Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes
Abstract
1. Introduction
2. Results
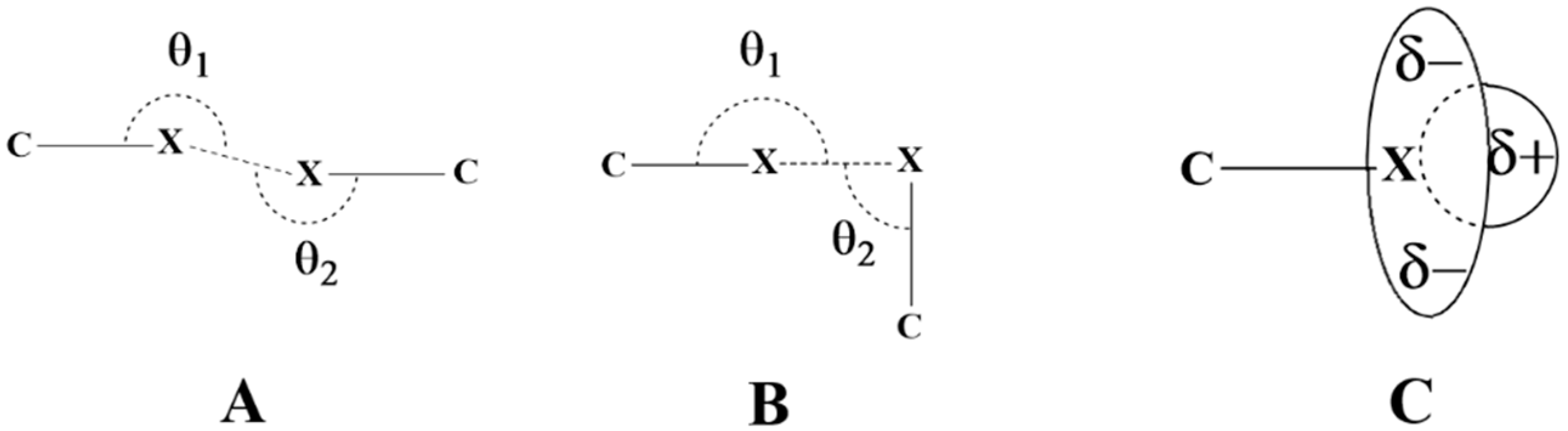
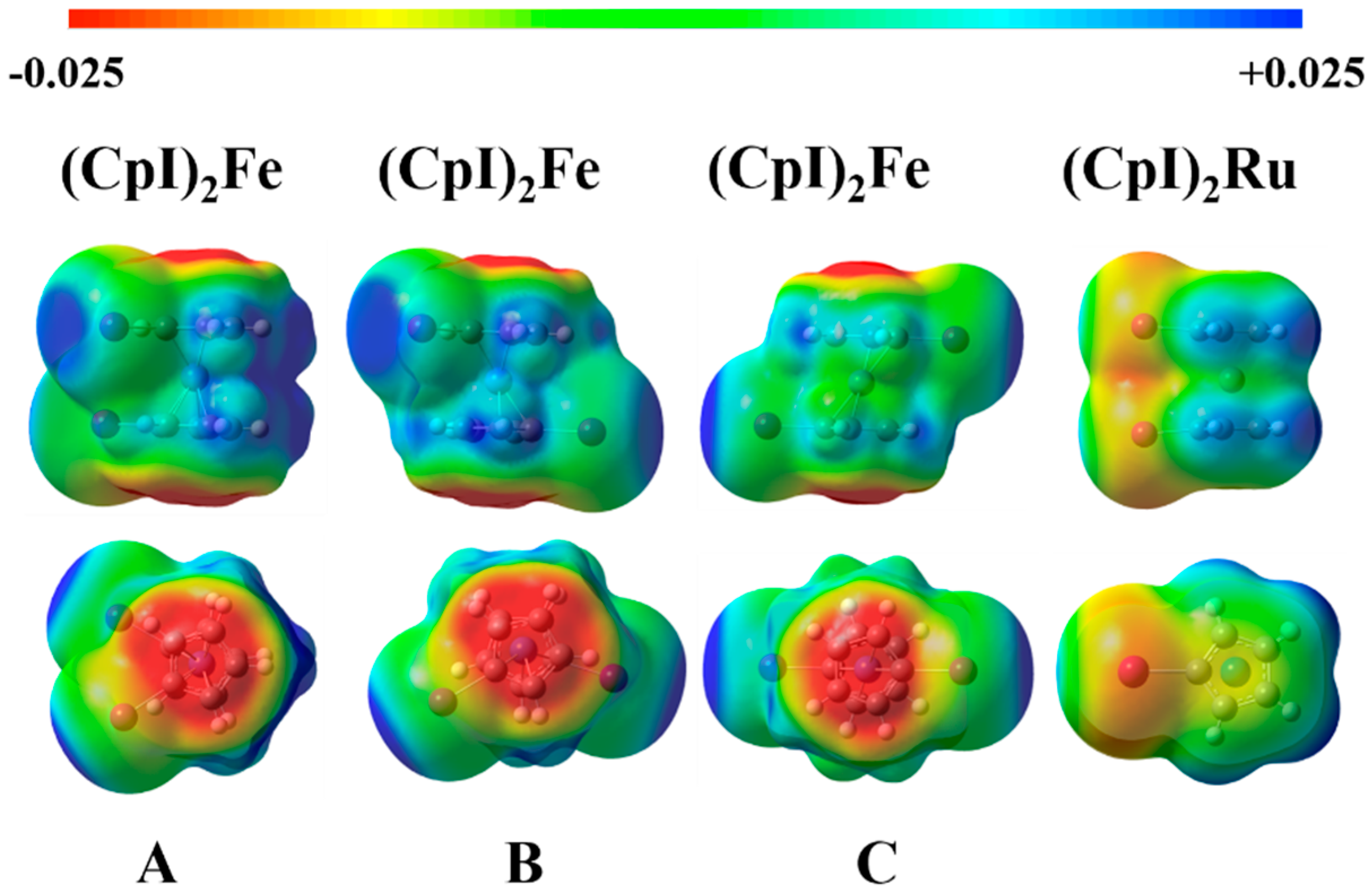
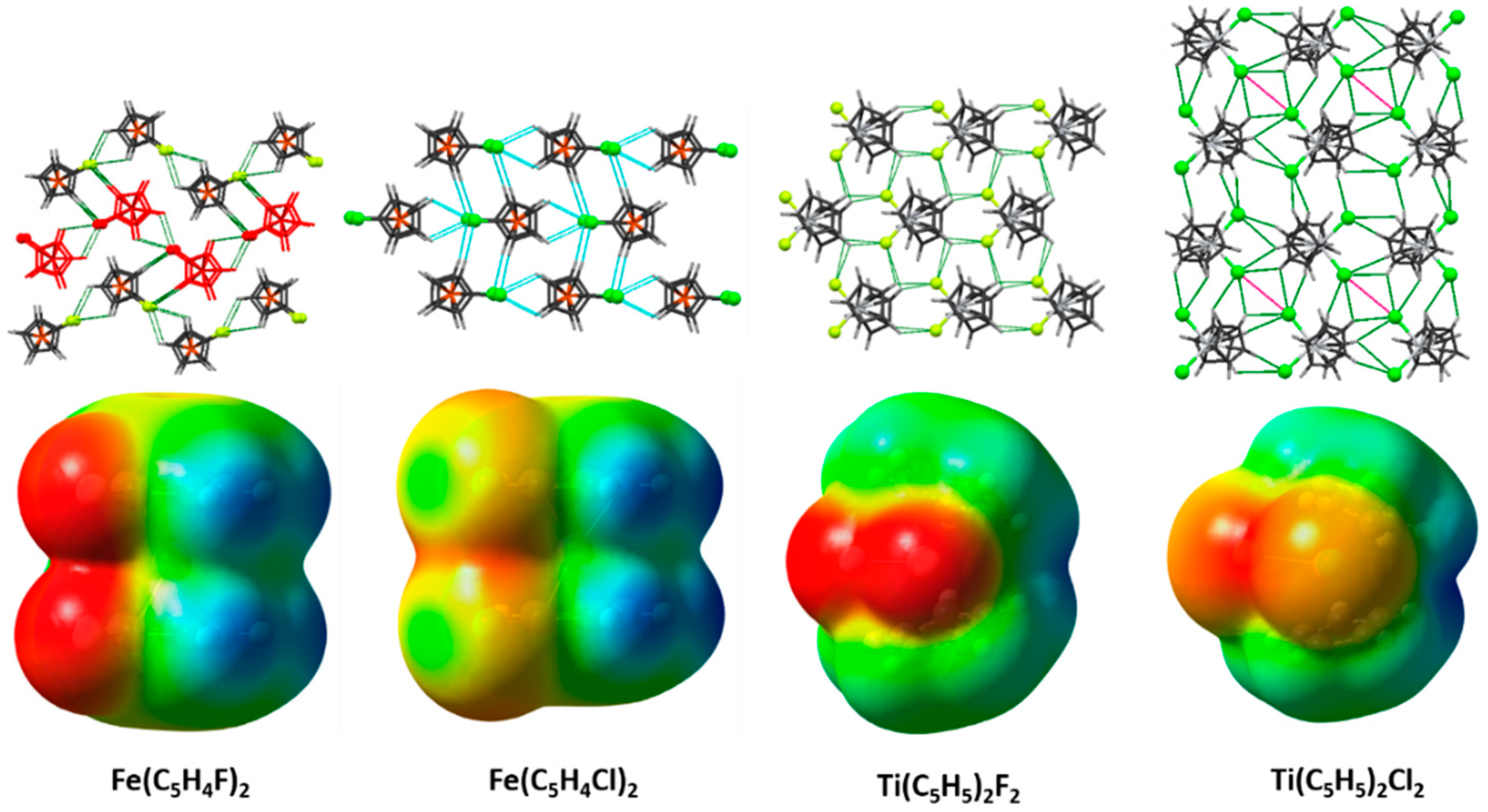
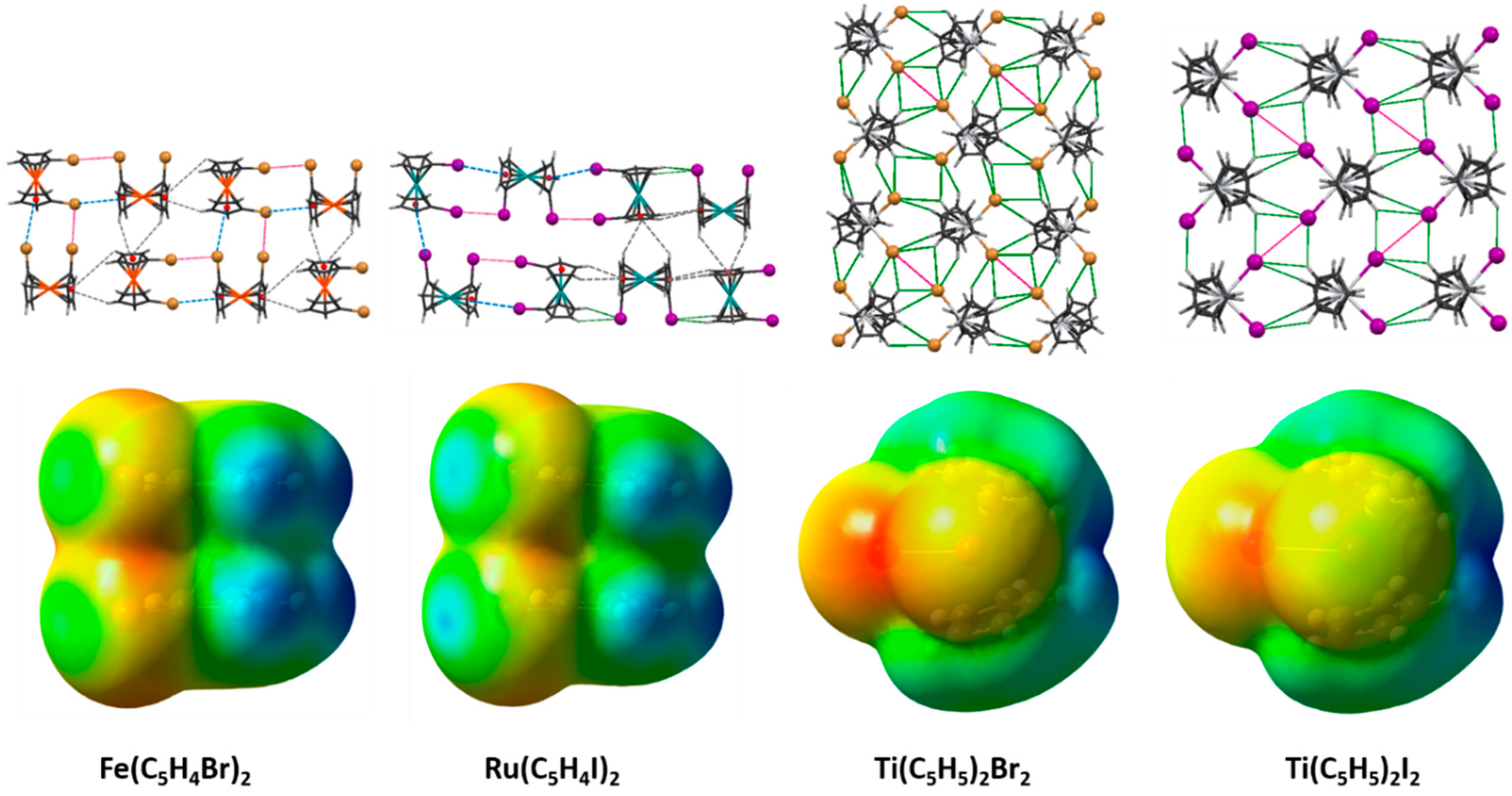
2.1. Electrostatic Potentials by DFT Calculations
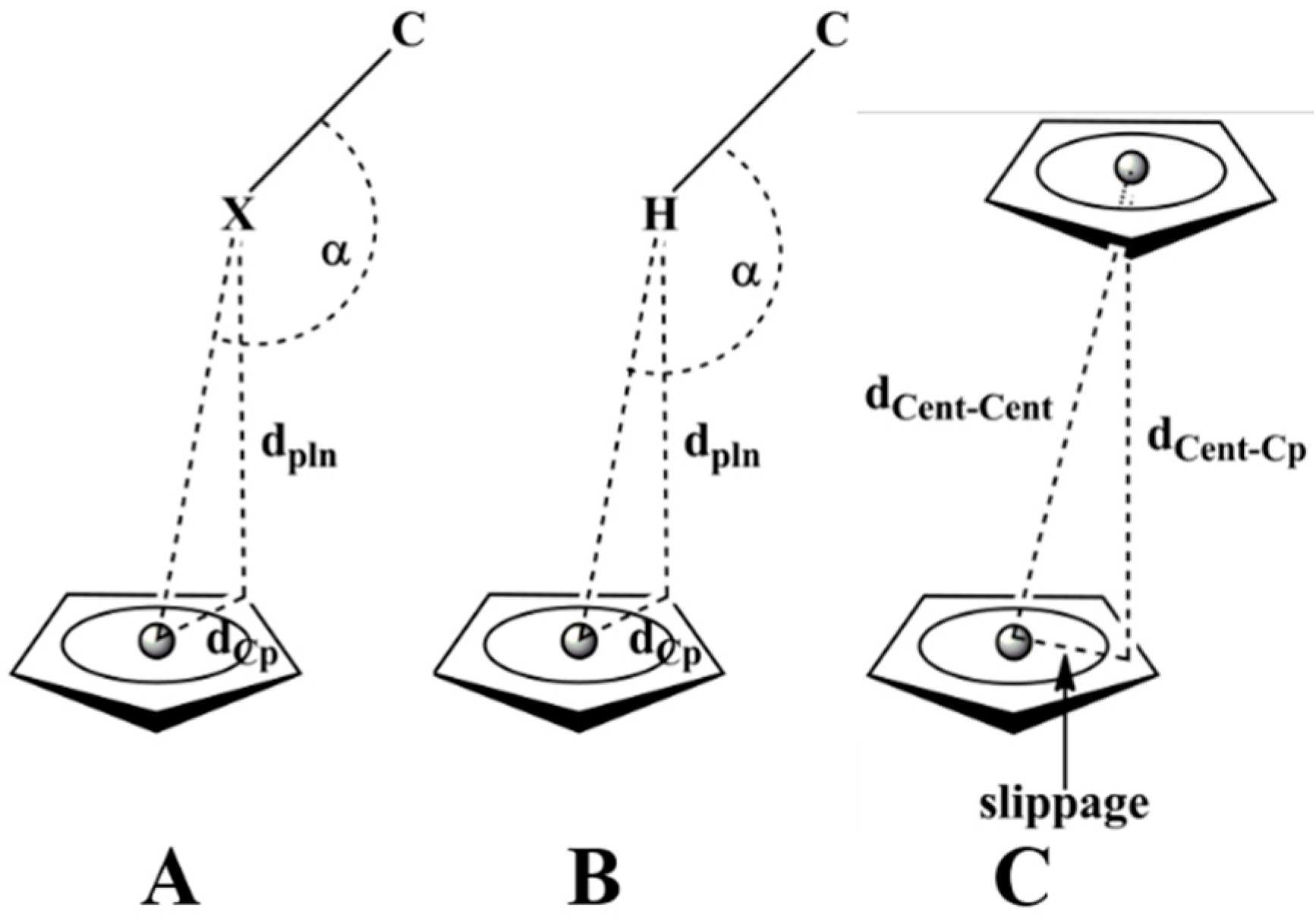
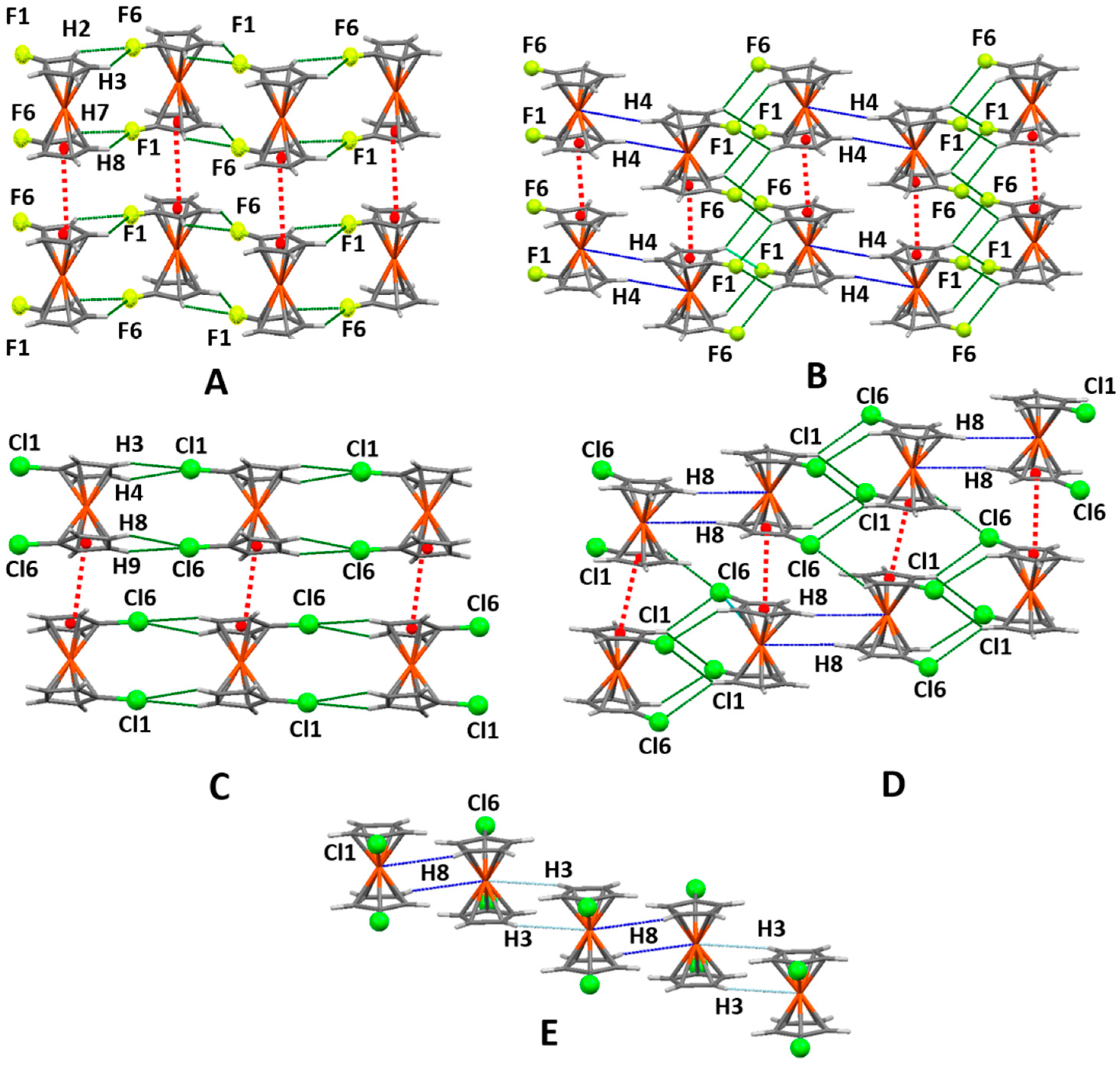
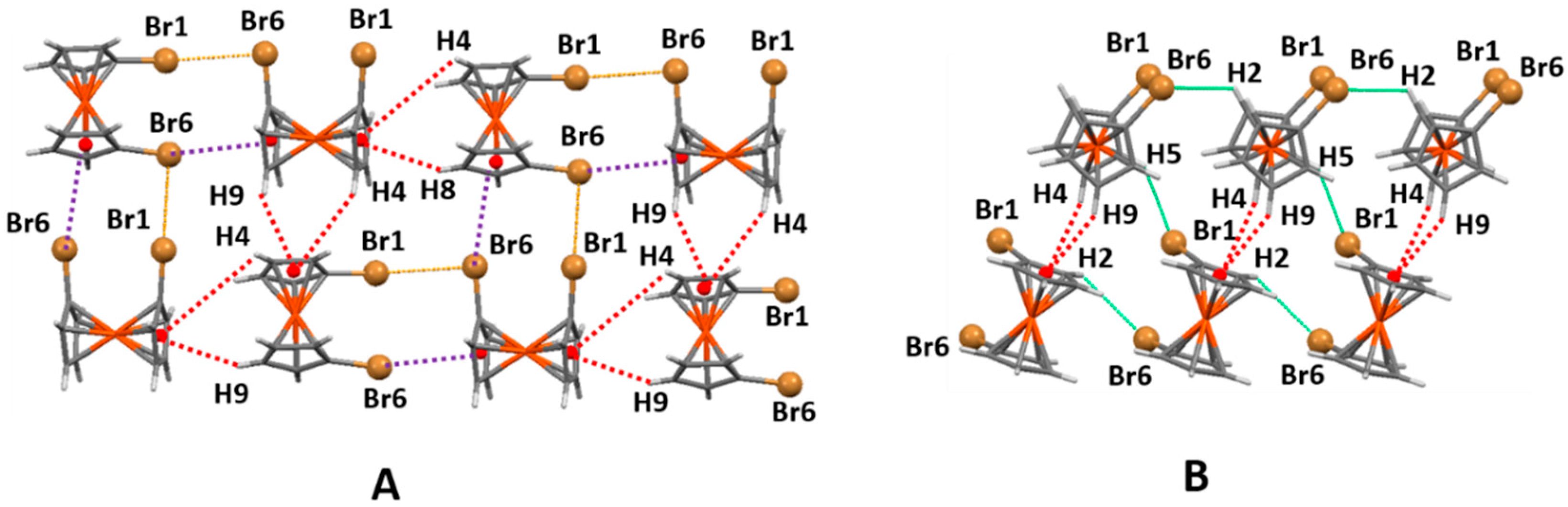
2.2. Analysis of Interactions Recurring to Hirshfeld Surfaces
2.3. Supramolecular Arrangements
2.4. 1,1′-Difluoroferrocene and 1,1′-Dichloroferrocene
2.5. 1,1′-Dibromoferrocene
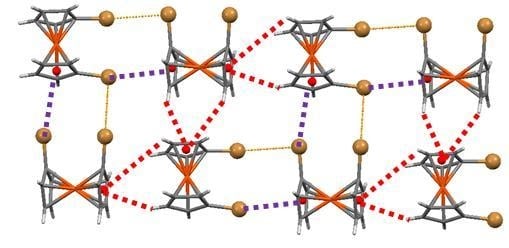
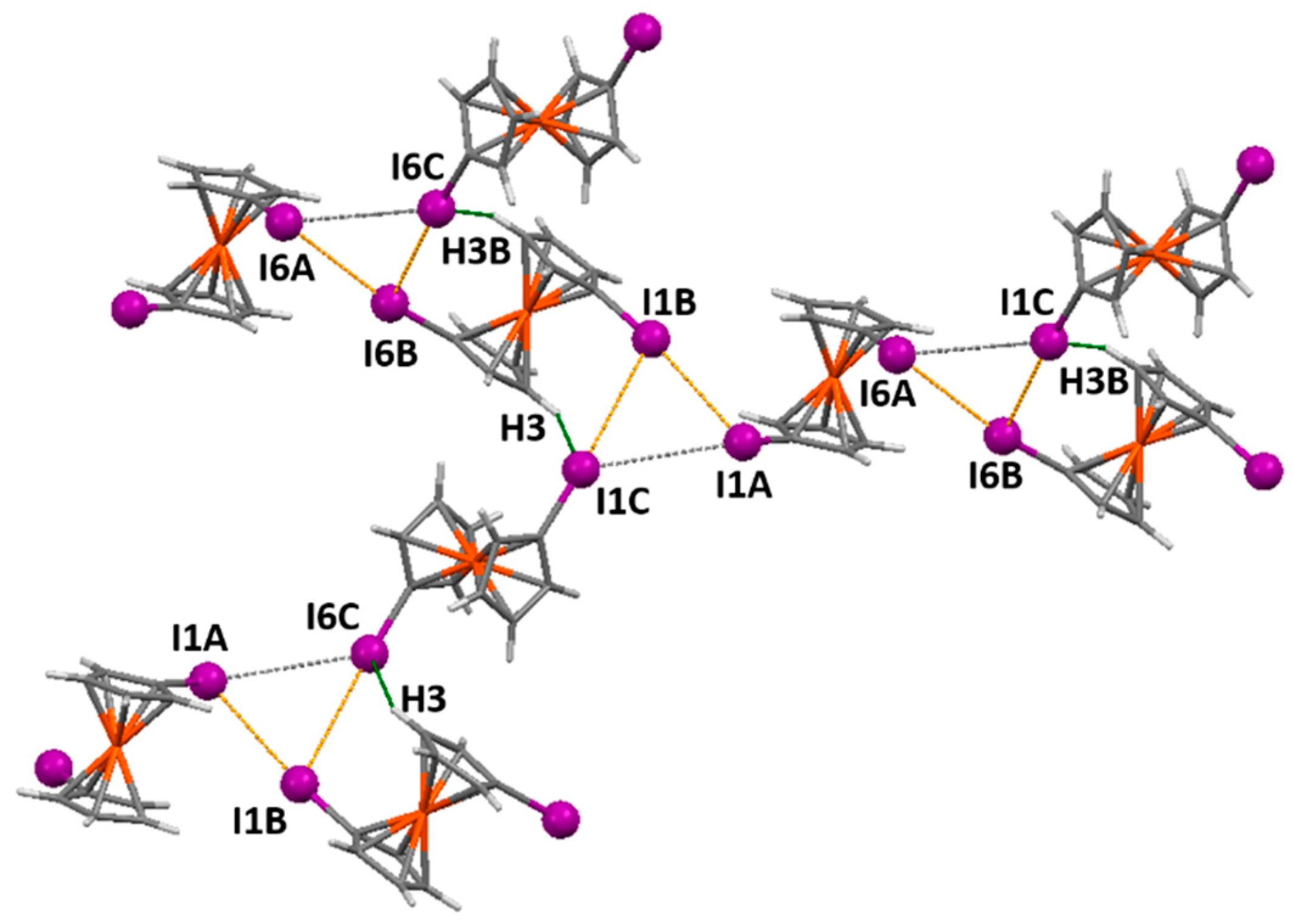
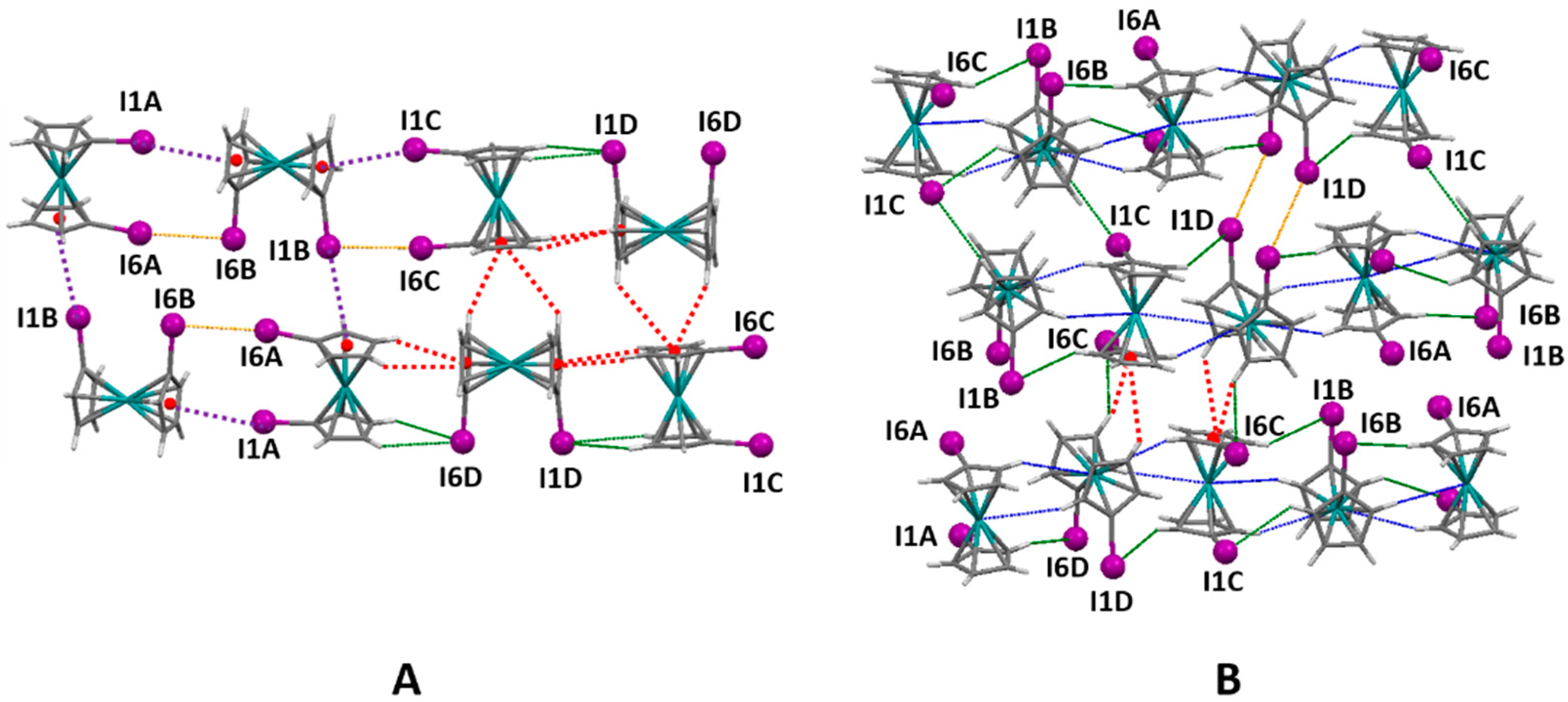
2.6. 1,1′-Diiodoferrocene
2.7. 1,1′-Diiodoruthenocene
3. Discussion
4. Methods
4.1. X-ray Crystallographic Analysis
4.2. Ab Initio Calculations
4.3. Hirshfeld Surface Calculations
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jeffrey, G.A. An Introduction to Hydrogen Bonding; Oxford University Press: Oxford, UK, 1997; pp. 1–6. ISBN 0195095499. [Google Scholar]
- Keesom, W.H. Van der Waals attractive force. Physik. Z. 1921, 22, 129–141. [Google Scholar]
- Debye, P. Van der Waals’ cohesion forces. Physik. Z. 1920, 21, 178–187. [Google Scholar]
- London, F. The general theory of molecular forces. Trans. Faraday Soc. 1937, 33, 8b. [Google Scholar] [CrossRef]
- Latimer, W.M.; Rodebush, W.H. Polarity and Ionization from the standpoint of the Lewis Theory of valence. J. Am. Chem. Soc. 1920, 42, 1419–1433. [Google Scholar] [CrossRef]
- Huggins, M.L. Electronic Structures of Atoms. J. Phys. Chem. 1922, 26, 601–625. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the chemical bond. II. The one-electron bond and the three-electron bond. J. Am. Chem. Soc. 1931, 53, 1367–1400. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond and the Structure of Molecules and Crystals: An Introduction to Modern Structural Chemistry, 3rd ed.; Cornell University Press: Ithaca, NY, USA, 1939; p. 449. [Google Scholar]
- Pimentel, G.C.; McClellan, A.L. The Hydrogen Bond; W.H. Freeman: San Francisco, SF, USA, 1960; p. 3. [Google Scholar]
