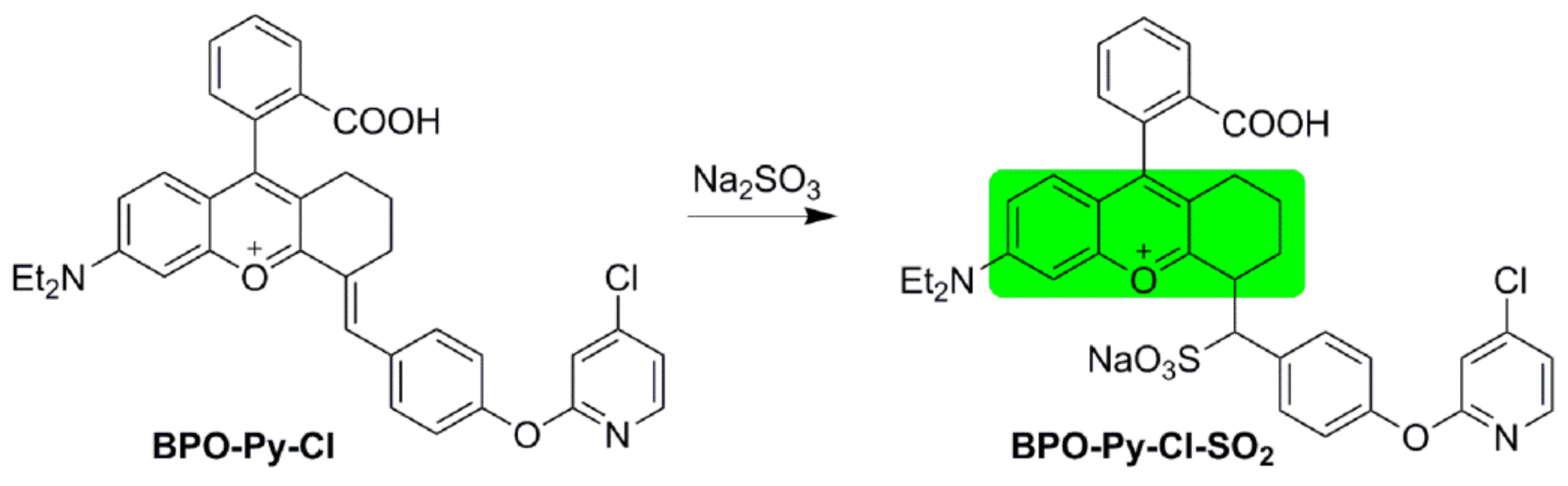
2.1. Synthesis and Characterization of BPO-Py-Cl
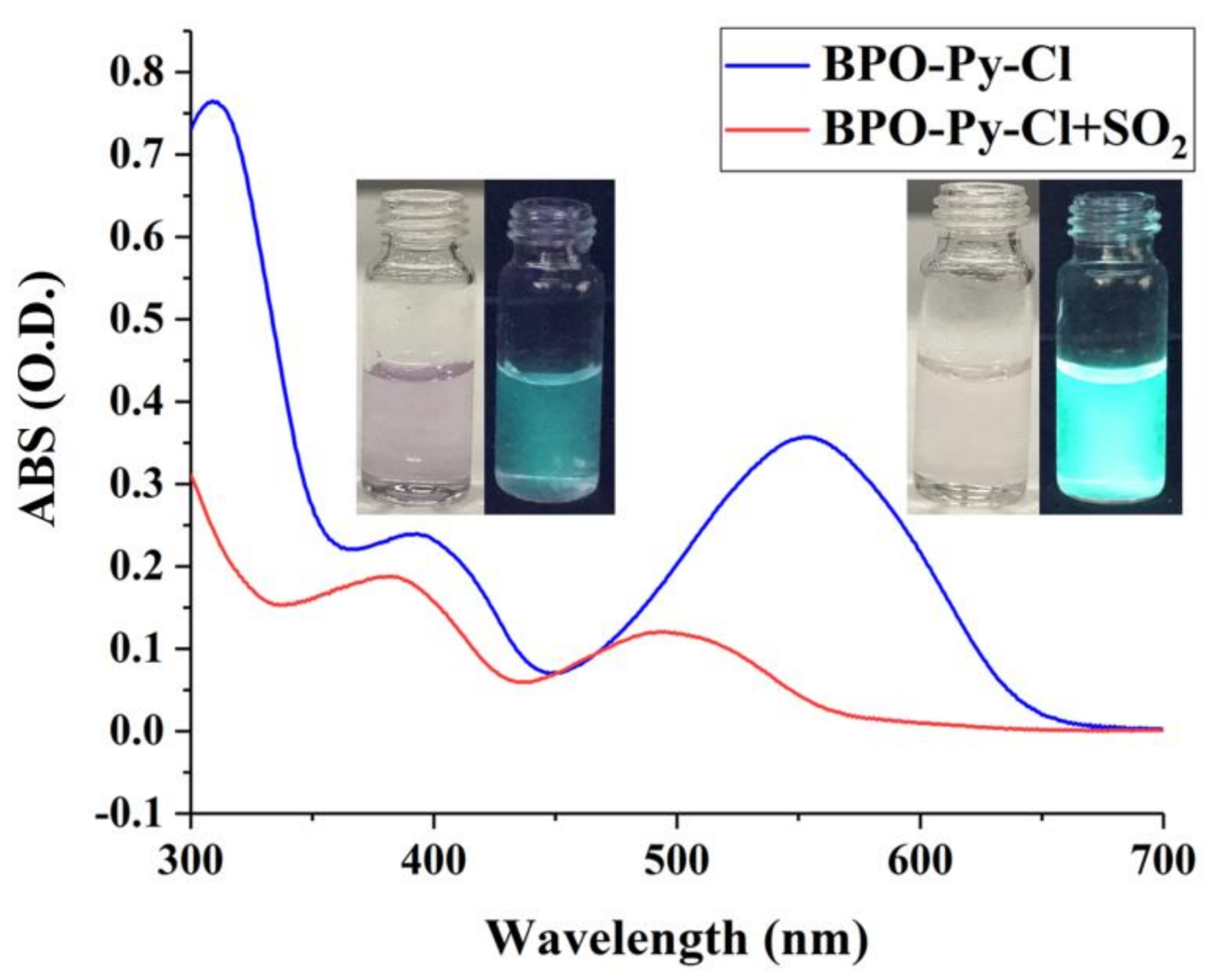
2.2. Optical Response of BPO-Py-Cl toward SO2
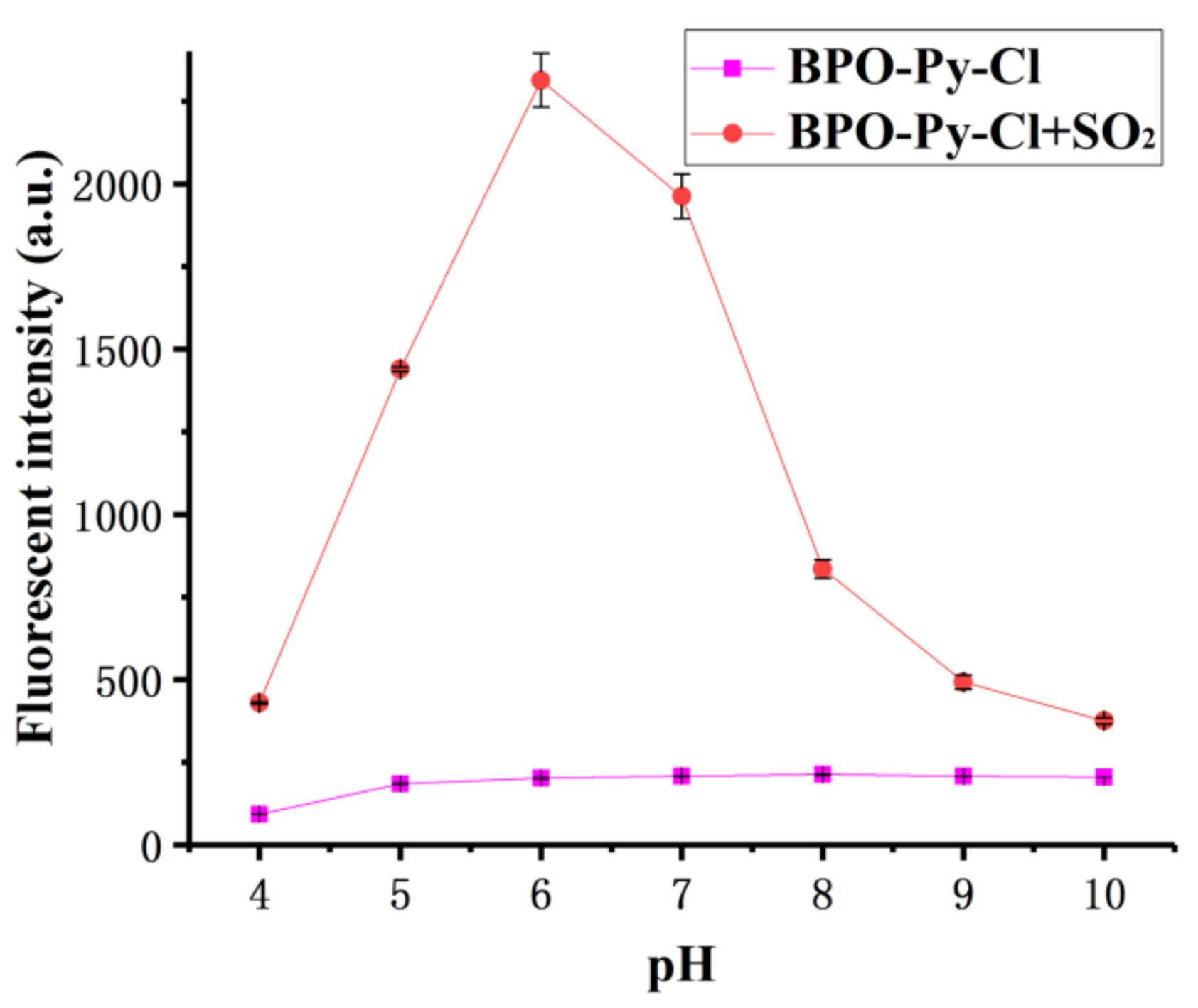
2.3. pH-Dependent Fluorescence Response of the Probe toward SO2
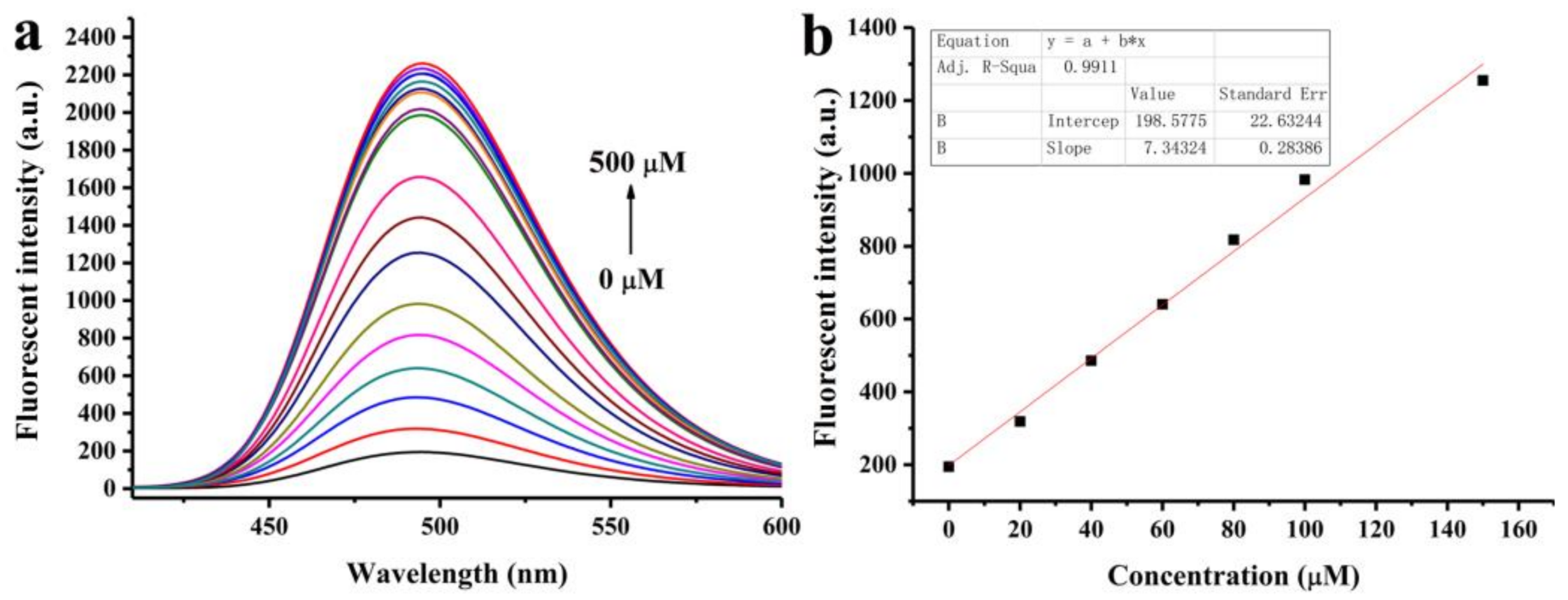
2.4. Quantitative Determination of SO2
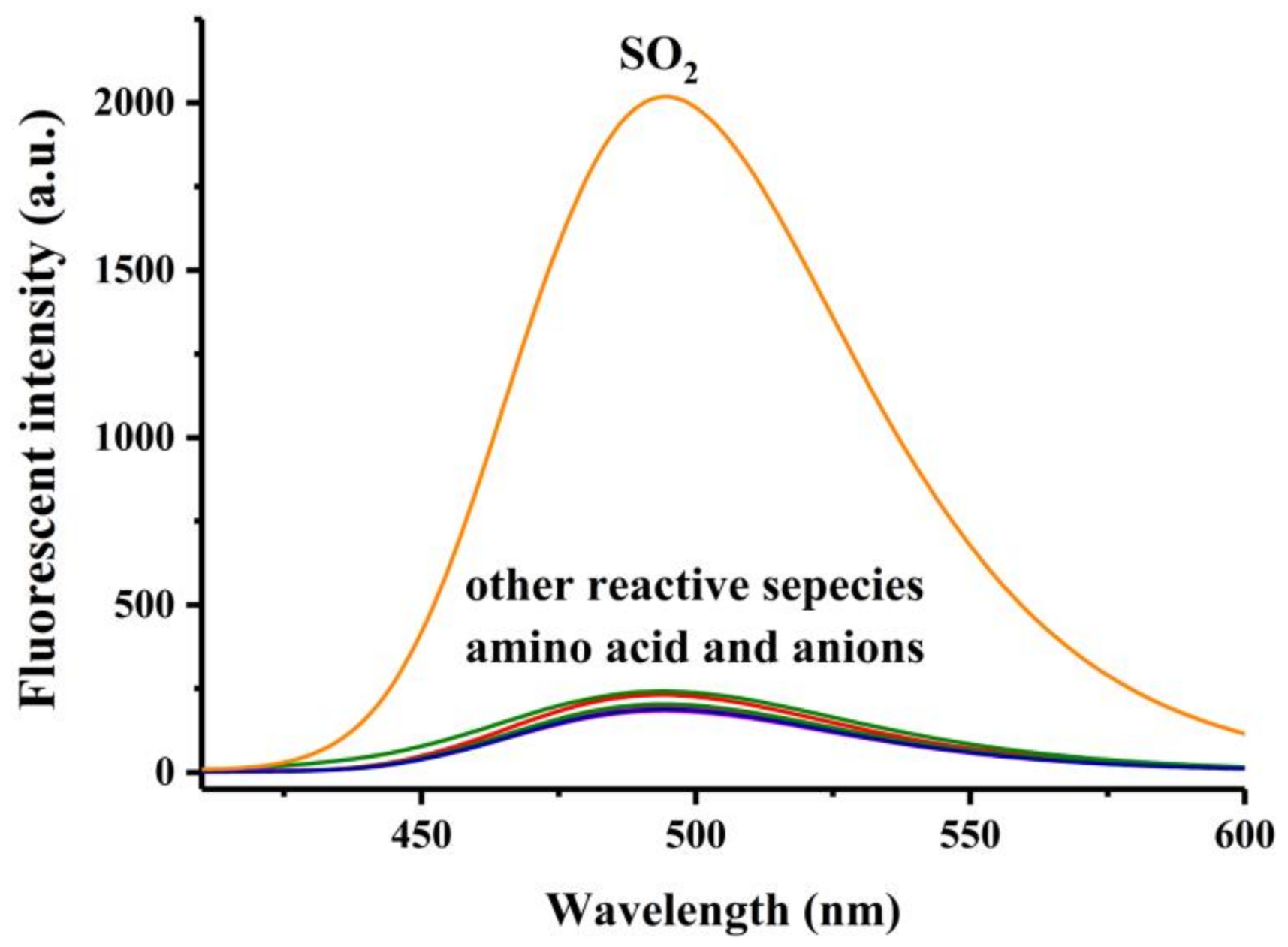
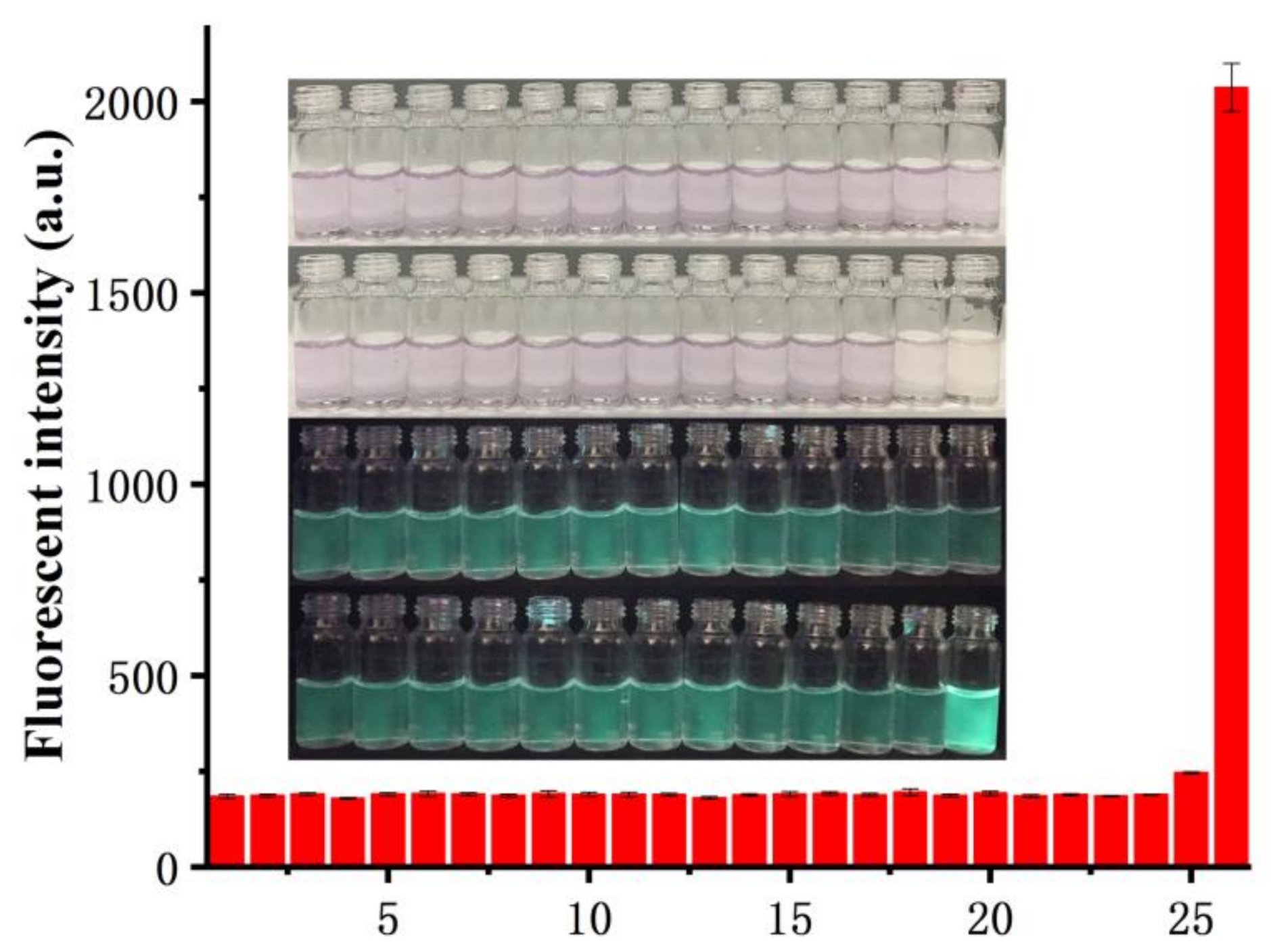
2.5. Selectivity Experiments
2.6. Possible Mechanism
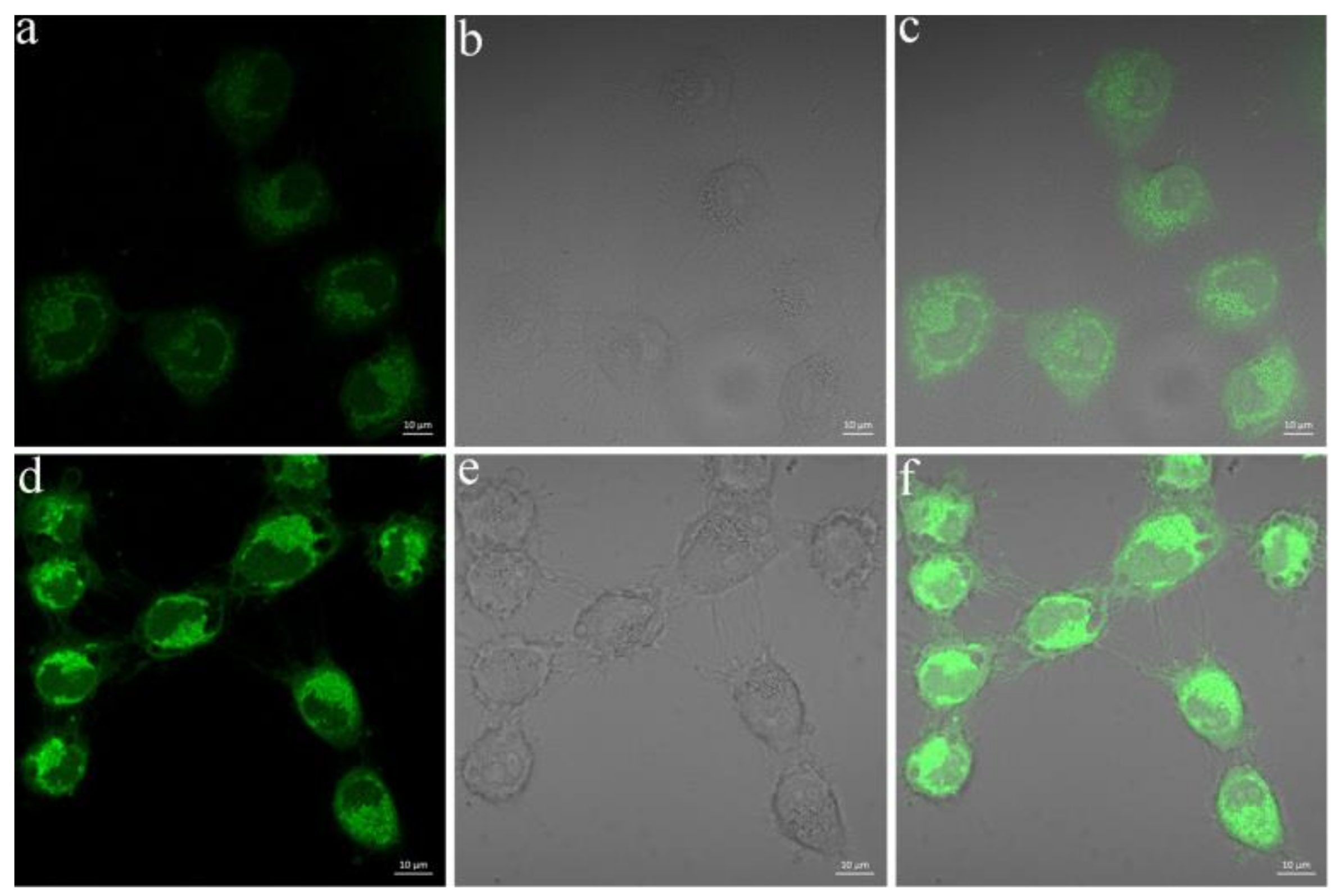
2.7. Living Cell Imaging
3. Materials and Methods
3.1. Materials and General Instruments
3.2. Synthesis and Characterization
3.3. Preparation of the Test Solutions
3.4. Quantum Yields
3.5. CCK-8 Assay for the Cell Cytotoxicity
3.6. Determination of the Detection Limit
3.7. Fluorescence Imaging of SO2 in Living Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chen, T.-M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor air pollution: Nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am. J. Med. Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bazer, F.W.; Gao, H.; Jobgen, W.; Johnson, G.A.; Li, P.; McKnight, J.R.; Satterfield, M.C.; Spencer, T.E.; Wu, G. Amino acids and gaseous signalling. Amino Acids 2009, 37, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Griffith, O.W. Mammalian sulfur amino acid metabolism: An overview. Methods Enzymol. 1987, 143, 366–376. [Google Scholar] [PubMed]
