Application of Multiple Regression and Design of Experiments for Modelling the Effect of Monoethylene Glycol in the Calcium Carbonate Scaling Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Data Acquision and Modelling
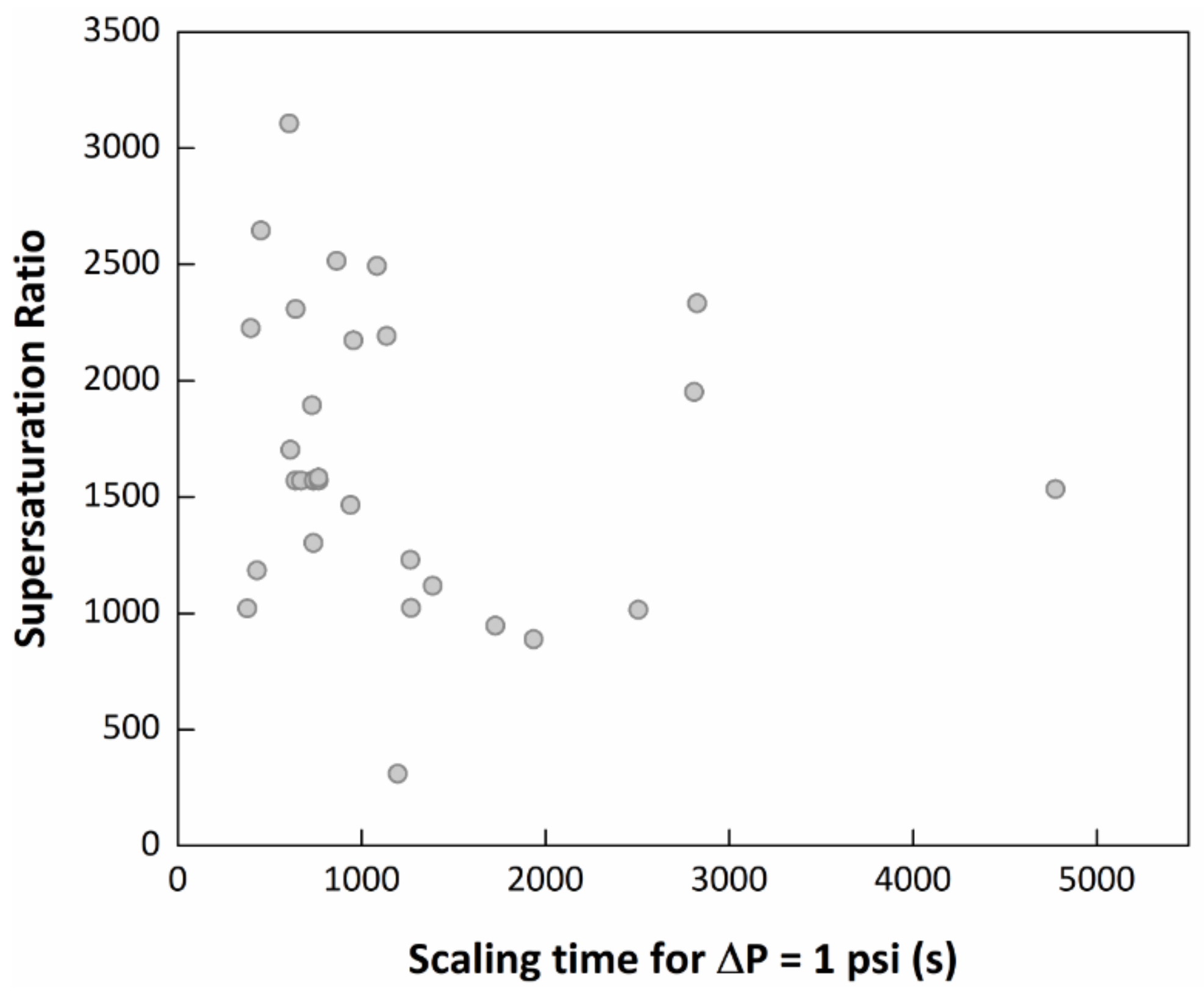
3.2. Evaluation of the MEG Effect over the Scaling Process
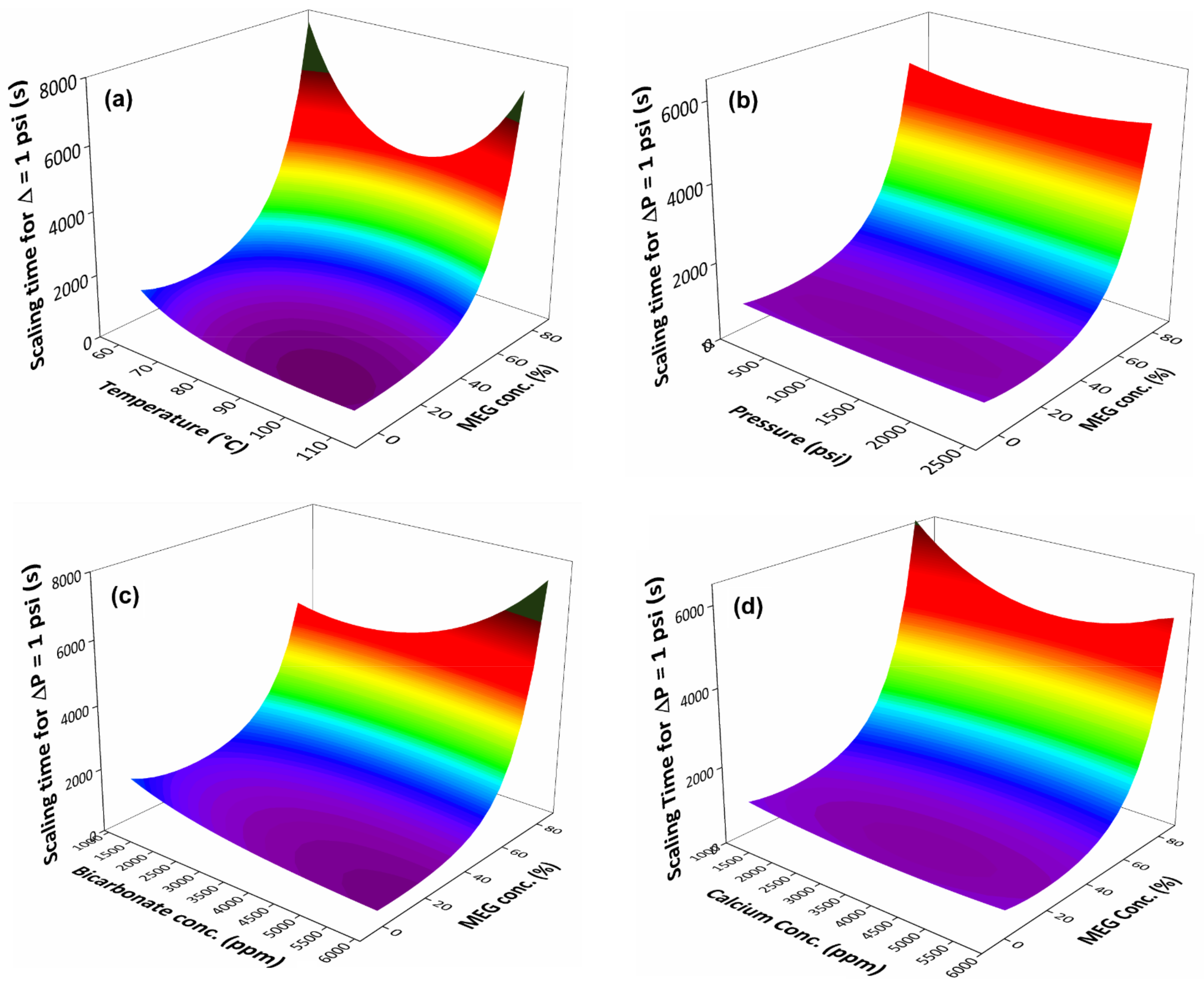
3.3. Evaluation the System’s Variables over the MEG Effect
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frenier, W.W.; Ziauddin, M. Formation, Removal, and Inhibition of Inorganic Scale in the Oilfield Environment; Society of Petroleum Engineers: Richardson, TX, USA, 2008. [Google Scholar]
- Costa Fraga, C.T.; Lara, A.Q.; Capeleiro Pinto, A.C.; Moreira Branco, C.C. Challenges and Solutions to Develop Brazilian Pre-Salt Deepwater Fields. In Proceedings of the 21st World Petroleum Congress, Moscow, Russia, 15–19 June 2014. [Google Scholar]