- Steiner, T.; Saenger, W. Role of C-H⋯O hydrogen bonds in the coordination of water molecules. Analysis of neutron diffraction data. J. Am. Chem. Soc. 1993, 115, 4540–4547. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond in Structural Chemistry and Biology; IUCR, Oxford University Press: Oxford, UK, 1999; pp. 1–19. ISBN 0198509707. [Google Scholar]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Defining the hydrogen bond: An account (IUPAC Technical Report). Pure Appl. Chem. 2011, 83, 1619–1636. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the hydrogen bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Borissova, A.O.; Antipin, M.Y.; Perekalin, D.S.; Lyssenko, K.A. Crucial role of Ru⋯H interactions in the crystal packing of ruthenocene and its derivatives. CrystEngComm 2008, 10, 827–832. [Google Scholar] [CrossRef]
- Wilkinson, G.; Birmingham, J.M. Bis-cyclopentadienyl Compounds of Ti, Zr, V, Nb and Ta. J. Am. Chem. Soc. 1954, 76, 4281–4284. [Google Scholar] [CrossRef]
- Ferreira da Silva, J.L.; Shimizu, K.; Duarte, M.T. The role of halogen interactions in the crystal structure of biscyclopentadienyl dihalides. CrystEngComm 2017, 19, 2802. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Parthasarathy, R. The nature of halogen⋯halogen interactions: Are short halogen contacts due to specific attractive forces or due to close packing of nonspherical atoms? J. Am. Chem. Soc. 1989, 111, 8725–8726. [Google Scholar] [CrossRef]
- Metrangolo, P.; Neukirch, H.; Pilati, T.; Resnatti, G. Halogen Bonding Based Recognition Processes: A World Parallel to Hydrogen Bonding. Acc. Chem. Res. 2005, 38, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Panikkattu, S.; Chopade, P.D.; Desper, J. Competing hydrogen-bond and halogen-bond donors in crystal engineering. CrystEngComm 2013, 15, 3125–3136. [Google Scholar] [CrossRef]
- Dunitz, J.D.; Taylor, R. Organic Fluorine Hardly Ever Accepts Hydrogen Bonds. Chem. Eur. J. 1997, 3, 89–98. [Google Scholar] [CrossRef]
- Rathore, R.S.; Karthikeyan, N.S.; Alekhya, Y.; Sathiyanarayanan, K.; Aravindan, F.G. The role of weak intermolecular C-H⋯F interactions in supramolecular assembly: Structural investigations on 3,5-dibenzylidene-piperidin-4-one and database analysis. J. Chem. Sci. 2011, 123, 403–409. [Google Scholar] [CrossRef]
- Desiraju, G.R.; Steiner, T. The Weak Hydrogen Bond: In Structural Chemistry and Biology; IUCR, Oxford University Press: Oxford, UK, 1999; pp. 202–224. ISBN 0198509707. [Google Scholar]
- Sakurai, T.; Sundaralingam, M.; Jeffrey, G.A. A nuclear quadrupole resonance and X-ray study of the crystal structure of 2,5-di-chloroaniline. Acta Crystallogr. 1963, 16, 354–363. [Google Scholar] [CrossRef]
