Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Collection of Macroalgae
4.2. Extraction of Bioactive Compounds from Algae Using Organic Solvents
4.3. Collection of Fungal Pathogens
4.3.1. Patients
4.3.2. Sputum Processing and Identification of Fungal Isolates
4.3.3. Determination of Antifungal Activity—Agar Well-Diffusion Method
4.4. Minimum Inhibitory Concentrations (MICs)—Broth Dilution Method
Inoculum Preparation
4.5. Determination of Minimal Fungicidal Concentration (MFC)
4.6. Antifungal Agents
4.7. Preparation of Microdilution Plates and Antifungal Assay
4.8. GC–MS/MS Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| µg | Microgram |
| AMR | Antimicrobial resistance |
| CLSI | Clinical and Laboratory Standards Institute |
| ELISA | Enzyme-linked immunosorbent assay |
| GC–MS/MS | Gas chromatography/tandem mass spectrometry |
| ICS | Inhaled corticosteroids |
| IgE | Immunoglobulin E |
| IU | International unit |
| MIC | Minimum inhibitory concentration |
| MFC | Minimum fungicidal concentration |
| mL | Milliliter |
| MW | Molecular weight |
| NIST | National Institute Standard and Technology |
| RPMI | Roswell Park Memorial Institute |
| RT | Retention time |
| SABA | Short-acting beta-agonist |
| UK | United Kingdom |
| USA | United States of America |
References
- Morgan, B.W.; Grigsby, M.R.; Siddharthan, T.; Chowdhury, M.; Rubinstein, A.; Gutierrez, L.; Irazola, V.; Miranda, J.J.; Bernabe-Ortiz, A.; Alam, D.; et al. Epidemiology and Risk Factors of Asthma-COPD Overlap in Low- and Middle-Income Countries. J. Allergy Clin. Immunol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Bleecker, E.R.; Panettieri, R.A., Jr.; Wenzel, S.E. Clinical Issues in Severe Asthma: Consensus and Controversies on the Road to Precision Medicine. Chest 2018, 154, 982–983. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Antifungal activity of selected Indian Medicinal Plant salts. J. Glob. Pharma Technol. 2010, 2, 71–74. [Google Scholar]
- Hayes Watson, C.; Nuss, H.; Celestin, M.; Tseng, T.S.; Parada, N.; Yu, Q.; Moody-Thomas, S. Health beliefs associated with poor disease self-management in smokers with asthma and/or COPD: A pilot study. J. Asthma 2018, 1–8. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial resistance: Tackling a crisis for the health and wealth of nations. Rev. Antimicrob. Resist. 2014, 11, 1–6. [Google Scholar]
- Roemer, T.; Krysan, D.J. Antifungal drug development: Challenges, unmet clinical needs, and new approaches. Cold Spring Harb. Perspect. Med. 2014, 4, a019703. [Google Scholar] [CrossRef] [PubMed]