- Sloan, E.D.; Koh, C.A.; Sum, A.K.; Ballard, A.L.; Shoup, G.J.; McMullen, N.; Creek, J.L.; Palemo, T. Hydrates: State of the Art Inside and Outside Flowlines. J. Pet. Technol. 2009, 61, 89–94. [Google Scholar] [CrossRef]
- Kvenvolden, K.A.; Lorenson, T.D. Global Occurrences of Gas Hydrate. In Proceedings of the Eleventh International Offshore and Polar Engineering Conference, Stavanger, Norway, 17–22 June 2011. [Google Scholar]
- Hight, M.A. State-of-the-Art Survey on Hydrate Formation. In Proceedings of the SPE Annual Technical Conference and Exhibition, New Orleans, LA, USA, 25–28 September 1994. [Google Scholar]
- Sivaraman, R. Flow Assurance: Understanding and Controlling Natural Gas Hydrate; Gas-TIPS; U.S. Department of Energy and Hart Publications, Inc.: Des Plaines, IL, USA, 2002. [Google Scholar]
- Kelland, M.A. History of the Development of Low Dosage Hydrate Inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Tomson, M.B.; Kan, A.T.; Fu, G.; Al-Thubaiti, M.; Shen, D.; Shipley, H.J. Scale Formation and Prevention in the Presence of Hydrate Inhibitors. In Proceedings of the International Symposium on Oilfield Chemistry, Houston, TX, USA, 5–7 February 2003. [Google Scholar]