- Li, J.; Meng, Z. The role of sulfur dioxide as an endogenous gaseous vasoactive factor in synergy with nitric oxide. Nitric Oxide 2009, 20, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, R.; Meng, Z. Sulfur dioxide upregulates the aortic nitric oxide pathway in rats. Eur. J. Pharmacol. 2010, 645, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Song, A.; Liao, Q.; Li, J.; Lin, F.; Liu, E.; Jiang, X.; Deng, L. Chronic exposure to sulfur dioxide enhances airway hyperresponsiveness only in ovalbumin-sensitized rats. Toxicol. Lett. 2012, 214, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, Y.; Wang, X.; Sun, Y.; Tang, C.; Du, J. Sulfur dioxide preconditioning increases antioxidative capacity in rat with myocardial ischemia reperfusion (I/R) injury. Nitric Oxide 2013, 32, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, C.; Jin, H.; Du, J. Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis 2011, 215, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, Y.; Prabha, M.; Liu, D.; Chen, S.; Zhang, R.; Liu, X.; Tang, C.; Tang, X.; Jin, H.; et al. Effects of sulfur dioxide on hypoxic pulmonary vascular structural remodelling. Lab. Investig. 2010, 90, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, D.; Ochs, T.; Tang, C.; Chen, S.; Zhang, S.; Geng, B.; Jin, H.; Du, J. Endogenous sulfur dioxide protects against isoproterenol-induced myocardial injury and increases myocardial antioxidant capacity in rats. Lab. Investig. 