- Alsaid-Habia, T.; McLeish, A.C.; Kraemer, K.M. Associations between distress tolerance and asthma symptoms and quality of life. J. Asthma 2018, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mickymaray, S.; Al Aboody, M.S.; Rath, P.K.; Annamalai, P.; Nooruddin, T. Screening and antibacterial efficacy of selected Indian medicinal plants. Asian Pac. J. Trop. Biomed. 2016, 6, 185–191. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Gheda, S.F.; El-Adawi, H.I.; EL-Deeb, N.M. Antiviral Profile of Brown and Red Seaweed Polysaccharides against Hepatitis C Virus. Iran. J. Pharm. Res. 2016, 15, 483–491. [Google Scholar] [PubMed]
- De Alencar, D.B.; de Carvalho, F.C.; Rebouças, R.H.; dos Santos, D.R.; dos Santos Pires-Cavalcante, K.M.; de Lima, R.L.; Baracho, B.M.; Bezerra, R.M.; Viana, F.A.; dos Fernandes Vieira, R.H.; et al. Bioactive extracts of red seaweeds Pterocladiella capillacea and Osmundaria obtusiloba (Floridophyceae: Rhodophyta) with antioxidant and bacterial agglutination potential. Asian Pac. J. Trop. Med. 2016, 9, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Rodríguez, A.G.; Juárez-Portilla, C.; Olivares-Bañuelos, T.; Zepeda, R.C. Anticancer activity of seaweeds. Drug Discov. Today 2017, 23, 434–447. [Google Scholar] [CrossRef] [PubMed]
- Roberton, R.C.; Guihéneuf, F.; Bahar, B.; Schmid, M.; Stengel, D.B.; Fitzgerald, G.F.; Paul Ross, R.; Stanton, C. The Anti-Inflammatory effect of algae-derived lipid extracts on lipopolysaccharide (LPS)-stimulated human THP-1 macrophages. Mar. Drugs 2015, 13, 5402–5424. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.; Vale, C.; Rauter, A. Halogenated compounds from marine algae. Mar. Drugs 2010, 8, 2301–2317. [Google Scholar] [CrossRef] [PubMed]
- Michalak, I.; Chojnacka, K. Algae as production systems of bioactive compounds. Eng. Life Sci. 2015, 15, 160–176. [Google Scholar] [CrossRef]
- Bouhlal, R.; Riadi, H.; Martínez, J.; Bourgougnon, N. The antibacterial potential of the seaweeds (Rhodophyceae) of the Strait of Gibraltar and the Mediterranean coast of Morocco. Afr. J. Biotechnol. 2010, 9, 6365–6372. [Google Scholar]
- Pierre, G.; Sopena, V.; Juin, C.; Mastouri, A.; Graber, M.; Maugard, T. Antibacterial activity of a sulfated galactan extracted from the marine alga Chaetomorpha aerea against Staphylococcus aureus. Biotechnol. Bioprocess Eng. 2011, 16, 937–945. [Google Scholar] [CrossRef]
