Heterogeneity of Circulating Tumor Cells in Neoadjuvant Chemotherapy of Breast Cancer
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Research Materials
3.2. Flow Cytometry
3.3. Fluorescence-Activated Cell Sorting
3.4. CTCs Spiking Experiment
3.5. Confocal Microscopy
3.6. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Giordano, A.; Gao, H.; Anfossi, S.; Cohen, E.; Mego, M.; Lee, B.N.; Tin, S.; De Laurentiis, M.; Parker, C.A.; Alvarez, R.H.; et al. Epithelial-mesenchymal transition and stem cell markers in patients with HER2-positive metastatic breast cancer. Mol. Cancer Ther. 2012, 11, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Kaigorodova, E.; Tarabanovskaya, N.; Simolina, E.; Perelmuter, V.; Stakheeva, M.; Cherdyntsevа, N.; Saveleva, O.; Tashireva, L. Circulating tumor cells and bone marrow progenitor cells in the blood of breast cancer patients in the dynamics of neoadjuvant chemotherapy. EJC Suppl. 2015, 13, 22. [Google Scholar] [CrossRef][Green Version]
- Kaigorodova, E.V. Circulating tumor cells: Clinical significance in breast cancer (Review). Ann. Russ. Acad. Med. Sci. 2017, 72, 450–457. [Google Scholar] [CrossRef]
- Lv, Q.B.; Fu, X.; Jin, H.M.; Xu, H.C.; Huang, Z.Y.; Xu, H.Z.; Chi, Y.L.; Wu, A.M. The relationship between weight change and risk of hip fracture: Meta-analysis of prospective studies. Sci. Rep. 2015, 5, 16030. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Mani, S.A.; Lee, B.N.; Li, C.; Evans, K.W.; Cohen, E.N.; Gao, H.; Jackson, S.A.; Giordano, A.; Hortobagyi, G.N.; et al. Expression of epithelial-mesenchymal transition-inducing transcription factors in primary breast cancer: The effect of neoadjuvant therapy. Int. J. Cancer 2012, 130, 808–816. [Google Scholar] [CrossRef] [PubMed]
- Kaigorodova, E.V.; Tarabanovskaya, N.A.; Staheeva, M.N.; Savelieva, O.E.; Tashireva, L.A.; Denisov, E.V.; Perelmuter, V.M. Effect of minor and major surgical injury on the level of different populations of circulating tumor cells in the blood of breast cancer patients. Neoplasma 2017, 64, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Tripathy, D.; Frenkel, E.P.; Shete, S.; Naftalis, E.Z.; Huth, J.F.; Beitsch, P.D.; Leitch, M.; Hoover, S.; Euhus, D.; et al. Circulating tumor cells in patients with breast cancer dormancy. Clin. Cancer Res. 2004, 10, 8152–8162. [Google Scholar] [CrossRef] [PubMed]
- Kasimir-Bauer, S.; Hoffmann, O.; Wallwiener, D.; Kimmig, R.; Fehm, T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012, 14, R15. [Google Scholar] [CrossRef] [PubMed]