2011, 91, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Li, G.K.; Sang, N. Delayed rectifier potassium channels are involved in SO2 derivative-induced hippocampal neuronal injury. Ecotoxicol. Environ. Saf. 2009, 72, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Migliore, L.; Coppedè, F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutat. Res. 2009, 674, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Sang, N.; Yun, Y.; Yao, G.; Li, H.; Guo, L.; Li, G. SO2-induced neurotoxicity is mediated by cyclooxygenases-2-derived prostaglandin E2 and its downstream signaling pathway in rat hippocampal neurons. Toxicol. Sci. 2011, 124, 400–413. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Bu, D.; Liu, A.D.; Holmberg, L.; Jia, Y.; Tang, C.; Du, J.; Jin, H. Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling. Cell Death Dis. 2014, 5, e1251. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Han, X.; Chen, L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem. Commun. 2014, 50, 12234–12249. [Google Scholar] [CrossRef] [PubMed]
- Lin, V.S.; Chen, W.; Xian, M.; Chang, C.J. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem. Soc. Rev. 2015, 44, 4596–4618. [Google Scholar] [CrossRef] [PubMed]
- Jiao, X.; Li, Y.; Niu, J.; Xie, X.; Wang, X.; Tang, B. Small-molecule fluorescent probes for imaging and detection of reactive oxygen, nitrogen, and sulfur species in biological systems. Anal. Chem. 2018, 90, 533–555. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Guo, S.; Hu, C.; Fan, J.; Peng, X. Recent development of chemosensors based on cyanine platforms. Chem. Rev. 2016, 116, 7768–7817. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.