- Al-Saif, S.S.A.; Abdel-Raouf, N.; El-Wazanani, H.A.; Aref, I.A. Antibacterial substances from marine algae isolated from Jeddah coast of Red sea, Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Kavita, K.; Singh, V.K.; Jha, B. 24-Branched delta 5 sterols from Laurencia papillosa red seaweed with antibacterial activity against human pathogenic bacteria. Microbiol. Res. 2014, 169, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.E.; Abushady, A.M.; Elshobary, M.E. In vitro screening of antimicrobial activity of extracts of some macroalgae collected from Abu-Qir bay Alexandria, Egypt. Afr. J. Biotechnol. 2010, 9, 7203–7208. [Google Scholar]
- Adaikalaraj, G.; Patric, R.D.; Johnson, M.; Janakiraman, N.; Babu, A. Antibacterial potential of selected red seaweeds from Manapad coastal areas, Thoothukudi, Tamil Nadu, India. Asian Pac. J. Trop. Biomed. 2012, 2, S1077–S1080. [Google Scholar] [CrossRef]
- Jassbi, A.R.; Mohabati, M.; Eslami, S.; Sohrabipour, J.; Miri, R. Biological activity and chemical constituents of red and brown algae from the Persian Gulf. Iran. J. Pharm. Res. 2013, 12, 339–348. [Google Scholar] [PubMed]
- De Jesus Raposo, M.F.; de Morais, A.M.B.; de Morais, R.M.S.C. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef] [PubMed]
- Krish, S.; Das, A. In-vitro bioactivity of marine seaweed, Cladophora rupestris. Int. J. Pharm. Biol. Sci. 2014, 5, 898–908. [Google Scholar]
- Karthikeyan, K.; Shweta, K.; Jayanthi, G.; Prabhu, K.; Thirumaran, G. Antimicrobial and antioxidant potential of selected seaweeds from Kodinar, Southern Coast of Saurashtra, Gujarat, India. J. Appl. Pharm. Sci. 2015, 5, 35–40. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Gruner, M.; Andriamanantoanina, H.; Andriamihaja, B.; Bauer, I.; Knölker, H.J. Red algae (Rhodophyta) from the coast of Madagascar: Preliminary bioactivity studies and isolation of natural products. Mar. Drugs 2015, 13, 4197–4216. [Google Scholar] [CrossRef] [PubMed]
- Alarif, W.M.; Al-Lihaibi, S.S.; Abdel-Lateff, A.; Ayyad, S.E. New antifungal cholestane and aldehyde derivatives from the red alga Laurencia papillosa. Nat. Prod. Commun. 2011, 6, 1821–1824. [Google Scholar] [PubMed]
- Chowdhury, M.M.H.; Kubra, K.; Hossain, M.B.; Mustafa, M.G.; Jainab, T.; Karim, M.R.; Mehedy, M.E. Screening of Antibacterial and Antifungal Activity of Freshwater and Marine Algae as a Prominent Natural Antibiotic Available in Bangladesh. Int. J. Pharmacol. 2015, 11, 828–833. [Google Scholar] [CrossRef]