- Kaasa, B.; Sandengen, K.; Ostvold, T. Thermodynamic Predictions of Scale Potential, pH and Gas Solubility in Glycol Containing Systems. In Proceedings of the SPE International Symposium on Oilfield Scale, Aberdeen, UK, 11–12 May 2005. [Google Scholar]
- Shipley, H.J.; Kan, A.T.; Fu, G.; Shen, D.; Tomson, M.B. Effect of hydrate inhibitors on calcite, sulfates and halite scale formation. In Proceedings of the SPE International Oilfield Scale Symposium, Aberdeen, UK, 31 May–1 June 2006. [Google Scholar]
- Flaten, E.M.; Seiersten, M.; Andreassen, J.-P. Growth of the calcium carbonate polymorph vaterite in mixtures of water and ethylene glycol at conditions of gas processing. J. Cryst. Growth 2010, 312, 953–960. [Google Scholar] [CrossRef]
- Flaten, E.M.; Ma, X.; Seiersten, M.; Aanonsen, C.; Beck, R.; Andreassen, J.-P. Impact of Monoethylene Glycol and Fe2+ on Crystal Growth of CaCO3. In Proceeding of the CORROSION 2015, Dallas, TX, USA, 15–19 March 2015. [Google Scholar]
- Beck, R.; Nergaard, M.; Andreassen, J.P. Crystal growth of calcite at conditions of gas processing in solvent mixtures of monoethylene glycol and water. Trans. Tianjin Univ. 2013, 19, 79–85. [Google Scholar] [CrossRef]
- Flaten, E.M.; Watterud, G.; Andreassen, J.-P.; Seiersten, M.E. Precipitation of Iron And Calcium Carbonate in Pipelines At Varying Meg Contents. In Proceedings of the SPE International Oilfield Scale Conference, Aberdeen, UK, 28–29 May 2008. [Google Scholar]
- Flaten, E.M.; Seiersten, M.; Andreassen, J.-P. Induction time studies of calcium carbonate in ethylene glycol and water. Chem. Eng. Res. Des. 2010, 88, 1659–1668. [Google Scholar] [CrossRef]
- Storm, T.D.; Hazleton, R.A.; Lahti, L.E. Some effects of solvent properties on nucleation. J. Cryst. Growth 1970, 7, 55–60. [Google Scholar] [CrossRef]
