Differential Effects of the Flavonolignans Silybin, Silychristin and 2,3-Dehydrosilybin on Mesocestoides vogae Larvae (Cestoda) under Hypoxic and Aerobic In Vitro Conditions
Abstract
1. Introduction
2. Results
2.1. Assessment of Morphology and Motility
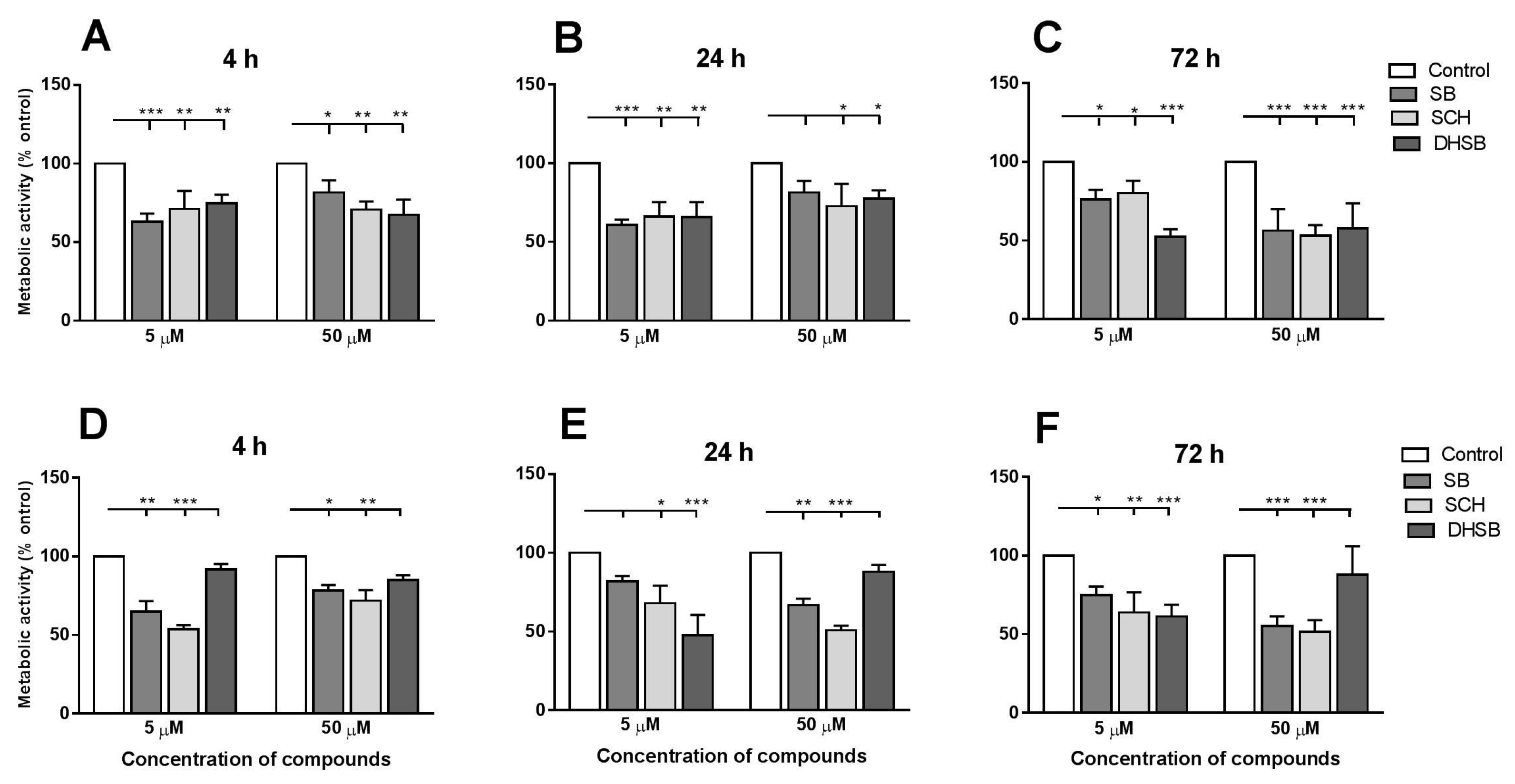
2.2. Metabolic Activity of Larvae
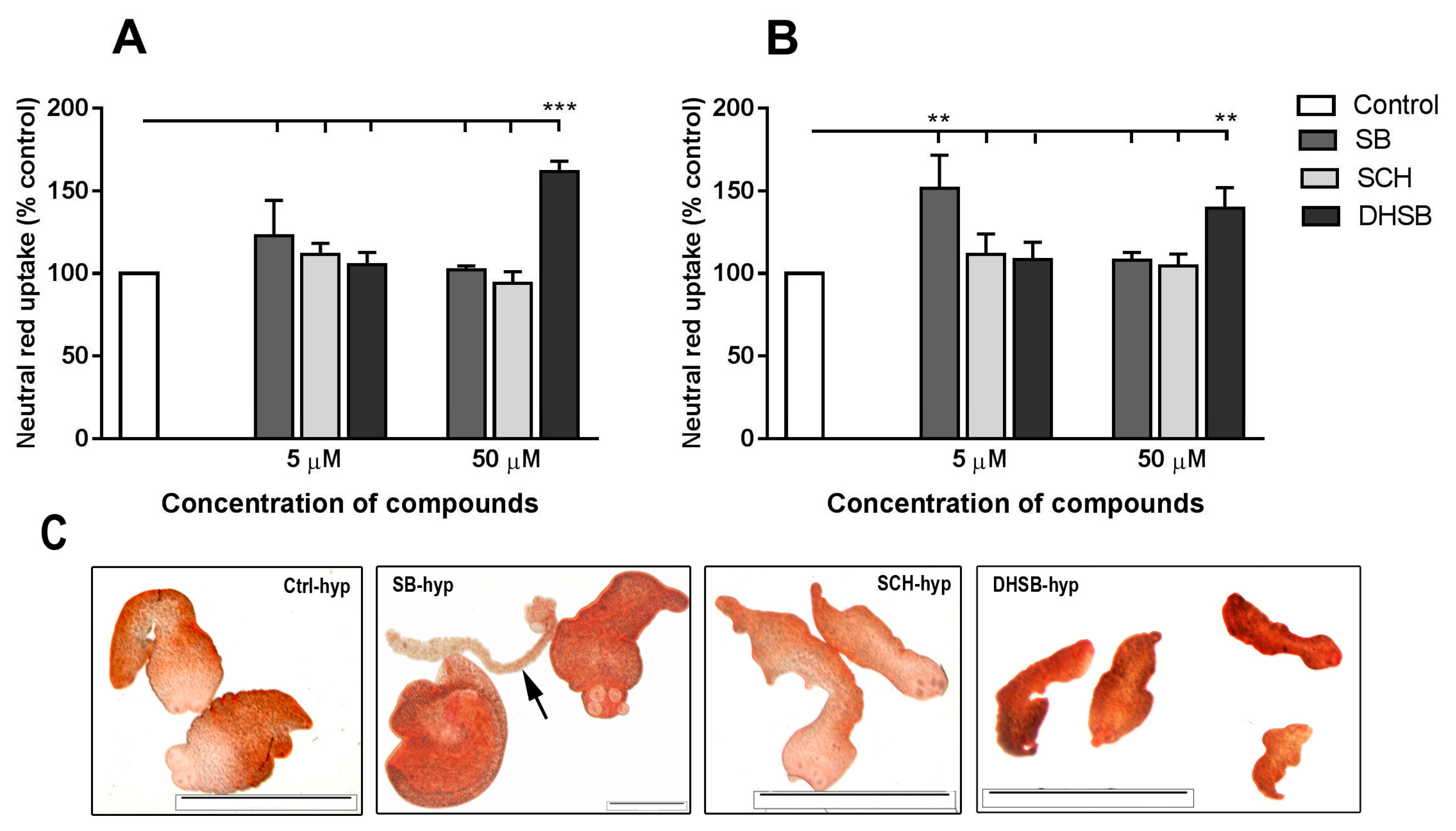
2.3. Neutral Red Uptake
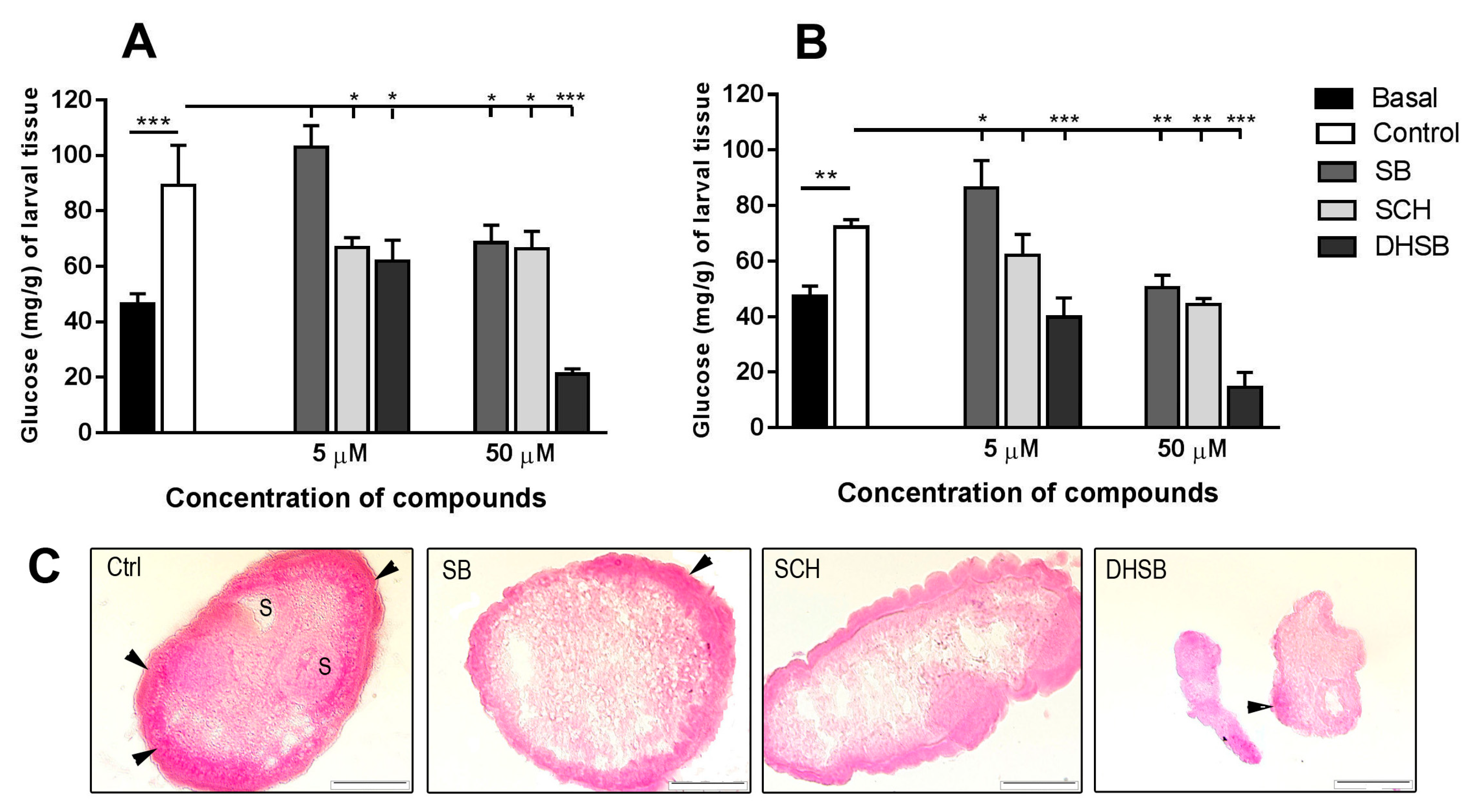
2.4. Concentration of Glucose in Larvae and Glycogen Localization
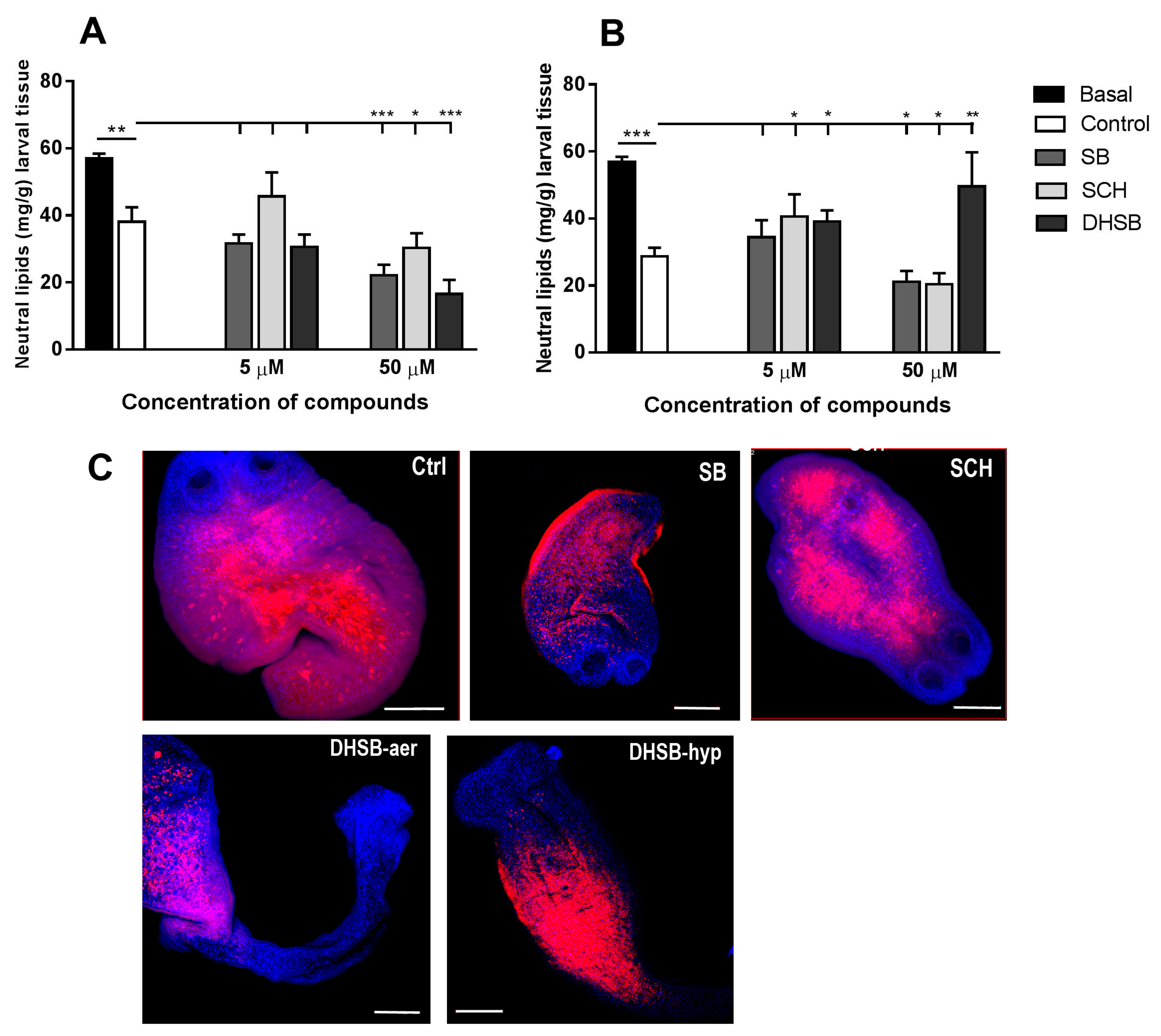
2.5. Neutral Lipid Concentrations and Their Distribution
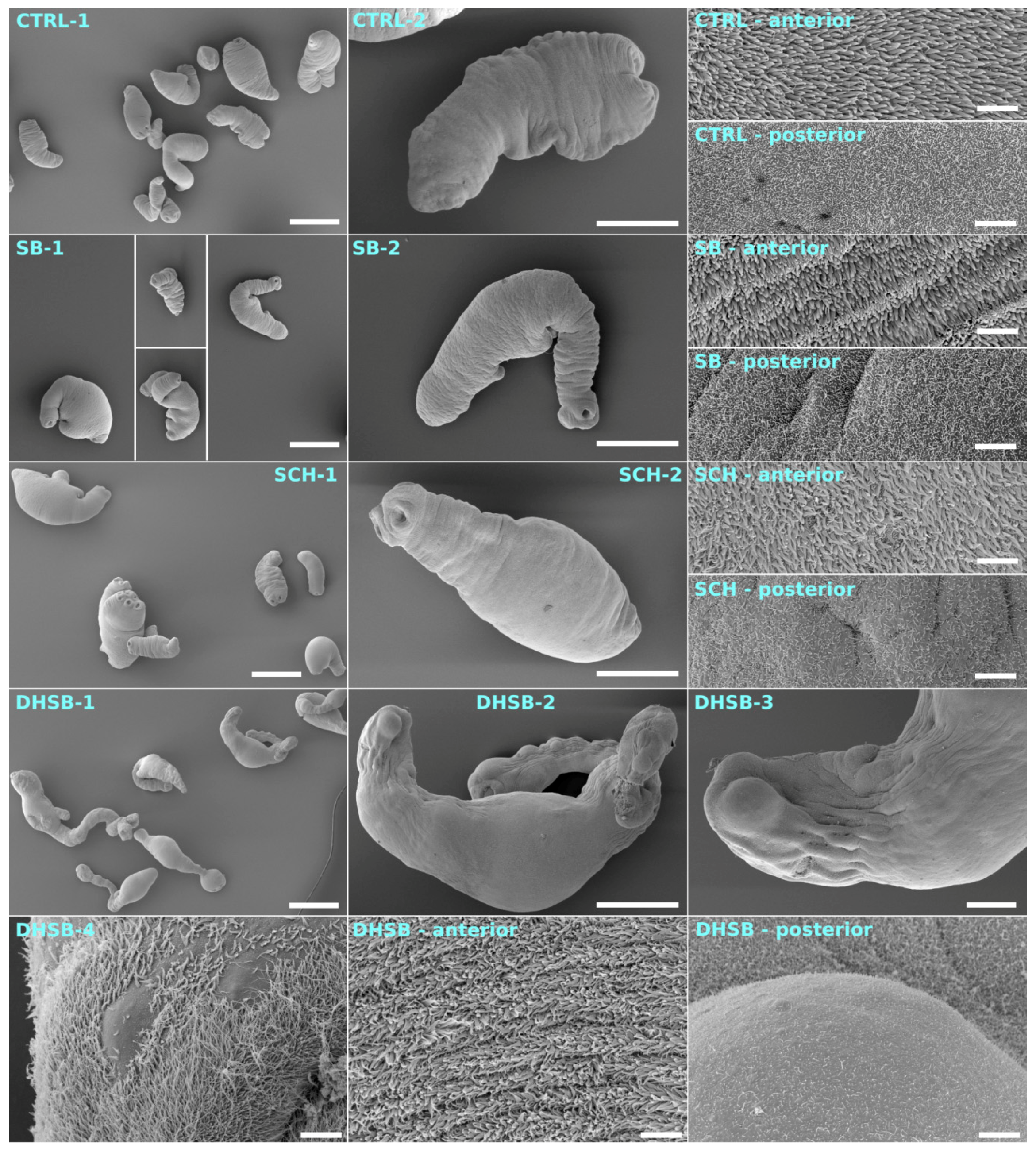
2.6. Scanning Electron Microscopy of Larvae
3. Discussion
4. Materials and Methods
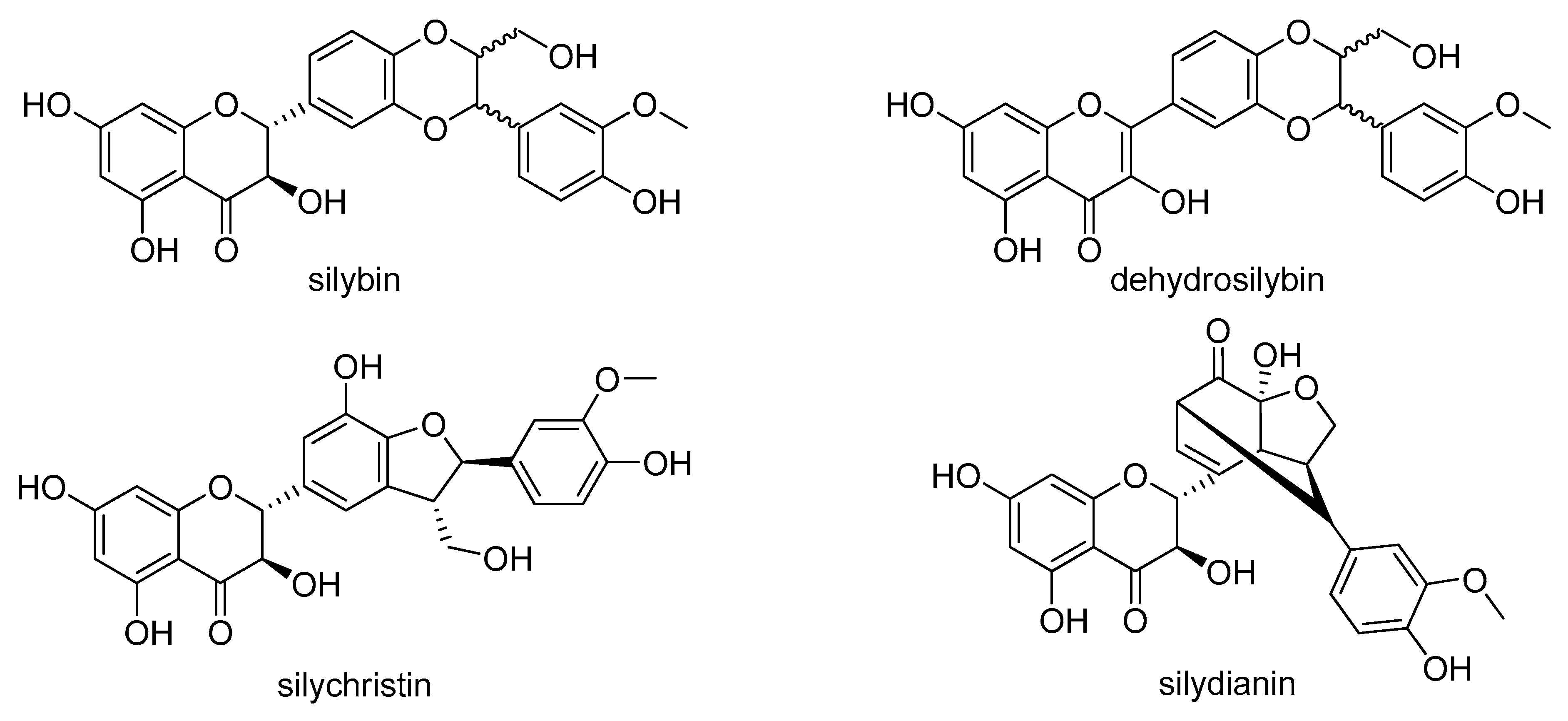
4.1. Silymarin Flavonolignans and Other Chemicals
4.2. Parasites and In Vitro Culture System
4.3. Quantification of Effects of FLs in Larval Cultures
4.4. Metabolic Activity of Larvae
4.5. Neutral Red Uptake
4.6. Motility of Larvae and Morphological Alterations of Larvae
4.7. Glucose Concentration and Localization of Glycogen in Larvae
4.8. Neutral Lipids Concentration
4.9. Confocal Laser Scanning Microscopy for Localization of Neutral Lipids
4.10. Scanning Electron Microscopy of Larvae
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadou, S.; Githiori, J.; Kyriazakis, I. Medicinal plants for helminth parasite control: Facts and fiction. Animal 2007, 1, 1392–1400. [Google Scholar] [CrossRef] [PubMed]
- Hrčková, G.; Velebný, S. Pharmacological Potential of Selected Natural Compounds in the Control of Parasitic Diseases; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Irum, S.; Ahmed, H.; Mirza, B.; Donskow-Łysoniewska, K.; Muhammad, A.; Qayyum, M.; Simsek, S. In vitro and in vivo anthelmintic activity of extracts from Artemisia parviflora and A. sieversiana. Helminthologia 2017, 54, 218–224. [Google Scholar] [CrossRef]