- Ge, F.; Zhang, H.; Wang, D.D.; Li, L.; Lin, P.P. Enhanced detection and comprehensive in situ phenotypic characterization of circulating and disseminated heteroploid epithelial and glioma tumor cells. Oncotarget 2015, 6, 27049–27064. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.P.; Gires, O.; Wang, D.D.; Li, L.; Wang, H. Comprehensive in situ co-detection of aneuploid circulating endothelial and tumor cells. Sci. Rep. 2017, 7, 9789. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Fong, D.; Wurm, M.; Ensinger, C.; Obrist, P.; Hofer, C.; Mazzoleni, G.; Gastl, G.; Went, P. EpCAM expression in primary tumour tissues and metastases: An immunohistochemical analysis. J. Clin. Pathol. 2011, 64, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Ladwein, M.; Pape, U.F.; Schmidt, D.S.; Schnölzer, M.; Fiedler, S.; Langbein, L.; Franke, W.W.; Moldenhauer, G.; Zöller, M. The cell-cell adhesion molecule EpCAM interacts directly with the tight junction protein claudin-7. Exp. Cell Res. 2005, 309, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Guillemot, J.C.; Naspetti, M.; Malergue, F.; Montcourrier, P.; Galland, F.; Naquet, P. Ep-CAM transfection in thymic epithelial cell lines triggers the formation of dynamic actin-rich protrusions involved in the organization of epithelial cell layers. Histochem. Cell Biol. 2001, 116, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Maetzel, D.; Denzel, S.; Mack, B.; Canis, M.; Went, P.; Benk, M.; Kie, C.; Papior, P.; Baeuerle, P.A.; Munz, M.; et al. Nuclear signalling by tumour-associated antigen EpCAM. Nat. Cell Biol. 2009, 11, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Osta, W.A.; Chen, Y.; Mikhitarian, K.; Mitas, M.; Salem, M.; Hannun, Y.A.; Cole, D.J.; Gillanders, W.E. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res. 2004, 64, 5818–5824. [Google Scholar] [CrossRef] [PubMed]
- Münz, M.; Kieu, C.; Mack, B.; Schmitt, B.; Zeidler, R.; Gires, O. The carcinoma-associated antigen EpCAM upregulates c-myc and induces cell proliferation. Oncogene 2004, 23, 5748–5758. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Gires, O. EpCAM (CD326) finding its role in cancer. Br. J. Cancer 2007, 96, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Spizzo, G.; Went, P.; Dirnhofer, S.; Obrist, P.; Moch, H.; Baeuerle, P.A.; Mueller-Holzner, E.; Marth, C.; Gastl, G.; Zeimet, A.G. Overexpression of epithelial cell adhesion molecule (Ep-CAM) is an independent prognostic marker for reduced survival of patients with epithelial ovarian cancer. Gynecol. Oncol. 2006, 103, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Went, P.; Vasei, M.; Bubendorf, L.; Terracciano, L.; Tornillo, L.; Riede, U.; Kononen, J.; Simon, R.; Sauter, G.; Baeuerle, P.A. Frequent high-level expression of the immunotherapeutic target Ep-CAM in colon, stomach, prostate and lung cancers. Br. J. Cancer 2006, 94, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.A.; Koo, G.B.; Han, H.; Sohn, J.; Choi, W.; Kim, S.I.; Jung, H.I.; Kim, Y.S. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget 2016, 7, 24677–24687. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Liao, M.Y.; Lin, W.W.; Wang, Y.P.; Lu, T.Y.; Wu, H.C. Epithelial Cell Adhesion Molecule Regulates Tumor Initiation and Tumorigenesis via Activating Reprogramming Factors and Epithelial-Mesenchymal Transition Gene Expression in Colon Cancer. J. Biol. Chem. 2012, 287, 39449–39459. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, W.M.; Duhé, R.J. Overview: Cellular plasticity, cancer stem cells and metastasis. Cancer Lett. 2013, 341, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Mishra, L.; Li, S. EMT, CTCs and CSCs in tumor relapse and drug-resistance. Oncotarget 2015, 6, 10697–10711. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Kao, A.P.; Lin, T.C.; Chang, C.C.; Kuo, T.C. Promotion of epithelial-mesenchymal transition and tumor growth by 17beta-estradiol in an ER(+)/HER2(+) cell line derived from human breast epithelial stem cells. Biotechnol. Appl. Biochem. 2012, 59, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Bhat-Nakshatri, P.; Goswami, C.P.; Badve, S.; Sledge, G.W., Jr.; Nakshatri, H. Identification of FDA approved drugs targeting breast cancer stem cells along with biomarkers of sensitivity. Sci. Rep. 2013, 3, 2530. [Google Scholar] [CrossRef] [PubMed]
- Morel, A.P.; Lievre, M.; Thomas, C.; Hinkal, G.; Ansieau, S.; Puisieux, A. Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 2008, 3, e2888. [Google Scholar] [CrossRef] [PubMed]
- Creighton, C.J.; Li, X.; Landis, M.; Dixon, J.M.; Neumeister, V.M.; Sjolund, A.; Rimm, D.L.; Wong, H.; Rodriguez, A.; Herschkowitz, J.I.; et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc. Natl. Acad. Sci. USA 2009, 106, 13820–13825. [Google Scholar] [CrossRef] [PubMed]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Asiedu, M.K.; Ingle, J.N.; Behrens, M.D.; Radisky, D.C.; Knutson, K.L. TGFbeta/TNF(alpha)-mediated epithelial-mesenchymal transition generates breast cancer stem cells with a claudin-low phenotype. Cancer Res. 2011, 71, 4707–4719. [Google Scholar] [CrossRef] [PubMed]
- Thompson, E.W.; Haviv, I. The social aspects of EMT- MET plasticity. Nat. Med. 2011, 17, 1048–1049. [Google Scholar] [CrossRef] [PubMed]
- Shibue, T.; Weinberg, R.A. EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 611–629. [Google Scholar] [CrossRef] [PubMed]
- Brabletz, T.; Kalluri, R.; Nieto, M.A.; Weinberg, R.A. EMT in cancer. Nat. Rev. Cancer 2018, 18, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Weinberg, R.A. Epithelial-Mesenchymal Plasticity: A Central Regulator of Cancer Progression. Trends Cell Biol. 2015, 25, 675–686. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Fan, D. The epithelial-mesenchymal transition and cancer stem cells: Functional and mechanistic links. Curr. Pharm. Des. 2015, 21, 1279–1291. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cong, Y.; Wang, D.; Sun, Y.; Deng, L.; Liu, Y.; Martin-Trevino, R.; Shang, L.; McDermott, S.P.; Landis, M.D.; et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem. Cell Rep. 2013, 2, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Perelmuter, V.M.; Manskikh, V.N. The Concept of a Preniche for Localization of Future Metastases. In Tumors of the Central Nervous System; Hayat, M., Ed.; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Chapter 11; Volume 13, pp. 93–106. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
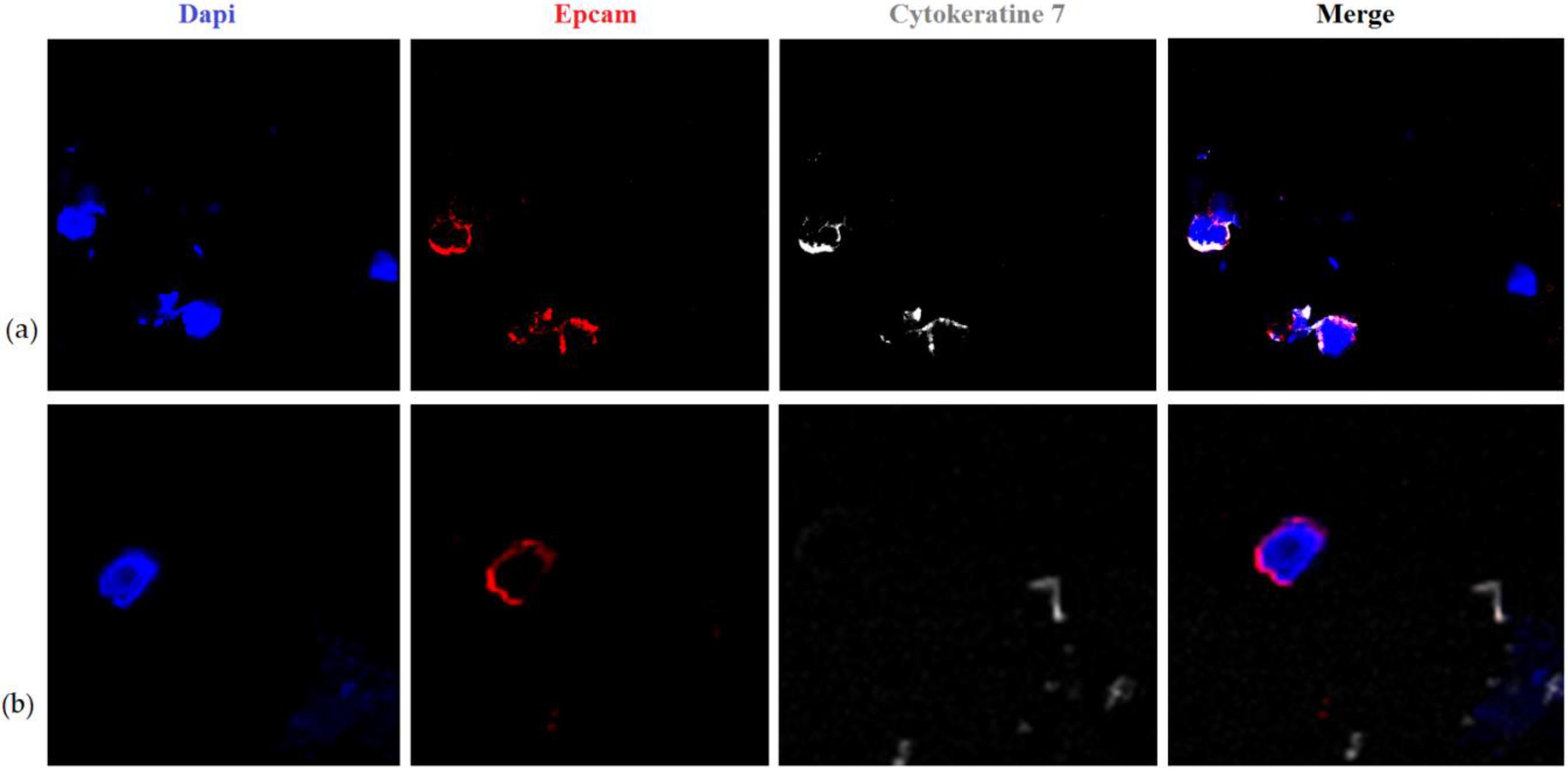
| N | СTC Total | CTC-1 | CTC-2 | CTC-3 | CTC-4 | CTC-5 | CTC-6 |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Breast cancer patients | |||||||
| 27 | 2.85 (0.51–4.40) | 0.54 (0.00–1.11) p2-1 = 0.00003 * | 0.09 (0.00–0.70) p3-1 = 0.00009 * p3-2 = 0.47 * | 0.00 (0.00–0.43) p4-1 = 0.00002 * p4-2 = 0.0011 * p4-3 = 0.11 * | 0.02 (0.00–0.63) p5-1 = 0.00009 * p5-2 = 0.33 * p5-3 = 0.43 * p5-4 = 0.68 * | 0.18 (0.00–2.41) p6-1 = 0.00003 * p6-2 = 0.14 * p6-3 = 0.67 * p6-4 = 0.011 * p6-5 = 0.23 * | 0.04 (0.00–0.34) p7-1 = 0.00006 * p7-2 = 0.57 * p7-3 = 0.47 * p7-4 = 0.79 * p7-5 = 0.58 * p7-6 = 0.19 * |
| Healthy donors | |||||||
| 7 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Level after Biopsy | Level after 1 Course of NACT | Level after 2 Course of NACT | Level after 3 Course of NACT | Level before Surgical Treatment |
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 |
| СTC total (EpCam+/-CD45-CD44+/-CD24-Ncadherin+/-) | ||||
| 2.97 (0.93–5.85) | 2.51 (1.85–21.45) p2-1 = 0.32 | 11.04 (3.46–21.96) p3-1 = 0.77 p3-2 = 0.20 | 19.94 (9.28–130.86) p4-1 = 0.068 p4-2 = 0.26 p4-3 = 0.32 | 14.06 (4.51–74.60) p5-1 = 0.017 p5-2 = 0.035 p5-3 = 0.16 p5-4 = 0.77 |
| CTC-1 without stemness and EMT properties (EpCam+CD45-CD44-CD24-Ncadherin-) | ||||
| 0.90 (0.00–1.73) | 1.25 (0.07–5.21) p2-1 = 0.074 | 3.75 (0.29–5.79) p3-1 = 0.027 p3-2 = 0.916 | 0.74 (0.31–4.80) p4-1 = 0.176 p4-2 = 0.735 p4-3 = 0.735 | 3.45 (0.00–7.09) p5-1 = 0.028 p5-2 = 0.600 p5-3 = 0.310 p5-4 = 0.310 |
| CTC-2 without stemness and with EMT properties (EpCam+CD45-CD44-CD24-Ncadherin+) | ||||
| 1.03 (0.45–22.50) | 0.28 (0.17–0.66) p2-1 = 0.345 | 0.87 (0.39–5.99) p3-1 = 0.916 p3-2 = 0.345 | 0.83 (0.29–1.84) p4-1 = 0.463 p4-2 = 0.735 p4-3 = 0.600 | 0.28 (0.00–1.28) p5-1 = 0.865 p5-2 = 0.046 p5-3 = 0.753 p5-4 = 0.753 |
| CTC-3 with stemness and without EMT properties (EpCam+CD45-CD44+CD24-Ncadherin-) | ||||
| 0.02 (0.00–0.22) | 0.12 (0.00–1.09) p2-1 = 0.310 | 0.20 (0.00–1.63) p3-1 = 0.176 p3-2 = 0.079 | 0.50 (0.00–1.79) p4-1 = 0.115 p4-2 = 0.115 p4-3 = 0.916 | 0.00 (0.00–2.57) p5-1 = 0.498 p5-2 = 0.224 p5-3 = 0.892 p5-4 = 0.753 |
| CTC-4 with stemness and EMT properties (EpCam+CD45-CD44+CD24-Ncadherin+) | ||||