- Kartnaller, V.; Romualdo, M.V.; Lobo, V.T.V.; Cajaiba, J. Kinetic Modeling of a Heat Generator for the Fluidization of Paraffin Deposits Using In-line Infrared Spectroscopy with the Development of a Graphical User Interface. Energy Fuels 2016, 30, 3660–3665. [Google Scholar] [CrossRef]
- Yang, M.; Stipp, S.L.S.; Harding, J. Biological Control on Calcite Crystallization by Polysaccharides. Cryst. Growth Des. 2008, 8, 4066–4074. [Google Scholar] [CrossRef]
- Henriksen, K.; Young, J.R.; Bown, P.R.; Stipp, S.L.S. Coccolith biomineralisation studied with atomic force microscopy. Palaeontology 2004, 47, 725–743. [Google Scholar] [CrossRef]
- Sand, K.K.; Yang, M.; Makovicky, E.; Cooke, D.J.; Hassenkam, T.; Bechgaard, K.; Stipp, S.L. Binding of Ethanol on Calcite: The Role of the OH Bond and Its Relevance to Biomineralization. Langmuir 2010, 26, 15239–15247. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yue, L.-H.; Wang, F.; Wang, Q. Divisive Effect of Alcohol−Water Mixed Solvents on Growth Morphology of Calcium Carbonate Crystals. J. Phys. Chem. B 2008, 112, 10668–10674. [Google Scholar] [CrossRef] [PubMed]