- Moazeni, M.; Saharkhiz, M.J.; Hosseini, A.A. In vitro lethal effect of ajowan (Trachyspermum ammi L.) essential oil on hydatid cyst protoscoleces. Vet. Parasitol. 2012, 187, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Tandon, V. Anthelmintic efficacy of Flemingia vestita (leguminoceae): Genistein-induced alterations in the activity of tegumental enzymes in the cestode, Raillietina echinobothrida. Parasitol. Int. 1998, 47, 233–243. [Google Scholar] [CrossRef]
- Tandon, V.; Pal, P.; Roy, B.; Rao, H.S.P.; Reddy, K.S. In vitro anthelmintic activity of root-tuber extract of Flemingia vestita, an indigenous plant in Shillong, India. Parasitol. Int. 1997, 492–498. [Google Scholar] [CrossRef]
- Naguleswaran, A.; Spicher, M.; Vonlaufen, N.; Ortega-Mora, L.M.; Torgerson, P.; Gottstein, B.; Hemphill, A. In vitro metacestodicidal activities of genistein and other isoflavones against Echinococcus multilocularis and Echinococcus granulosus. Antimicrob. Agents Chemother. 2006, 50, 3770–3778. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.G.; Machado, C.B.; Morais, E.R.; de Carvalho Moreira, É.B.; Soares, C.S.; da Silva, S.H.; Da Silva Filho, A.A.; Rodrigues, V. In vitro schistosomicidal activity of curcumin against Schistosoma mansoni adult worms. Parasitol. Res. 2009, 104, 1197–1201. [Google Scholar] [CrossRef] [PubMed]
- Gažák, R.; Walterová, D.; Křen, V. Silybin and silymarin—New and emerging applications in medicine. Cur. Med. Chem. 2007, 14, 315–338. [Google Scholar] [CrossRef]
- Esmaeil, N.; Anaraki, S.B.; Gharagozloo, M.; Moayedi, B. Silymarin impacts on immune system as an immunomodulator: One key for many locks. Int. Immunopharmacol. 2017, 50, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, T. The nuclear factor NF-κB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 2009, 1, a001651. [Google Scholar] [CrossRef]