| 0.22 (0.00–0.55) | 0.00 (0.00–0.26) p2-1 = 0.715 | 0.05 (0.00–0.22) p3-1 = 0.345 p3-2 = 0.500 | 0.00 (0.00–1.64) p4-1 = 0.144 p4-2 = 0.108 p4-3 = 0.224 | 0.01 (0.00–0.71) p5-1 = 0.043 p5-2 = 0.043 p5-3 = 0.115 p5-4 = 0.892 |
| CTC-5 with stemness, without EMT properties and without EpCAM membrane expression (EpCam-CD45-CD44+CD24-Ncadherin-) | ||||
| 0.00 (0.00–0.49) | 0.46 (0.00–2.61) p2-1 = 0.892 | 1.08 (0.13–5.32) p3-1 = 0.310 p3-2 = 0.310 | 5.62 (2.12–9.57) p4-1 = 0.068 p4-2 = 0.123 p4-3 = 0.025 | 2.39 (0.10–12.80) p5-1 = 0.086 p5-2 = 0.498 p5-3 = 0.128 p5-4 = 0.207 |
| CTC-6 with stemness and EMT properties and without EpCAM membrane expression (EpCam-CD45-CD44+CD24-Ncadherin+) | ||||
| 0.16 (0.04–1.59) | 0.32 (0.06–0.87) p2-1 = 1.00 | 0.21 (0.02–2.35) p3-1 = 0.498 p3-2 = 0.865 | 2.95 (1.25–13.28) p4-1 = 0.027 p4-2 = 0.062 p4-3 = 0.310 | 3.19 (0.47–9.41) p5-1 = 0.027 p5-2 = 0.090 p5-3 = 0.345 p5-4 = 0.779 |
| Clinicopathological Parameters | N (%) |
|---|---|
| Age (year) (Me (Q1–Q3)) | |
| 49 (43–57) | 27 (100%) |
| Molecular type of breast cancer | |
| Luminal A | 6/27 (22%) |
| Luminal В1 | 12/27 (44%) |
| Luminal В2 | 1/27 (4%) |
| HER2-positive | 1/27 (4%) |
| Triple-negative | 7/27 (26%) |
| Tumor size | |
| T1 | 6/27 (22%) |
| T2 | 18/27 (67%) |
| T3 | 1/27 (4%) |
| T4 | 2/27 (7%) |
| Lymph node status | |
| N0 | 14/27 (52%) |
| N1 | 8/27 (30%) |
| N2 | 3/27 (11%) |
| N3 | 2/27 (7%) |
| Neoadjuvant chemotherapy (NACT) | |
| NO | 13/27 (48%) |
| YES | 14/27 (52%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaigorodova, E.V.; Savelieva, O.E.; Tashireva, L.A.; Tarabanovskaya, N.A.; Simolina, E.I.; Denisov, E.V.; Slonimskaya, E.M.; Choynzonov, E.L.; Perelmuter, V.M. Heterogeneity of Circulating Tumor Cells in Neoadjuvant Chemotherapy of Breast Cancer. Molecules 2018, 23, 727. https://doi.org/10.3390/molecules23040727
Kaigorodova EV, Savelieva OE, Tashireva LA, Tarabanovskaya NA, Simolina EI, Denisov EV, Slonimskaya EM, Choynzonov EL, Perelmuter VM. Heterogeneity of Circulating Tumor Cells in Neoadjuvant Chemotherapy of Breast Cancer. Molecules. 2018; 23(4):727. https://doi.org/10.3390/molecules23040727
Chicago/Turabian StyleKaigorodova, Evgeniya V., Olga E. Savelieva, Liubov A. Tashireva, Natalia A. Tarabanovskaya, Elena I. Simolina, Evgeny V. Denisov, Elena M. Slonimskaya, Evgeny L. Choynzonov, and Vladimir M. Perelmuter. 2018. "Heterogeneity of Circulating Tumor Cells in Neoadjuvant Chemotherapy of Breast Cancer" Molecules 23, no. 4: 727. https://doi.org/10.3390/molecules23040727
APA StyleKaigorodova, E. V., Savelieva, O. E., Tashireva, L. A., Tarabanovskaya, N. A., Simolina, E. I., Denisov, E. V., Slonimskaya, E. M., Choynzonov, E. L., & Perelmuter, V. M. (2018). Heterogeneity of Circulating Tumor Cells in Neoadjuvant Chemotherapy of Breast Cancer. Molecules, 23(4), 727. https://doi.org/10.3390/molecules23040727