- Shobier, A.H.; Ghani, S.A.A.; Barakat, K.M. GC/MS spectroscopic approach and antifungal potential of bioactive extracts produced by marine macroalgae. Egypt J. Aquat. Res. 2016, 42, 289–299. [Google Scholar] [CrossRef]
- Mashjoor, S.; Yousefzadi, M.; Esmaeili, M.A.; Rafiee, R. Cytotoxicity and antimicrobial activity of marine macro algae (Dictyotaceae and Ulvaceae) from the Persian Gulf. Cytotechnology 2016, 68, 1717–1726. [Google Scholar] [CrossRef] [PubMed]
- Ertürk, Ö.; Taş, B. Antibacterial and antifungal effects of some marine algae. Kafkas Univ. Vet. Fak. Derg. 2011, 17, S121–S124. [Google Scholar]
- Guedes, E.A.; Araújo, M.A.; Souza, A.K.; de Souza, L.I.; de Barros, L.D.; Maranhão, F.C.; Sant’Ana, A.E. Antifungal activities of different extracts of marine macroalgae against dermatophytes and Candida species. Mycopathologia 2012, 174, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Pandian, P.; Selvamuthukumar, S.; Manavalan, R.; Parthasarathy, V. Screening of antibacterial and antifungal activities of red marine algae Acanthaphora specifera (Rhodophyceae). J. Biomed. Sci. Res. 2011, 3, 444–448. [Google Scholar]
- Genovese, G.; Leitner, S.; Minicante, S.A.; Lass-Flörl, C. The Mediterranean red alga Asparagopsis taxiformis has antifungal activity against Aspergillus species. Mycoses 2013, 56, 516–519. [Google Scholar] [CrossRef] [PubMed]
- Suresh, M.; Rath, P.K.; Panneerselvam, A.; Dhanasekaran, D.; Thajuddin, N. Anti-mycobacterial effect of leaf extract of Centella asiatica. Res. J. Pharm Technol. 2010, 3, 872–876. [Google Scholar]
- Sun, J.; Shi, D.; Ma, M.; Li, S.; Wang, S.; Han, L.; Yang, Y.; Fan, X.; Shi, J.; He, L. Sesquiterpenes from the red alga Laurencia tristicha. J. Nat. Prod. 2005, 68, 915–919. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Li, X.M.; Ding, L.P.; Wang, B.G. Diterpenes, sesquiterpenes, and a C15-acetogenin from the marine red alga Laurencia mariannensis. J. Nat. Prod. 2007, 70, 1901–1905. [Google Scholar] [CrossRef] [PubMed]
- Ji, N.Y.; Li, X.M.; Ding, L.P.; Wang, B.G. Aristolane sesquiterpenes and highly brominated indoles from the marine red alga Laurencia similis (Rhodomelaceae) Helv. Chim. Acta 2007, 90, 385–391. [Google Scholar] [CrossRef]
- Ji, N.Y.; Li, X.M.; Li, K.; Wang, B.G. Halogenated sesquiterpenes from the marine red alga Laurencia saitoi (Rhodomelaceae) Helv. Chim. Acta 2009, 92, 1873–1879. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.M.; Cui, C.M.; Li, C.S.; Wang, B.G. A new rearranged chamigrane sesquiterpene from Laurencia okamurai. Chin. Chem. Lett. 2009, 20, 190–192. [Google Scholar] [CrossRef]