- Yoo, H.G.; Jung, S.N.; Hwang, Y.S.; Park, J.S.; Kim, M.H.; Jeong, M.; Ahn, S.J.; Ahn, B.W.; Shin, B.A.; Park, R.K. Involvement of NF-κB and caspases in silibinin-induced apoptosis of endothelial cells. Int. J. Mol. Med. 2004, 13, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, D.; Buchta, M.; Holečková, V.; Sedlák, D.; Valentová, K.; Cvačka, J.; Bednárová, L.; Křenková, A.; Kuzma, M.; Škuta, C.; et al. Silychristin: Skeletal alterations and biological activities. J. Nat. Prod. 2016, 79, 3086–3092. [Google Scholar] [CrossRef] [PubMed]
- Pyszková, M.; Biler, M.; Biedermann, D.; Valentová, K.; Kuzma, M.; Vrba, J.; Ulrichová, J.; Sokolová, R.; Mojović, M.; Popovič-Bijelič, A.; et al. Flavonolignan 2,3-dehydroderivatives: Preparation, antiradical and cytoprotective activity. Free Radic Biol. Med. 2016, 90, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Roubalová, L.; Dinková-Kostová, A.T.; Biedermann, D.; Křen, V.; Ulrichová, J.; Vrba, J. Flavonolignan 2,3-dehydrosilydianin activates NRF2 and upregulates NAD(P)H: Quinone oxidoreductase 1 in Hepa1c1c7 cells. Fitoterapia 2017, 119, 115–120. [Google Scholar] [CrossRef] [PubMed]
- El-Lakkany, N.M.; Hammam, O.A.; El-Maadawy, W.H.; Badawy, A.A.; Ain-Shoka, A.A.; Ebeid, F.A. Anti-inflammatory/anti-fibrotic effects of the hepatoprotective silymarin and the schistosomicide praziquantel against Schistosoma mansoni-induced liver fibrosis. Parasit. Vectors 2012, 5, 9. [Google Scholar] [CrossRef] [PubMed]
- Velebný, S.; Hrčková, G.; Kogan, G. Impact of treatment with praziquantel, silymarin and/or β-glucan on pathophysiological markers of liver damage and fibrosis in mice infected with Mesocestoides vogae (cestoda) tetrathyridia. J. Helminthol. 2008, 82, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Velebný, S.; Hrčková, G.; Königová, A. Reduction of oxidative stress and liver injury following silymarin and praziquantel treatment in mice with Mesocestoides vogae (cestoda) infection. Parasitol. Int. 2010, 59, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Köhler, P.; Hanselmann, K. Anaerobic and aerobic energy metabolism in the larvae (tetrathyridia) of Mesocestoides corti. Exp. Parasitol. 1974, 36, 178–188. [Google Scholar] [CrossRef]