-T.; Ren, W.X.; Li, K.; Seo, J.; Sharma, A.; Yu, X.-Q.; Kim, J.S. Fluorescent bioimaging of pH: From design to applications. Chem. Soc. Rev. 2017, 46, 2076–2090. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zeng, Z.; Jiang, J.-H.; Chang, Y.-T.; Yuan, L. Discerning the chemistry in individual organelles with small-molecule fluorescent probes. Angew. Chem. Int. Ed. 2016, 55, 13658–13699. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; He, T.; Li, K.; Wu, M.-B.; Huang, Z.; Yu, X.-Q. A real-time colorimetric and ratiometric fluorescent probe for sulfite. Analyst 2013, 138, 3018–3025. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Li, K.; Li, C.-Y.; Hou, J.-T.; Yu, X.-Q. A water-soluble near-infrared probe for colorimetric and ratiometric sensing of SO2 derivatives in living cells. Chem. Commun. 2014, 50, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, K.; Xie, K.-X.; Li, L.-L.; Yu, K.-K.; Wang, X.; Yu, X.-Q. A water-soluble and fast-response mitochondria-targeted fluorescent probe for colorimetric and ratiometric sensing of endogenously generated SO2 derivatives in living cells. Chem. Commun. 2016, 52, 3430–3433. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, K.; Wu, M.-Y.; Liu, Y.-H.; Xie, Y.-M.; Yu, X.-Q. A mitochondria-targeted colorimetric and ratiometric fluorescent probe for biological SO2 derivatives in living cells. Chem. Commun. 2015, 51, 10236–10239. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, K.; Hou, J.-T.; Li, L.-L.; Lu, C.-Y.; Xie, Y.-M.; Wang, X.; Yu, X.-Q. Novel tumor-specific and mitochondria-targeted near-infrared-emission fluorescent probe for SO2 derivatives in living cells. ACS Sens. 2016, 1, 166–172. [Google Scholar] [CrossRef]