- Zefirov, Y.V. Characteristic features of various intermolecular contacts x⋯x in crystals. J. Struct. Chem. 1981, 22, 307–309. [Google Scholar] [CrossRef]
- Zefirov, Y.V.; Porai-Koshits, M.A. Geometry of halogen-halogen specific interactions in organic crystals. J. Struct. Chem. 1986, 27, 239–244. [Google Scholar] [CrossRef]
- Bui, T.T.T.; Dahaoui, S.; Lecomte, C.; Desiraju, G.R.; Espinosa, E. The Nature of Halogen⋯Halogen Interactions: A Model Derived from Experimental Charge-Density Analysis. Angew. Chem. 2009, 121, 3896–3899. [Google Scholar] [CrossRef]
- Saha, B.K.; Nangia, A.; Nicoud, J. Using Halogen⋯Halogen Interactions to Direct Noncentrosymmetric Crystal Packing in Dipolar Organic Molecules. Cryst. Growth Des. 2006, 6, 1278–1280. [Google Scholar] [CrossRef]
- Tothadi, S.; Joseph, S.; Desiraju, G.R. Synthon Modularity in Cocrystals of 4-Bromobenzamide with n-Alkanedicarboxylic Acids: Type I and Type II Halogen⋯Halogen Interactions. Cryst. Growth Des. 2013, 13, 3242–3254. [Google Scholar] [CrossRef]
- Mukherjee, A.; Desiraju, G.R. Halogen bonds in some dihalogenated phenols: Applications to crystal engineering. IUCrJ 2014, 1, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Ho, P.S.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Metrangolo, P.; Resnati, G. Type II halogen···halogen contacts are halogen bonds. IUCrJ 2014, 1, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen Bonding: A Paradigm in Supramolecular Chemistry. Chem. Eur. J. 2001, 7, 2511–2519. [Google Scholar] [CrossRef]
- Clark, T.; Henneman, M.; Murray, J.S.; Politzer, P. Halogen bonding: The sigma-hole. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.; Resnati, G.; Metrangolo, P.; Weber, E.; Hulliger, J. Organic fluorine compounds: A great opportunity for enhanced materials properties. Chem. Soc. Rev. 2011, 40, 3496–3508. [Google Scholar] [CrossRef] [PubMed]
- Marushima, Y.; Uchiumi, Y.; Ogu, K.; Hori, A. Intermolecular π-stacking and F⋯F interactions of fluorine-substituted meso-alkynylporphyrin. Acta Crystallogr. Sect. C 2010, 66, o406–o409. [Google Scholar] [CrossRef] [PubMed]
- Brinck, T.; Murray, J.S.; Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quantum Chem. Quantum Biol. Symp. 1992, 44, 57–64. [Google Scholar] [CrossRef]