- Bovet, N.; Yang, M.; Javadi, M.S.; Stipp, S.L.S. Interaction of alcohols with the calcite surface. Phys. Chem. Chem. Phys. 2015, 17, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Navrotsky, A. Probing the energetics of organic-nanoparticle interactions of ethanol on calcite. Proc. Natl. Acad. Sci. USA 2015, 112, 5314–5318. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |
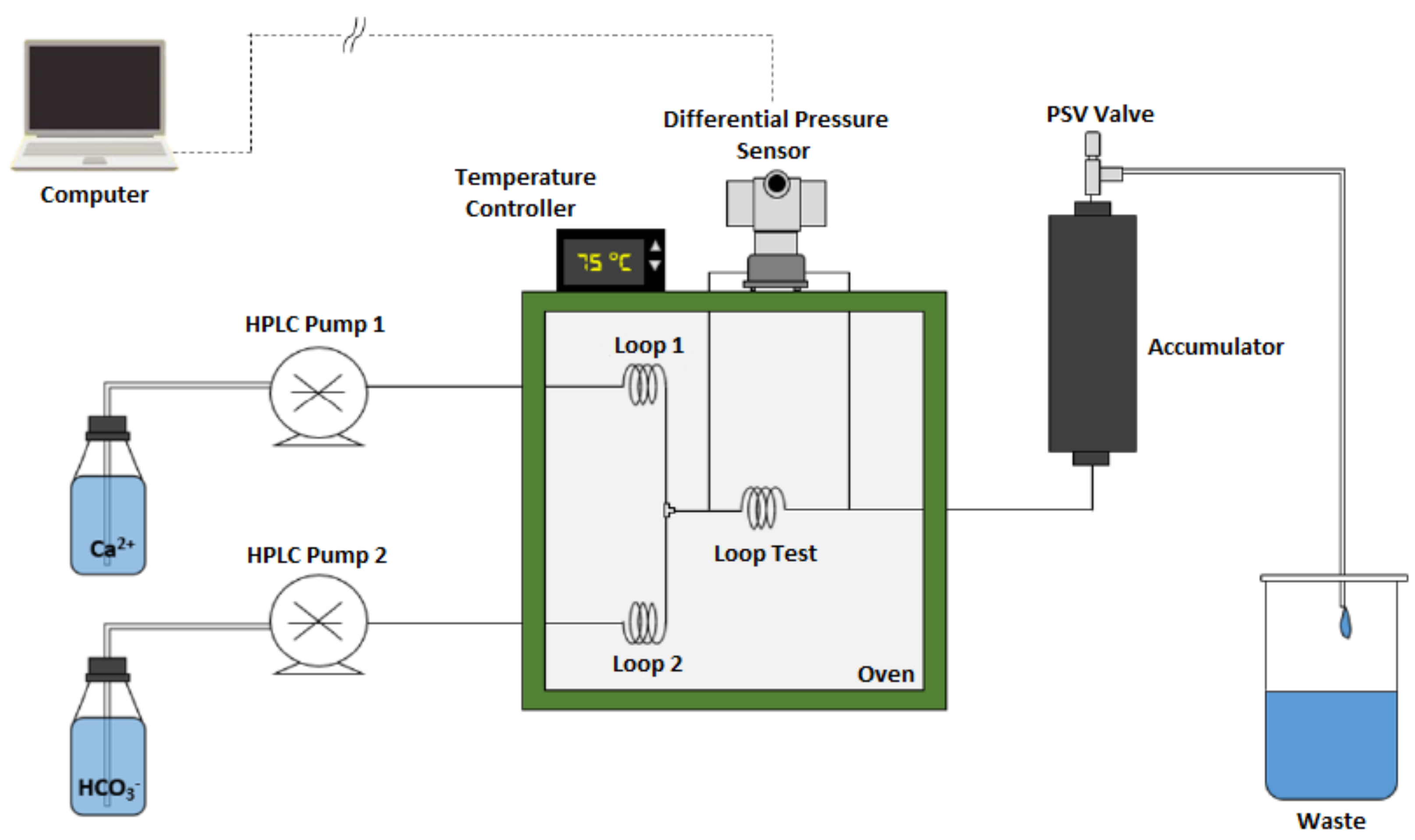
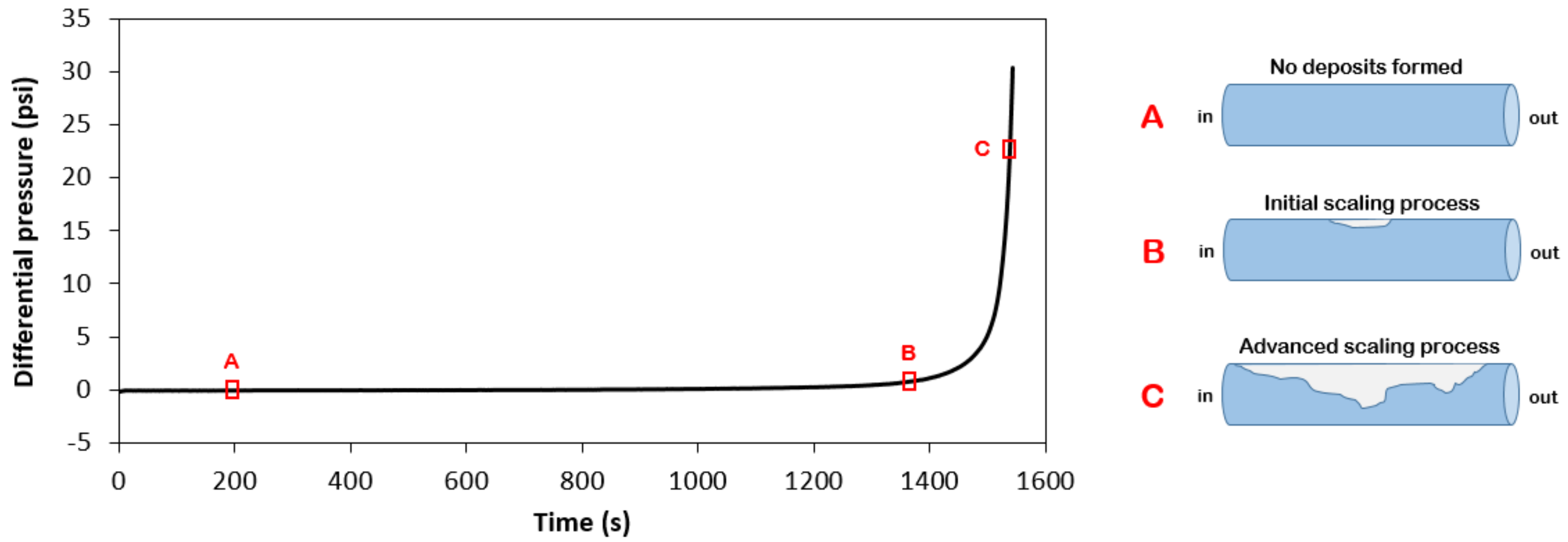

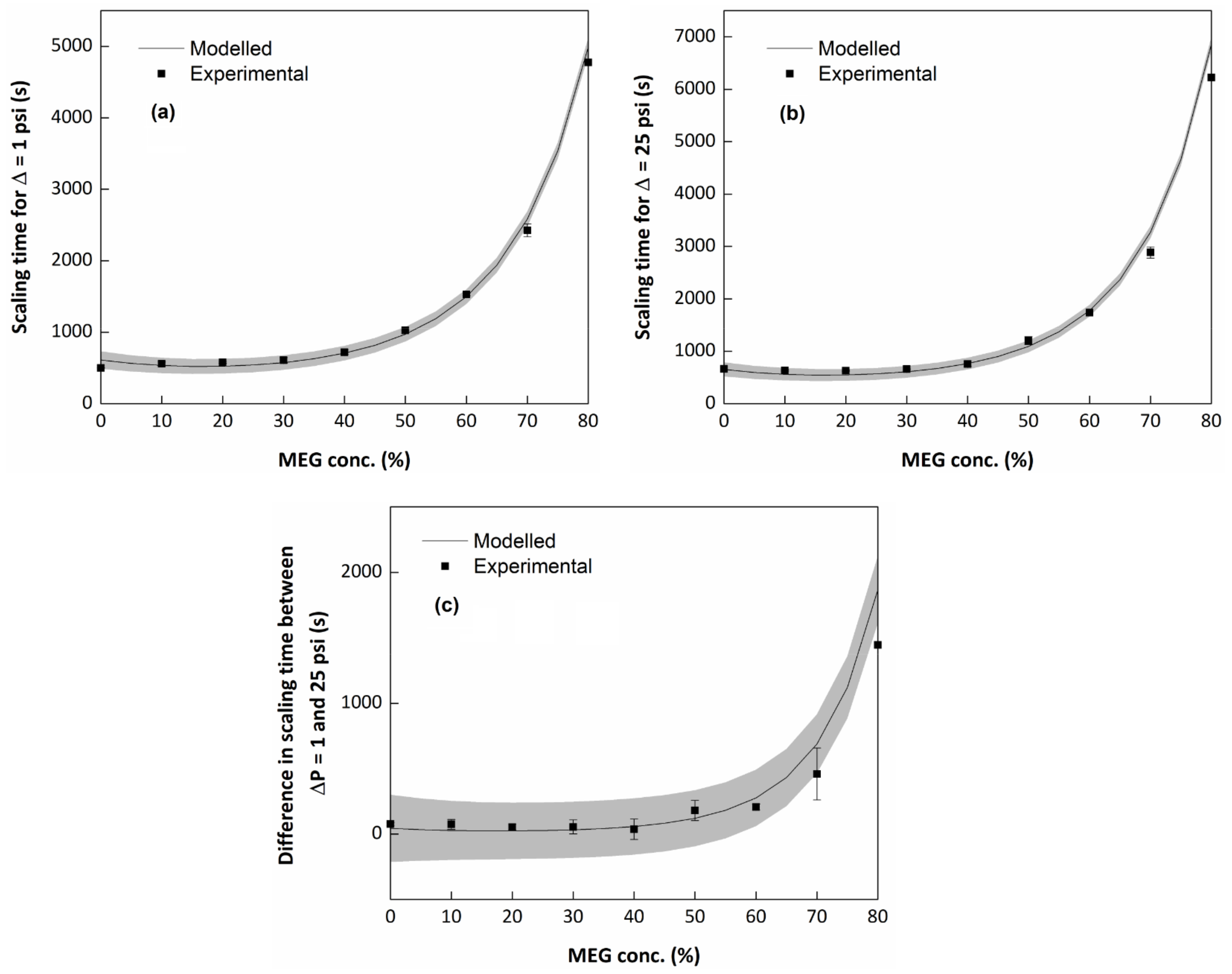
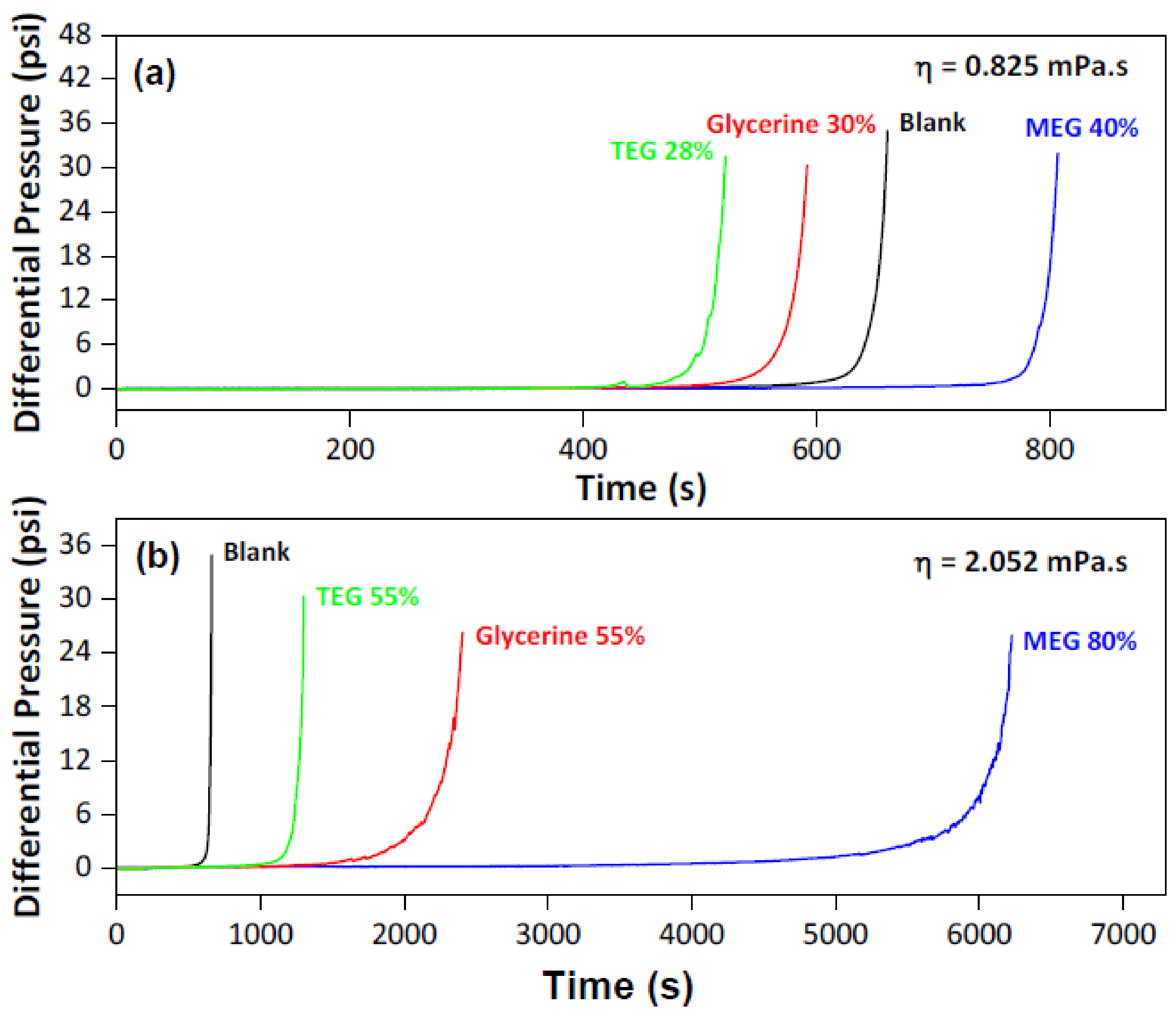
| Experiment # | Pressure (psi) | Temperature (°C) | MEG Conc. (v/v %) | (ppm) | (ppm) |
|---|---|---|---|---|---|
| 1 | 714 | 60 | 23 | 2449 | 4551 |
| 2 | 1751 | 60 | 23 | 2449 | 2449 |
| 3 | 714 | 90 | 23 | 2449 | 2449 |
| 4 | 1751 | 90 | 23 | 2449 | 4551 |
| 5 | 714 | 60 | 57 | 2449 | 2449 |
| 6 | 1751 | 60 | 57 | 2449 | 4551 |
| 7 | 714 | 90 | 57 | 2449 | 4551 |