- Xu, W.; Teoh, C.L.; Peng, J.; Su, D.; Yuan, L.; Chang, Y.-T. A mitochondria-targeted ratiometric fluorescent probe to monitor endogenously generated sulfur dioxide derivatives in living cells. Biomaterials 2015, 56, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-Q.; Liu, J.; Zhang, J.; Yang, T.; Guo, W. Fluorescent probe for biological gas SO2 derivatives bisulfite and sulfite. Chem. Commun. 2013, 49, 2637–2639. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-L.; Wang, Z.-Y.; Dai, X.; Miao, J.-Y.; Zhao, B.-X. An effective colorimetric and ratiometric fluorescent probe based FRET with a large Stokes shift for bisulfite. Sci. Rep. 2016, 6, 25315. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-P.; Wang, Z.-Y.; Cao, X.-J.; Cui, J.; Wang, X.; Cui, H.-Z.; Miao, J.-Y.; Zhao, B.-X. A mitochondria-targeted fluorescent probe for ratiometric detection of endogenous sulfur dioxide derivatives in cancer cells. Chem. Commun. 2016, 52, 2760–2763. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Chen, Y.; Wang, J.; Wu, J.; Gasser, G.; Ji, L.; Chao, H. Direct imaging of biological sulfur dioxide derivatives in vivo using a two-photon phosphorescent probe. Biomaterials 2015, 63, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tang, Y.; Zhao, Y.; Gao, S.; Lin, W. Two-photon and deep-red emission ratiometric fluorescent