- Liang, Y.; Li, X.M.; Cui, C.M.; Li, C.S.; Sun, H.; Wang, B.G. Sesquiterpene and acetogenin derivatives from the marine red alga Laurencia okamurai. Mar. Drugs 2012, 10, 2817–2825. [Google Scholar] [CrossRef] [PubMed]
- Abbassy, M.A.; Marzouk, M.A.; Rabea, E.I.; Abd-Elnabi, A.D. Insecticidal and fungicidal activity of Ulva lactuca Linnaeus (Chlorophyta) extracts and their fractions. Annu. Res. Rev. Biol. 2014, 4, 2252–2262. [Google Scholar] [CrossRef]
- Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Colellaa, M.T.; Olaizolaa, C.; Ontiverosb, Y. Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 2004, 8, 39–45. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Method for Antifungal Disk Diffusion Susceptibility Testing of Nondermatophyte Filamentous Fungi; Approved Guideline; CLSI Document M51-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2010. [Google Scholar]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi; Approved Standard; CLSI Document M38-A; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2002. [Google Scholar]
- Borman, A.M.; Fraser, M.; Palmer, M.D.; Szekely, A.; Houldsworth, M.; Patterson, Z.; Johnson, E.M. MIC Distributions and Evaluation of Fungicidal Activity for Amphotericin B, Itraconazole, Voriconazole, Posaconazole and Caspofungin and 20 Species of Pathogenic Filamentous Fungi Determined Using the CLSI Broth Microdilution Method. J. Fungi 2017, 3, E27. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumar, R.; Sandle, T.; Al-Aboody, M.S.; AlFonaisan, M.K.; Alturaiki, W.; Mickymaray, S.; Premanathan, M.; Alsagaby, S.A. Distribution of biocide resistant genes and biocides susceptibility in multidrug-resistant Klebsiella pneumoniae, Pseudomonas aeruginosa and Acinetobacter baumannii––A first report from the Kingdom of Saudi Arabia. J. Infect. Public Health 2018, 11, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Kumaravel, S. Unraveling the potential phytochemical compounds of Gymnema sylvestre through GC-MS study. Int. J. Pharm. Pharm. Sci. 2016, 8, 1–4. [Google Scholar]
Sample Availability: Not available. |


| Fungal Isolates | Ethanol Fractions: Zone of Inhibition (mm) with Standard Deviation for Triplicates (1000 µg/mL) | Standard antibiotic | ||||
|---|---|---|---|---|---|---|
| Acanthophora spicifera | Cladophoropsis sp. | Laurencia paniculat | Tydemania sp. | Ulva prolifera | (amphotericin B; 100 units) | |