| 8 | 1751 | 90 | 57 | 2449 | 2449 |
| 9 | 714 | 60 | 23 | 4551 | 2449 |
| 10 | 1751 | 60 | 23 | 4551 | 4551 |
| 11 | 714 | 90 | 23 | 4551 | 4551 |
| 12 | 1751 | 90 | 23 | 4551 | 2449 |
| 13 | 714 | 60 | 57 | 4551 | 4551 |
| 14 | 1751 | 60 | 57 | 4551 | 2449 |
| 15 | 714 | 90 | 57 | 4551 | 2449 |
| 16 | 1751 | 90 | 57 | 4551 | 4551 |
| 17–22 | 1233 | 75 | 40 | 3500 | 3500 |
| 23 | 0 | 75 | 40 | 3500 | 3500 |
| 24 | 2466 | 75 | 40 | 3500 | 3500 |
| 25 | 1233 | 40 | 40 | 3500 | 3500 |
| 26 | 1233 | 110 | 40 | 3500 | 3500 |
| 27 | 1233 | 75 | 0 | 3500 | 3500 |
| 28 | 1233 | 75 | 80 | 3500 | 3500 |
| 29 | 1233 | 75 | 40 | 1000 | 3500 |
| 30 | 1233 | 75 | 40 | 6000 | 3500 |
| 31 | 1233 | 75 | 40 | 3500 | 1000 |
| 32 | 1233 | 75 | 40 | 3500 | 6000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kartnaller, V.; Venâncio, F.; F. do Rosário, F.; Cajaiba, J. Application of Multiple Regression and Design of Experiments for Modelling the Effect of Monoethylene Glycol in the Calcium Carbonate Scaling Process. Molecules 2018, 23, 860. https://doi.org/10.3390/molecules23040860
Kartnaller V, Venâncio F, F. do Rosário F, Cajaiba J. Application of Multiple Regression and Design of Experiments for Modelling the Effect of Monoethylene Glycol in the Calcium Carbonate Scaling Process. Molecules. 2018; 23(4):860. https://doi.org/10.3390/molecules23040860
Chicago/Turabian StyleKartnaller, Vinicius, Fabrício Venâncio, Francisca F. do Rosário, and João Cajaiba. 2018. "Application of Multiple Regression and Design of Experiments for Modelling the Effect of Monoethylene Glycol in the Calcium Carbonate Scaling Process" Molecules 23, no. 4: 860. https://doi.org/10.3390/molecules23040860
APA StyleKartnaller, V., Venâncio, F., F. do Rosário, F., & Cajaiba, J. (2018). Application of Multiple Regression and Design of Experiments for Modelling the Effect of Monoethylene Glycol in the Calcium Carbonate Scaling Process. Molecules, 23(4), 860. https://doi.org/10.3390/molecules23040860