probe with a large emission shift and signal ratios for sulfur dioxide: Ultrafast response and applications in living cells, brain tissues, and zebrafishes. Anal. Chem. 2017, 89, 9388–9393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, T.; Huo, F.; Ning, P.; Meng, X.; Yin, C. Reversible ratiometric fluorescent probe for sensing bisulfate/H2O2 and Its application in zebrafish. Anal. Chem. 2017, 89, 8079–8083. [Google Scholar] [CrossRef] [PubMed]
- Dou, K.; Fu, Q.; Chen, G.; Yu, F.; Liu, Y.; Cao, Z.; Li, G.; Zhao, X.; Xia, L.; Chen, L.; et al. A novel dual-ratiometric-response fluorescent probe for SO2/ClO− detection in cells and in vivo and its application in exploring the dichotomous role of SO2 under the ClO− induced oxidative stress. Biomaterials 2017, 133, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Du, W.; Zhang, M.; Xu, Y.; Song, L.; Zhang, Q.; Tian, X.; Zhou, H.; Wu, J.; Tian, Y. A series of water-soluble A–π–A′ typological indolium derivatives with two-photon properties for rapidly detecting HSO3−/SO32− in living cells. J. Mater. Chem. B 2017, 5, 3862–3869. [Google Scholar] [CrossRef]
- Che, S.; Dao, R.; Zhang, W.; Lv, X.; Li, H.; Wang, C. Designing an anion-functionalized fluorescent ionic liquid as an efficient and reversible turn-off sensor for detecting SO2. Chem. Commun. 2017, 53, 3862–3865. [Google Scholar] [CrossRef] [PubMed]
- Dou, K.; Chen, G.; Yu, F.; Sun, Z.; Li, G.; Zhao, X.; Chen, L.; You, J. A two-photon ratiometric fluorescent probe for the synergistic detection of the mitochondrial SO2/HClO crosstalk in cells and in vivo. J. Mater. Chem. B 2017, 5, 8389–8398. [Google Scholar] [CrossRef]