- Wilcken, R.; Zimmermann, M.O.; Lange, A.; Joerger, A.C.; Boeckler, F.M. Principles and Applications of Halogen Bonding in Medicinal Chemistry and Chemical Biology. J. Med. Chem. 2013, 56, 1363–1388. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Resnati, G. Halogen Bonding: Fundamentals and Applications. Struct. Bond. 2008, 126, 17. [Google Scholar]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Politzer, P. A predicted new type of directional noncovalent interaction. Int. J. Quantum Chem. 2007, 107, 3046–3052. [Google Scholar] [CrossRef]
- Murray, J.S.; Clark, T.; Lane, P.; Politzer, P. σ-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the σ-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Metrangolo, P.; Meyer, F.; Pilati, T.; Resnati, G.; Terraneo, G. Halogen bonding in supramolecular chemistry. Angew. Chem. Int. Ed. 2008, 47, 6114–6127. [Google Scholar] [CrossRef] [PubMed]
- Auffinger, P.; Hays, F.A.; Westhof, E.; Ho, P.S. Halogen bonds in biological molecules. Proc. Natl. Acad. Sci. USA 2004, 101, 16789–16794. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen bonding in metal–organic–supramolecular networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Pennington, W.T.; Resnati, G.; Taylor, M.S. Halogen bonding: From self-assembly to materials and biomolecules. CrystEngComm 2013, 15, 3057. [Google Scholar] [CrossRef]
- Metrangolo, P.; Pilati, T.; Resnati, G. Halogen bonding and other noncovalent interactions involving halogens: A terminology issue. CrystEngComm 2006, 8, 946–947. [Google Scholar] [CrossRef]
- Dumele, O.; Trapp, N.; Diederich, F. Halogen Bonding Molecular Capsules. Angew. Chem. Int. Ed. 2015, 54, 12339–12344. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zang, S.-Q.; Wang, L.-Y.; Mak, T.C.W. Halogen bonding: A. powerful, emerging tool for constructing high-dimensional metal-containing supramolecular networks. Coord. Chem. Rev. 2016, 308, 1–21. [Google Scholar] [CrossRef]
- Imai, Y.N.; Inoue, Y.; Yamamoto, Y. Propensities of Polar and Aromatic Amino Acids in Noncanonical Interactions: Nonbonded Contacts Analysis of Protein−Ligand Complexes in Crystal Structures. J. Med. Chem. 2007, 50, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Matter, H.; Nazare, M.; Gussregen, S.; Will, D.; Schreuder, H.; Bauer, A.; Urmann, M.; Ritter, K.; Wagner, M.; Wehner, V. Evidence for C-Cl/C-Br⋯π Interactions as an Important Contribution to Protein–Ligand Binding Affinity. Angew. Chem. Int. Ed. 2009, 48, 2911–2916. [Google Scholar] [CrossRef] [PubMed]