- Hrckova, G.; Velebny, S. Effects of free and liposomized praziquantel on worm burden and antibody-response in mice infected with Mesocestoides corti tetrathyridia. J. Helminthol. 1995, 69, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Shinohara, M.; Komori, K.; Sakai, Y.; Matsui, H.; Osada, T. The importance of physiological oxygen concentrations in the sandwich cultures of rat hepatocytes on gas-permeable membranes. Biotechnol. Prog. 2014, 30, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Lukiewicz, S.; Hyde, J.S. Murine in vivo L-band ESR spin-label oximetry with a loop-gap resonator. Magn. Reson. Med. 1986, 3, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Antal, P.; Sipka, S.; Suranyi, P.; Csipo, I.; Seres, T.; Marodi, L.; Szegedi, G. Flow cytometric assay of phagocytic-activity of human neutrophils and monocytes in whole-blood by neutral red uptake. Ann. Hematol. 1995, 70, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.; Behm, C.A. Biochemical Adaptation in Parasites; Chapman and Hall Ltd.: London, UK, 1989. [Google Scholar] [CrossRef]
- Frayha, G.J.; Smyth, J.D.; Baker, J.R.; Muller, R. Lipid metabolism in parasitic helminths. In Advances in Parasitology; Academic Press: Cambridge, MA, USA, 1983; Volume 22, pp. 309–387. [Google Scholar] [CrossRef]
- Etges, F.J. The proliferative tetrathyridium of Mesocestoides vogae sp. N.(cestoda). J. Helminthol. Soc. Wash. 1991, 58, 181–185. [Google Scholar]
- Eleni, C.; Scaramozzino, P.; Busi, M.; Ingrosso, S.; D’Amelio, S.; De Liberato, C. Proliferative peritoneal and pleural cestodiasis in a cat caused by metacestodes of Mesocestoides sp. anatomohistopathological findings and genetic identification. Parasite 2007, 14, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.; Papini, R.; Ferrini, N.; Gasser, R.B. Use of a molecular approach for the definitive diagnosis of proliferative larval mesocestoidiasis in a cat. Infect. Genet. Evol. 2012, 12, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Wirtherle, N.; Wiemann, A.; Ottenjann, M.; Linzmann, H.; van