| Aspergillus niger | 10.6 ± 1.15 a | 11.6 ± 0.57 a,b | 16.3 ± 0.57 | - | 16 ± 2 | 16.33 ± 0.57 |
| Candida albicans | 10 ± 2 a | 11 ± 2 a,b | 17.6 ± 0.57 | - | 17.3 ± 1.15 | 17 ± 1 |
| Mucor sp. | - | 10.6 ± 1.15 a | 17 ± 1 | 10 ± 1 a | 17 ± 1 | 17.33 ± 0.57 |
| Paecilomyces sp. | 10.6 ± 1.15 a | 11.3 ± 1.15 a | 17.3 ± 1.15 b | - | 16.6 ± 1.15 | 16.66 ± 0.57 |
| S.No | Fungal Pathogens | L. paniculata | U. prolifera | ||||
|---|---|---|---|---|---|---|---|
| MIC μg/mL * | MFC μg/mL ** | Fungicidal Ratio | MIC μg/mL * | MFC μg/mL ** | Fungicidal Ratio | ||
| 1. | A. niger | 250 | 500 | 1:2 | 500 | 1000 | 1:2 |
| 2. | C. albicans | 125 | 125 | 1:1 | 125 | 125 | 1:1 |
| 3. | Mucor sp. | 250 | 500 | 1:1 | 500 | 1000 | 1:2 |
| 4 | Paecilomyces sp. | 250 | 500 | 1:2 | 500 | 500 | 1:1 |
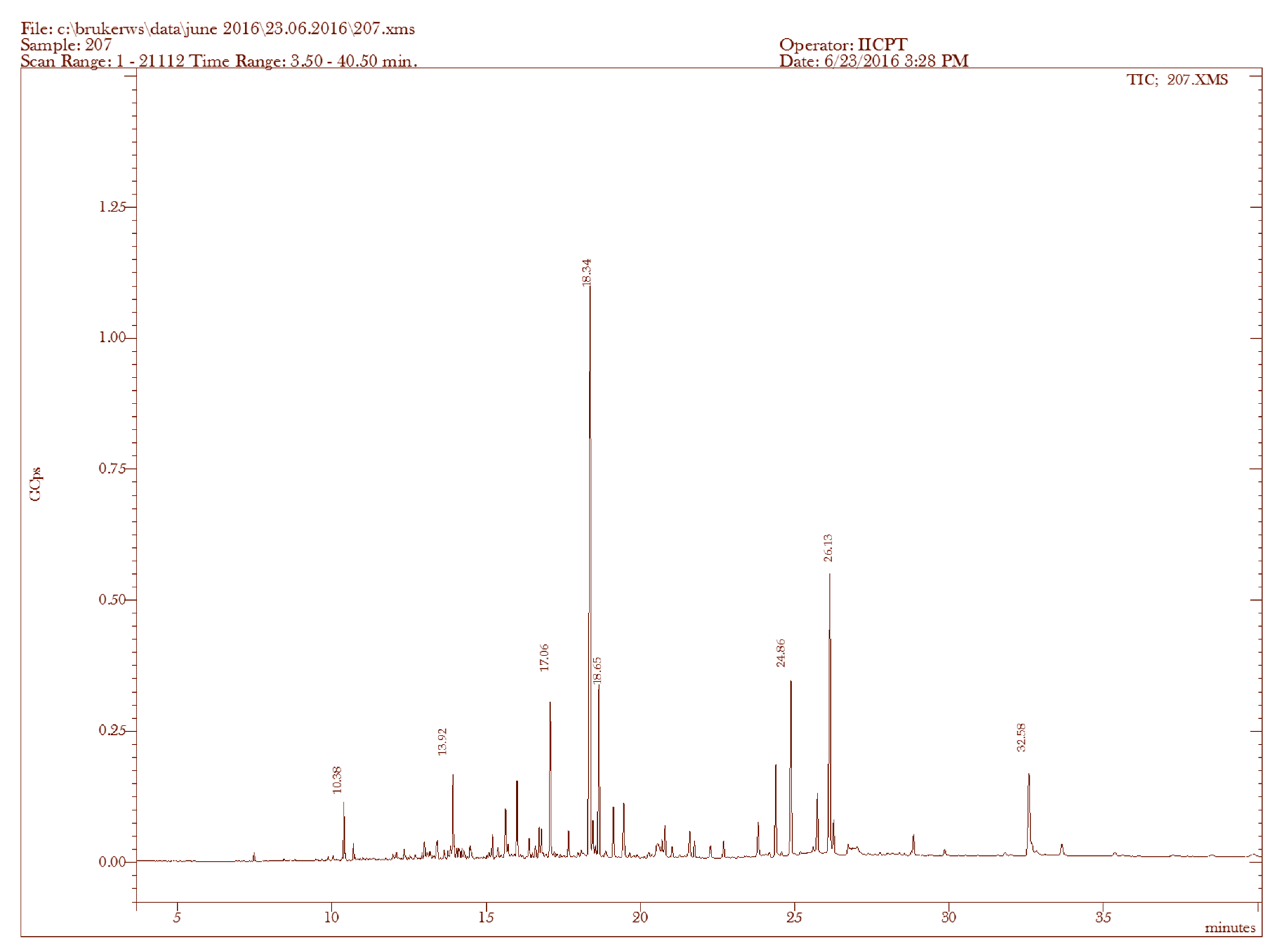
| Sample | RT (min) | Name of the Compound | Molecular Formula | MW (g/mol) | Peak Area % | Compound Nature | ** Activity |
|---|---|---|---|---|---|---|---|
| 1. | 7.47 | (−)-Aristolene | C15H24 | 204 | 0.32 | Sesquiterpene | Antibacterial, anti-inflammatory, fungicidal |
| 2. | 9.88 | 2-Naphthalenemethanol, decahydro-α,α,4a-trimethyl-8-methylene-, [2R-(2α,4aα,8aβ)]- | C15H26O | 222 | 0.18 | Sesquiterpene alcohol | Antibacterial, anti-inflammatory, fungicidal |
| 3. | 10.38 | 1-Naphthalenemethanol, 1,4,4a,5,6,7,8,8a-octahydro-2,5,5,8a-tetramethyl- | C15H26O | 222 | 1.73 | Sesquiterpene alcohol | Antibacterial, anti-inflammatory, fungicidal |
| 4. | 10.71 | 1,6,10-Dodecatrien-3-ol, 3,7,11-trimethyl-, (E)- | C15H26O | 222 | 0.67 | Sesquiterpene alcohol | Antibacterial, anti-inflammatory, fungicidal |
| 5. | 13.92 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296 | 7.32 | Terpene alcohol | Antimicrobial, anti-inflammatory |
| 6. | 17.66 | Phytol | C20H40O | 296 | 2.03 | Diterpene | Antimicrobial, anti-inflammatory Anticancer, |
| 7. | 18.65 | Androst-5-en-17-one, 3-(acetyloxy)-19-hydroxy-, (3β)- | C21H30O4 | 346 | 5.98 | Steroid | antimicrobial, anti-inflammatory Anticancer, antiasthma |
| 8. | 21.74 | trans-Z-α-Bisabolene epoxide | C15H24O | 220 | 1.35 | Sesquiterpene alcohol | Anti-tumor, antibacterial, anti-inflammatory, fungicidal. |
| 9. | 22.69 | Dasycarpidan-1-methanol, acetate (ester) | C20H26N2O2 | 326 | 0.76 | Nitrogen compound | Antimicrobial |
| 10. | 28.85 | Cholesta-3,5-diene | C27H44 | 368 | 0.50 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