- Girão, E.C.; Liebold-Ribeiro, Y.; Batista, J.A.; Barros, E.B.; Fagan, S.B.; Mendes Filho, J.; Dresselhaus, M.S.; Souza Filho, A.G. Functionalization of single-wall carbon nanotubes through chloroform adsorption: Theory and experiment. Phys. Chem. Chem. Phys. 2010, 12, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Brown, P.A.; Lu, J.; Shuford, K.L. Electronic Properties of Halogen-Adsorbed Graphene. J. Phys. Chem. C 2015, 119, 17271–17277. [Google Scholar] [CrossRef]
- Riley, K.E.; Hobza, P. Investigations into the Nature of Halogen Bonding Including Symmetry Adapted Perturbation Theory Analyses. J. Chem. Theory Comput. 2008, 4, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Wallnoefer, H.; Fox, T.; Liedl, K.; Tautermann, C.S. Dispersion dominated halogen–π interactions: Energies and locations of mínima. Phys. Chem. Chem. Phys. 2010, 12, 14941–14949. [Google Scholar] [CrossRef] [PubMed]
- Forni, A.; Pieraccini, S.; Rendine, S.; Gabas, F.; Sironi, M. Halogen-Bonding Interactions with π Systems: CCSD(T), MP2, and DFT Calculations. ChemPhysChem 2012, 13, 4224–4234. [Google Scholar] [CrossRef] [PubMed]
- Forni, A.; Pieraccini, S.; Rendine, S.; Sironi, M. Halogen bonds with benzene: An assessement of DFT functionals. J. Comput. Chem. 2014, 35, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Youn, I.S.; Kim, D.Y.; Cho, W.J.; Madridejos, J.M.L.; Lee, H.M.; Kolaski, M.; Lee, J.; Baig, C.; Shin, S.K.; Filatov, M.; et al. Halogen-π Interactions between Benzene and X2/CX4 (X = Cl, Br): Assessment of Various Density Functionals with Respect to CCSD(T). J. Phys. Chem. A 2016, 120, 9305–9314. [Google Scholar] [CrossRef] [PubMed]
- Kodama, Y.; Nishihata, K.; Nishio, M.; Nakagawa, N. Attractive interaction between aliphatic and aromatic systems. Tetrahedron Lett. 1977, 2105–2108. [Google Scholar] [CrossRef]
- Tamres, M. Aromatic Compounds as Donor Molecules in Hydrogen Bonding. J. Am. Chem. Soc. 1952, 74, 3375–3378. [Google Scholar] [CrossRef]
- Nishio, M.; Umezawa, Y.; Honda, K.; Tsuboyama, S.; Suezawa, H. CH/π hydrogen bonds in organic and organometallic chemistry. CrystEngComm 2009, 11, 1757–1788. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Tedesco, E. X-H⋯π (X = O, N, C) Hydrogen Bonds in Organometallic Crystals. Organometallics 1998, 17, 2669–2672. [Google Scholar] [CrossRef]
- Sakaki, S.; Kato, K.; Miyazaki, T.; Musashi, Y.; Ohkubo, K.; Ihara, H.; Hirayama, C. Structures and binding energies of benzene—Methane and benzene—Benzene complexes. An ab initio SCF/MP2 study. J. Chem. Soc. Faraday Trans. 1993, 89, 659–664. [Google Scholar] [CrossRef]