der Grinten, E.; Kohn, B.; Gruber, A.D.; Clausen, P.H. First case of canine peritoneal larval cestodosis caused by Mesocestoides lineatus in Germany. Parasitol. Int. 2007, 56, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Vendelova, E.; Hrčková, G.; Lutz, M.B.; Brehm, K.; Nono, J.K. In vitro culture of Mesocestoides corti metacestodes and isolation of immunomodulatory excretory-secretory products. Parasite Immunol. 2016, 38, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.D.; McManus, D.P. The Physiology and Biochemistry of Cestodes; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar] [CrossRef]
- Sakai, C.; Tomitsuka, E.; Esumi, H.; Harada, S.; Kita, K. Mitochondrial fumarate reductase as a target of chemotherapy: From parasites to cancer cells. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Hrčková, G.; Velebný, S.; Halton, D.W.; Day, T.A.; Maule, A.G. Pharmacological characterisation of neuropeptide F (NPF)-induced effects on the motility of Mesocestoides corti (syn. Mesocestoides vogae) larvae. Int. J. Parasitol. 2004, 34, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Terenina, N.B.; Reuter, M.; Gustafsson, M.K.S. An experimental, NADPH-diaphorase histochemical and immunocytochemical study of Mesocestoides vogae tetrathyridia. Int. J. Parasitol. 1999, 29, 787–793. [Google Scholar] [CrossRef]
- Halton, D.W. Nutritional adaptations to parasitism within the platyhelminthes. Int. J. Parasitol. 1997, 27, 693–704. [Google Scholar] [CrossRef]
- Hess, E. Ultrastructural-study of the tetrathyridium of Mesocestoides corti Hoeppli, 1925: Tegument and parenchyma. Parasitol. Res. 1980, 61, 135–159. [Google Scholar] [CrossRef]
- Matsumoto, J.; Sakamoto, K.; Shinjyo, N.; Kido, Y.; Yamamoto, N.; Yagi, K.; Miyoshi, H.; Nonaka, N.; Katakura, K.; Kita, K.; et al. Anaerobic NADH-fumarate reductase system is predominant in the respiratory chain of Echinococcus multilocularis, providing a novel target for the chemotherapy of alveolar echinococcosis. Antimicrob. Agents Chemother. 