| 11. | 32.58 | Cholesterol | C27H46O | 386 | 2.06 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
| 12. | 35.36 | Cholest-4-en-3-one | C27H44O | 384 | 0.05 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
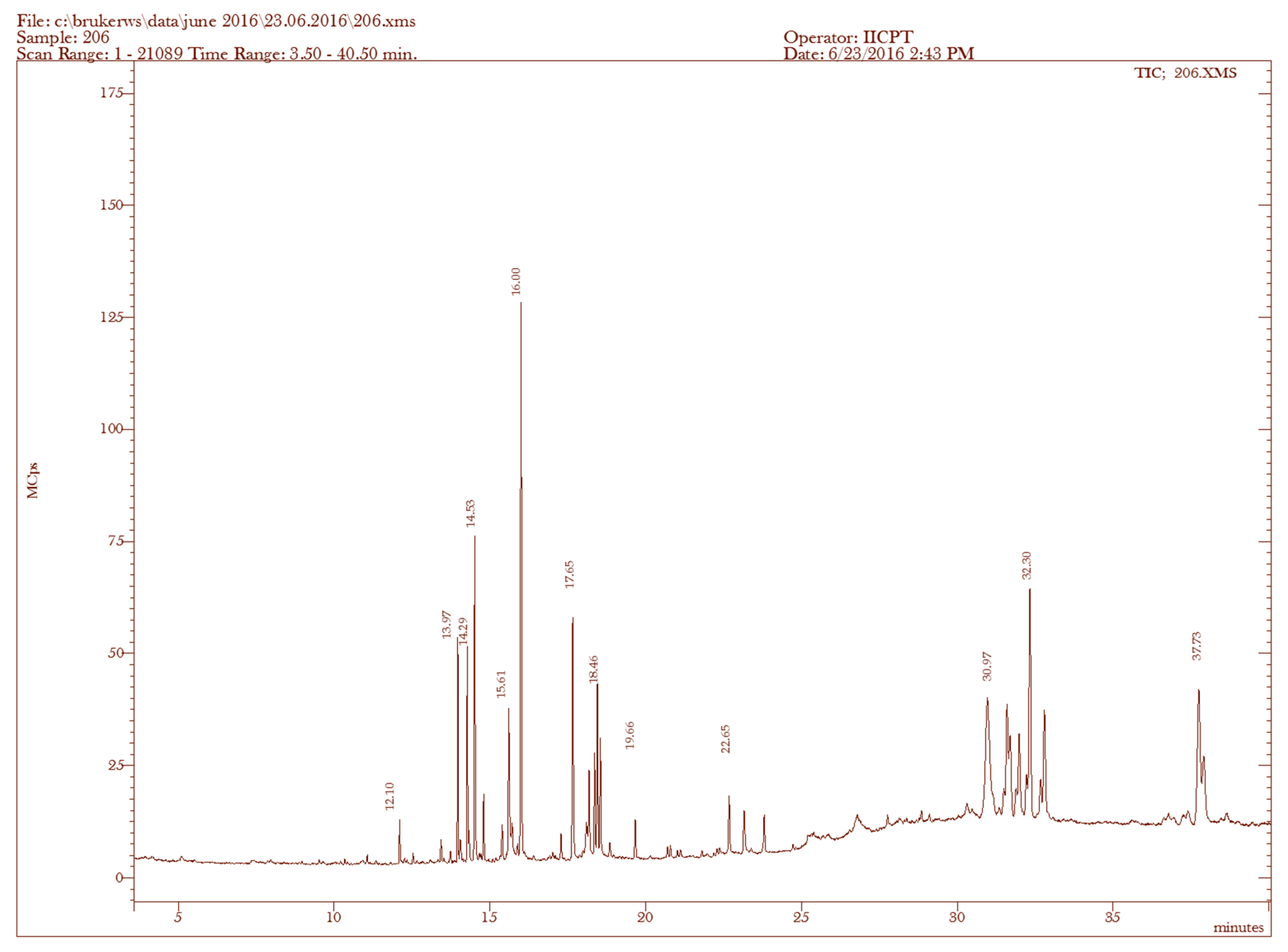
| Sample | RT (min) | Name of the Compound | Molecular Formulae | MW (g/mol) | Peak Area % | Compound Nature | ** Activity |
|---|---|---|---|---|---|---|---|
| 1. | 12.10 | n-Heptadecanol-1 | C17H36O | 256 | 0.75 | Alcoholic compound | Antimicrobial |
| 2. | 13.97 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | C20H40O | 296 | 3.38 | Terpene alcohol | Antimicrobial, anti-inflammatory |
| 3. | 17.65 | Phytol | C20H40O | 296 | 5.28 | Diterpene | Antimicrobial, anti-inflammatory, anticancer, diuretic |
| 4. | 22.65 | Dasycarpidan-1-methanol acetate (ester) | C20H26N2O2 | 326 | 1.54 | Nitrogen compound | Antimicrobial |
| 5. | 30.97 | Cholestan-3-ol, 2-methylene-, (3β,5α)- | C28H48O | 400 | 13.91 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
| 6. | 31.63 | Stigmasta-5,22-dien-3-ol, acetate, (3β)- | C31H50O2 | 454 | 4.85 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
| 7. | 31.98 | Pregn-5-en-20-one, 3-(acetyloxy)-17-hydroxy-, (3β)- | C23H34O4 | 374 | 5.24 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
| 8. | 32.30 | Allopregn-5,16-diene-3β-ol-20-one acetate | C23H32O3 | 356 | 9.93 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
| 9. | 32.76 | 5,16,20-Pregnatriene-3beta,20-diol diacetate | C25H34O4 | 398 | 6.04 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
| 10. | 37.73 | Stigmasta-5,24(28)-dien-3-ol, (3β,24Z)- | C29H48O | 412 | 8.33 | Steroid | Antimicrobial, anti-inflammatory, anticancer, antiasthma |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickymaray, S.; Alturaiki, W. Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules 2018, 23, 3032. https://doi.org/10.3390/molecules23113032
Mickymaray S, Alturaiki W. Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules. 2018; 23(11):3032. https://doi.org/10.3390/molecules23113032
Chicago/Turabian StyleMickymaray, Suresh, and Wael Alturaiki. 2018. "Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases" Molecules 23, no. 11: 3032. https://doi.org/10.3390/molecules23113032
APA StyleMickymaray, S., & Alturaiki, W. (2018). Antifungal Efficacy of Marine Macroalgae against Fungal Isolates from Bronchial Asthmatic Cases. Molecules, 23(11), 3032. https://doi.org/10.3390/molecules23113032