- Akazome, M.; Hirabayashi, A.; Senda, K.; Ogura, K. Inclusion compounds of l,d-dipeptide with small sulfoxides: Flexible sheet structure of (S)-phenylglycyl-(R)-phenylglycine. Tetrahedron 2007, 63, 9933–9938. [Google Scholar] [CrossRef]
- Anderson, C.D.; Dudding, T.; Gordillo, R.; Houk, K.N. Origin of Enantioselection in Hetero-Diels−Alder Reactions Catalyzed by Naphthyl-TADDOL. Org. Lett. 2008, 10, 2749–2752. [Google Scholar] [CrossRef] [PubMed]
- Harigai, M.; Kataoka, M.; Imamoto, Y. A Single CH/π Weak Hydrogen Bond Governs Stability and the Photocycle of the Photoactive Yellow Protein. J. Am. Chem. Soc. 2006, 128, 10646–10647. [Google Scholar] [CrossRef] [PubMed]
- Nishio, M. The CH/π hydrogen bond in chemistry. Conformation, supramolecules, optical resolution and interactions involving carbohydrates. Phys. Chem. Chem. Phys. 2011, 13, 13873–13900. [Google Scholar] [CrossRef] [PubMed]
- Ferreira da Silva, J.L.; Harjivan, S.G.; Ferreira, A.P.; Shimizu, K.; Marques, M.M.; Duarte, M.T. Effect of substituents in the molecular and supramolecular architectures of 1-ferrocenyl-2-(aryl)thioethanones. CrystEngComm 2015, 17, 3089. [Google Scholar] [CrossRef]
- Choudhury, R.R.; Chitra, R. Stacking interaction between homostacks of simple aromatics and the factors influencing these interactions. CrystEngComm 2010, 12, 2113–2121. [Google Scholar] [CrossRef]
- Mignon, P.; Loverix, S.; Geerlings, P. Interplay between π-π interactions and the H-bonding ability of aromatic nitrogen bases. Chem. Phys. Lett. 2005, 401, 40–46. [Google Scholar] [CrossRef]
- Trifan, D.S.; Bacskai, R. Metal/hydrogen bonding in metallocene conompounds. J. Am. Chem. Soc. 1960, 82, 5010. [Google Scholar] [CrossRef]
- Roe, D.M.; Bailey, P.M.; Mosely, K.; Maitlis, P.M. Structure of bromobis(triphenylphosphine)-(1,2,3,4-tetrakismethoxycarbonylbuta-1,3-dienyl)palladium and evidence for a C–H⋯Pd interaction. J. Chem. Soc. Chem. Commun. 1972, 1273. [Google Scholar] [CrossRef]
- Cerichelli, G.; Illuminati, G.; Ortaggi, G.; Giuliani, A. The behaviour of ferrocene and ruthenocene in weakly to strongly protic media. Implications on the mechanism of substitutions involving proton as the electrophile. J. Organomet. Chem. 1977, 127, 357. [Google Scholar] [CrossRef]