2008, 52, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Valentová, K.; Purchartová, K.; Rydlová, L.; Roubalová, L.; Biedermann, D.; Petrásková, L.; Křenková, A.; Pelantová, H.; Holečková-Moravcová, V.; Tesařová, E.; et al. Sulfated metabolites of flavonolignans and 2,3-dehydroflavonolignans: Preparation and properties. Int. J. Mol. Sci. 2018, 19, 2349. [Google Scholar] [CrossRef] [PubMed]
- Maitrejean, M.; Comte, G.; Barron, D.; El Kirat, K.; Conseil, G.L.; Di Pietro, A. The flavanolignan silybin and its hemisynthetic derivatives, a novel series of potential modulators of P-glycoprotein. Bioorg. Med. Chem. Lett. 2000, 10, 157–160. [Google Scholar] [CrossRef]
- Kubala, M.; Čechová, P.; Geletičová, J.; Biler, M.; Štenclová, T.; Trouillas, P.; Biedermann, D. Flavonolignans as a novel class of sodium pump inhibitors. Front. Physiol. 2016, 7, 115. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.; Thongphasuk, P.; Erben, G.; Lehmann, W.-D.; Tuma, S.; Stremmel, W.; Chamulitrat, W. Significantly greater antioxidant anticancer activities of 2,3-dehydrosilybin than silybin. Biochim. Biophys. Acta Gen. Subj. 2008, 1780, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Płytycz, B.; Rózanowska, M.; Seljelid, R. Quantification of neutral red pinocytosis by small numbers of adherent cells: Comparative studies. Folia Biol. 1992, 40, 3–9. [Google Scholar]
- Maggiore, M.; Elissondo, M.C. In vitro cestocidal activity of thymol on Mesocestoides corti tetrathyridia and adult worms. Interdiscip. Perspect. Infect. Dis. 2014, 2014, 268135. [Google Scholar] [CrossRef]
- Shuhua, X.; Hotez, P.J.; Tanner, M. Artemether, an effective new agent for chemoprophylaxis against shistosomiasis in china: Its in vivo effect on the biochemical metabolism of the asian schistosome. Southeast Asian J. Trop. Med. Public Health 2000, 31, 724–732. [Google Scholar] [PubMed]