- Shang, H.; Liu, K.; Lin, W. Construction of a novel ratiometric near-infrared fluorescent probe for SO2 derivatives and its application for biological imaging. Anal. Methods 2017, 9, 3790–3794. [Google Scholar] [CrossRef]
- Chen, H.; Dong, B.; Tang, Y.; Lin, W. A unique “integration” strategy for the rational design of optically tunable near-infrared fluorophores. Acc. Chem. Res. 2017, 50, 1410–1422. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Song, X.; Kong, X.; Wang, C.; Tang, Y.; Liu, Y.; Lin, W. Simultaneous near-infrared and two-photon in vivo imaging of H2O2 using a ratiometric fluorescent probe based on the unique oxidative rearrangement of oxonium. Adv. Mater. 2016, 28, 8755–8759. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Yang, Y.; Chen, H. A unique class of near-infrared functional fluorescent dyes with carboxylic-acid-modulated fluorescence ON/OFF switching: Rational design, synthesis, optical properties, theoretical calculations, and applications for fluorescence imaging in living animals. J. Am. Chem. Soc. 2012, 134, 1200–1211. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Cheng, D.; Ren, T.; Li, Y.; Zeng, Z.; Yuan, L. Design of NIR chromenylium-cyanine fluorophore library for “switch-ON” and ratiometric detection of bio-active species in vivo. Anal. Chem. 2016, 88, 1842–1849. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Lin, W.; Chen, H. Analogs of Changsha near-infrared dyes with large Stokes Shifts for bioimaging. Biomaterials 2013, 34, 9566–9571. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.C.A.; Rees, W.T. Correction of fluorescence spectra and measurement of fluorescence quantum efficiency. Analyst 1960, 85, 587–600. [Google Scholar] [CrossRef]
- Joshi, B.P.; Park, J.; Lee, W.I.; Lee, K. Ratiometric and turn-on monitoring for heavy and transition metal ions in aqueous solution with a fluorescent peptide sensor. Talanta 2009, 78, 903–909. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds BPOH and BPO-Py-Cl are available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-Y.; Wu, J.; Wang, Y.; Liu, Y.-H.; Yu, X.-Q. A Novel Colorimetric Fluorescent Probe for SO2 and Its Application in Living Cells Imaging. Molecules 2018, 23, 871. https://doi.org/10.3390/molecules23040871
Wu M-Y, Wu J, Wang Y, Liu Y-H, Yu X-Q. A Novel Colorimetric Fluorescent Probe for SO2 and Its Application in Living Cells Imaging. Molecules. 2018; 23(4):871. https://doi.org/10.3390/molecules23040871
Chicago/Turabian StyleWu, Ming-Yu, Jing Wu, Yue Wang, Yan-Hong Liu, and Xiao-Qi Yu. 2018. "A Novel Colorimetric Fluorescent Probe for SO2 and Its Application in Living Cells Imaging" Molecules 23, no. 4: 871. https://doi.org/10.3390/molecules23040871
APA StyleWu, M.-Y., Wu, J., Wang, Y., Liu, Y.-H., & Yu, X.-Q. (2018). A Novel Colorimetric Fluorescent Probe for SO2 and Its Application in Living Cells Imaging. Molecules, 23(4), 871. https://doi.org/10.3390/molecules23040871