- Drago, R.S.; Norazi, M.S.; Klinger, R.J.; Chamberlain, C. Quantitative data on some oxidative addition reactions and on the Lewis basicity of bis(triphenylphosphine)carbonylchloroiridium(I) Ir(I)[(C6H5)3P]2(CO)Cl. Inorg. Chem. 1979, 18, 1254. [Google Scholar] [CrossRef]
- Yoshida, T.; Tani, K.; Yamagata, T.; Tatsuno, Y.; Saito, T. Preparation and structure of [Rh{(η5-C5H4(2-C5H4N))(η5-C5H4PPh2)}(cod)]PF6 and [Ir(H){Fe[η5-C5H3(2-C5H4N)](η5-C5H4PPh2)}(cod)]PF6; a RhI complex having a C–H⋯RhI interaction and a hydrido IrIII complex (where cod = cyclo-octa-1,5-diene). J. Chem. Soc. Chem. Comm. 1990, 292–294. [Google Scholar] [CrossRef]
- Brammer, L. Metals and hydrogen bonds. DaltonTrans. 2003, 16, 3145–3157. [Google Scholar] [CrossRef]
- Shubina, E.S.; Epstein, L.M. Regularities in formation of intramolecular hydrogen bonds with the metal atom Part I. α-Metallocenylcarbinols of the iron subgroup. J. Mol. Struct. 1992, 265, 367–384. [Google Scholar] [CrossRef]
- Shubina, E.S.; Krylov, A.N.; Kreindlin, A.Z.; Rybinskaya, M.I.; Epstein, L. Intermolecular hydrogen bonds with d-electrons of transition metal atoms. H-complexes with metallocenes of the iron subgroup. J. Mol. Struct. 1993, 301, 1–5. [Google Scholar] [CrossRef]
- Epstein, L.M.; Krylov, A.N.; Shubina, E.S. Novel types of hydrogen bonds involving transition metal atoms and proton transfer (XH⋯M, [MH]+⋯B, [MH]+⋯A−). J. Mol. Struct. 1994, 322, 345–352. [Google Scholar] [CrossRef]
- Bruno, G.; Lanza, S.; Nicolò, F. Structure of [Pt(C6H5)2(btz-N,N′)].CHCl3, btz = 2,2′-bi-5,6-dihydro-4H-1,3-thiazine. Acta Crystallogr. Sect. C 1990, 46, 765–767. [Google Scholar] [CrossRef]
- Brammer, L.; Charnock, J.M.; Goggin, P.L.; Goodfellow, R.J.; Orpen, A.G.; Koetzle, T. The role of transition metal atoms as hydrogen bond acceptors: A neutron diffraction study of [NPrn4]2[PtCl4]·cis-[PtCl2(NH2Me)2] at 20 K. J. Chem. Soc. Dalton Trans. 1991, 7, 1789–1798. [Google Scholar] [CrossRef]
- Braga, D.; Grepioni, F.; Tedesco, E. Hydrogen Bonding in Organometallic Crystals. 6. X−H⋯M Hydrogen Bonds and M⋯(H−X) Pseudo-Agostic Bonds. Organometallics 1997, 16, 1846–1856. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. Sect. B 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Inkpen, M.S.; Du, S.; Hildebrand, M.; White, A.J.P.; Harrison, N.M.; Albrecht, T.; Long, N.J. The Unusual Redox Properties of Fluoroferrocenes Revealed through a Comprehensive Study of the Haloferrocenes. Organometallics 2015, 34, 5461–5469. [Google Scholar] [CrossRef]