- Tandon, V.; Das, B.; Saha, N. Anthelmintic efficacy of Flemingia vestita (fabaceae): Effect of genistein on glycogen metabolism in the cestode, Raillietina echinobothrida. Parasitol. Int. 2003, 52, 179–183. [Google Scholar] [CrossRef]
- Lienhard, G.E.; Slot, J.W.; James, D.E.; Mueckler, M.M. How cells absorb glucose. Sci. Am. 1992, 266, 86–91. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Digel, M.; Küch, E.-M.; Stremmel, W.; Füllekrug, J. Silybin and dehydrosilybin decrease glucose uptake by inhibiting GLUT proteins. J. Cell. Biochem. 2011, 112, 849–859. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Sanchez, C.; Sanz, N.; Lopez-Novoa, J.M.; Leverve, X.; El-Mir, M.Y. Interrelation between the inhibition of glycolytic flux by silibinin and the lowering of mitochondrial ROS production in perifused rat hepatocytes. Life Sci. 2008, 82, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.C.; Coley, S.F.; Williams, J. Lipid and protein composition of the surface tegument from larvae of Taenia taeniaeformis. J. Parasitol. 1984, 197–207. [Google Scholar] [CrossRef]
- Alvite, G.; Esteves, A. Lipid binding proteins from parasitic platyhelminthes. Front. Physiol. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.-C.; Freitas, T.C.; Amiel, E.; Everts, B.; Pearce, E.L.; Lok, J.B.; Pearce, E.J. Fatty acid oxidation is essential for egg production by the parasitic flatworm Schistosoma mansoni. PLoS Pathog. 2012, 8, e1002996. [Google Scholar] [CrossRef] [PubMed]
- Křenek, K.; Marhol, P.; Peikerová, Ž.; Křen, V.; Biedermann, D. Preparatory separation of the silymarin flavonolignans by sephadex LH-20 gel. Food Res. Int. 2016, 65, 115–120. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
| Tested Compounds | Concentration (μM) | Aerobic Cultivation | Hypoxic Cultivation | ||||
|---|---|---|---|---|---|---|---|
| Motility Score/Days of Cultivation | |||||||
| Day 1 | Day 2 | Day 3 | Day 1 | Day 2 | Day 3 | ||
| Control | - | 3+ | 3+ | 3+ | 3+ | 3+ | 3+ |
| SB | 5 | 2+ | 2+ | 2+ | 3+ | 3+ | 3+ |
| 50 | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ | |
| SCH | 5 | 2+ | 2+ | 2+ | 2+ | 2+ | 2+ |
| 50 | 1+/2+ | 1+/2+ | 1+/2+ | 1+/2+ | 1+/2+ | 1+/2+ | |
| DHSB | 5 | 1+ | 1+ | 1+ | 1+/2+ | 1+ | 1+ |
| 50 | 4+ | 4+ | 4+ | 1+ | 1+ | 0.5+ | |
| Tested Compounds | Concentration [μM] | Proportions [%] under Cultivation Conditions | |
|---|---|---|---|
| Aerobic | Hypoxic | ||
| Control | - | 1.9 ± 0.8 | 1.4 ± 0.9 |
| SB | 5 | 1.1 ± 0.7 | 1.8 ± 0.8 |
| 50 | 2.4 ± 1.5 | 3.0 ± 1.2 | |
| SCH | 5 | 2.9 ± 2.0 | 2.9 ± 1.5 |
| 50 | 5.1 ± 2.3 | 4.7 ± 2.9 | |
| DHSB | 5 | 3.7 ± 2.1 | 5.4 ± 3.6 |
| 50 | 7.9 ± 3.3 | 15.1 ± 4.7 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hrčková, G.; Kubašková, T.M.; Benada, O.; Kofroňová, O.; Tumová, L.; Biedermann, D. Differential Effects of the Flavonolignans Silybin, Silychristin and 2,3-Dehydrosilybin on Mesocestoides vogae Larvae (Cestoda) under Hypoxic and Aerobic In Vitro Conditions. Molecules 2018, 23, 2999. https://doi.org/10.3390/molecules23112999
Hrčková G, Kubašková TM, Benada O, Kofroňová O, Tumová L, Biedermann D. Differential Effects of the Flavonolignans Silybin, Silychristin and 2,3-Dehydrosilybin on Mesocestoides vogae Larvae (Cestoda) under Hypoxic and Aerobic In Vitro Conditions. Molecules. 2018; 23(11):2999. https://doi.org/10.3390/molecules23112999
Chicago/Turabian StyleHrčková, Gabriela, Terézia Mačák Kubašková, Oldřich Benada, Olga Kofroňová, Lenka Tumová, and David Biedermann. 2018. "Differential Effects of the Flavonolignans Silybin, Silychristin and 2,3-Dehydrosilybin on Mesocestoides vogae Larvae (Cestoda) under Hypoxic and Aerobic In Vitro Conditions" Molecules 23, no. 11: 2999. https://doi.org/10.3390/molecules23112999
APA StyleHrčková, G., Kubašková, T. M., Benada, O., Kofroňová, O., Tumová, L., & Biedermann, D. (2018). Differential Effects of the Flavonolignans Silybin, Silychristin and 2,3-Dehydrosilybin on Mesocestoides vogae Larvae (Cestoda) under Hypoxic and Aerobic In Vitro Conditions. Molecules, 23(11), 2999. https://doi.org/10.3390/molecules23112999