- Hnetinka, C.A.; Hunter, A.D.; Zeller, M.; Lesley, M.J.G. 1,1′-Di-bromo-ferrocene. Acta Crystallogr. Sect. E 2004, 60, m1806. [Google Scholar] [CrossRef]
- Roemer, M.; Nijhuis, C.A. Syntheses and purification of the versatile synthons iodoferrocene and 1,1′-diiodoferrocene. Dalton Trans. 2014, 43, 11815–11818. [Google Scholar] [CrossRef] [PubMed]
- Hess, J.; Konatschnig, S.; Morard, S.; Pierroz, V.; Ferrari, S.; Spingler, B.; Gasser, G. Novel, Mercury-Free Synthetic Pathway for Trifluoromethylthio-Substituted Metallocenes. Inorg. Chem. 2014, 53, 3662–3667. [Google Scholar] [CrossRef] [PubMed]
- Dance, I. Distance criteria for crystal packing analysis of supramolecular motifs. New J. Chem. 2003, 27, 22–27. [Google Scholar] [CrossRef]
- Glaser, R.; Murphy, R.F.; Sui, Y.; Barnes, C.L.; Kim, S.H. Multifurcated halogen bonding involving Ph–Cl⋯H-CPh=N-R′ interactions and its relation to idioteloamphiphile layer architecture. CrystEngComm 2006, 8, 372–376. [Google Scholar] [CrossRef]
- Anthony, A.; Desiraju, G.R.; Jetti, R.K.R.; Kuduva, S.S.; Madhavi, N.N.L.; Nangia, A.; Thaimattam, R.; Thalladi, V.R. Crystal Engineering: Some Further Strategies. Cryst. Eng. 1998, 1, 1–18. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03; revision C.05; Gaussian Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648. [Google Scholar] [CrossRef]
- Wedig, U.; Dolg, M.; Stoll, H.; Preuss, H. Energy-Adjusted Pseudopotentials for Transition-Metal Elements. In Quantum Chemistry: The Challenge of Transition Metals and Coordination Chemistry; Veillard, A., Ed.; Springer: Dordrecht, The Netherlands, 1986; Volume 176, pp. 79–89. ISBN 978-90-277-2237-9. [Google Scholar]
- Petersson, G.A.; Bennett, A.; Tensfeldt, T.G.; Al-Laham, M.A.; Shirley, W.A.; Mantzaris, J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row atoms. J. Chem. Phys. 1988, 89, 2193–2218. [Google Scholar] [CrossRef]
- Petersson, G.A.; Al-Laham, M.A. A complete basis set model chemistry. II. Open-shell systems and the total energies of the first-row atoms. J. Chem. Phys. 1991, 94, 6081–6090. [Google Scholar] [CrossRef]
- Breneman, C.M.; Wiberg, K.B. Determining atom-centered monopoles from molecular electrostatic potentials. The need for high sampling density in formamide conformational analysis. J. Comput. Chem. 1990, 11, 361–373. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer17; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds not are available from the authors. |


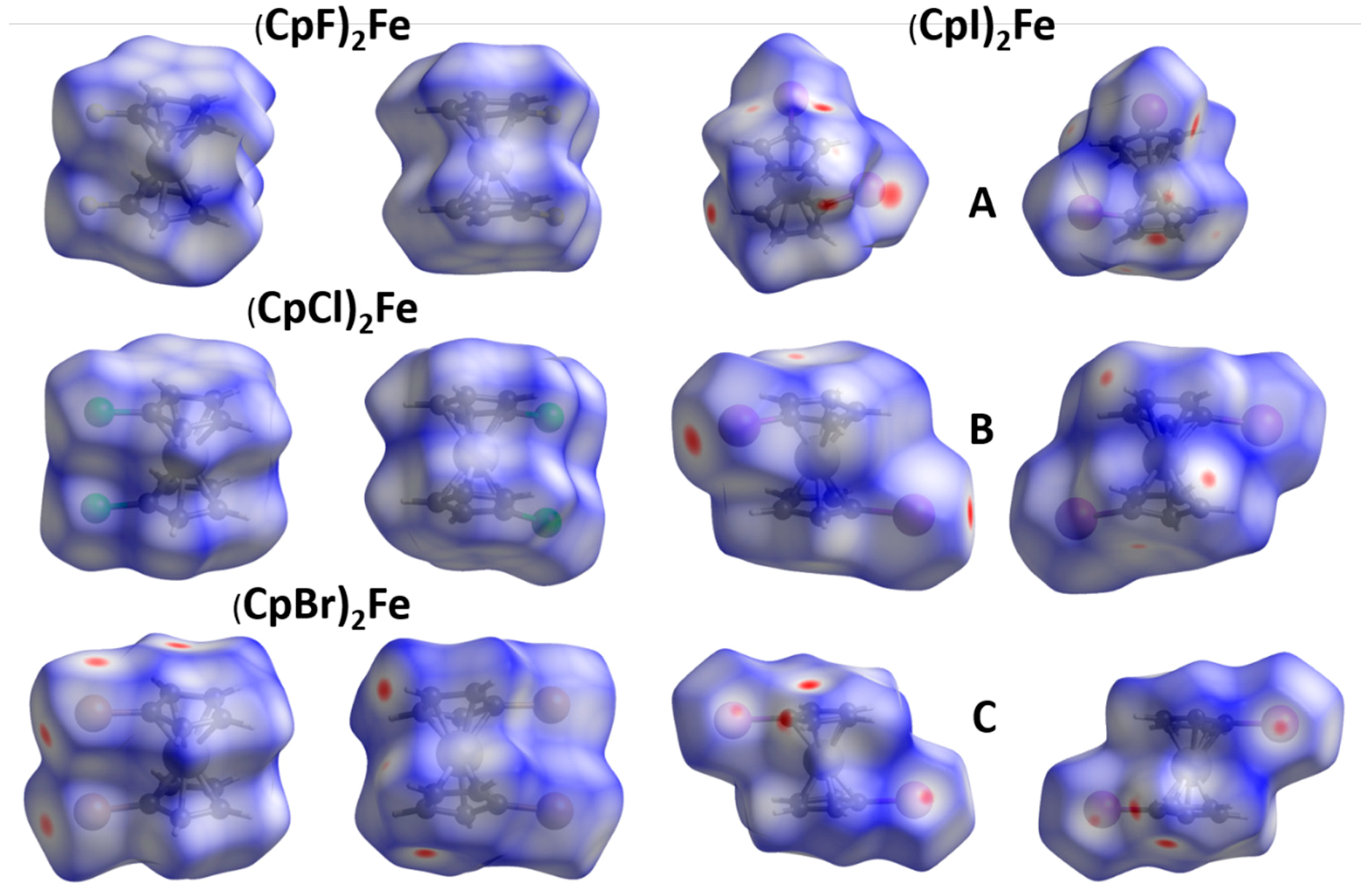
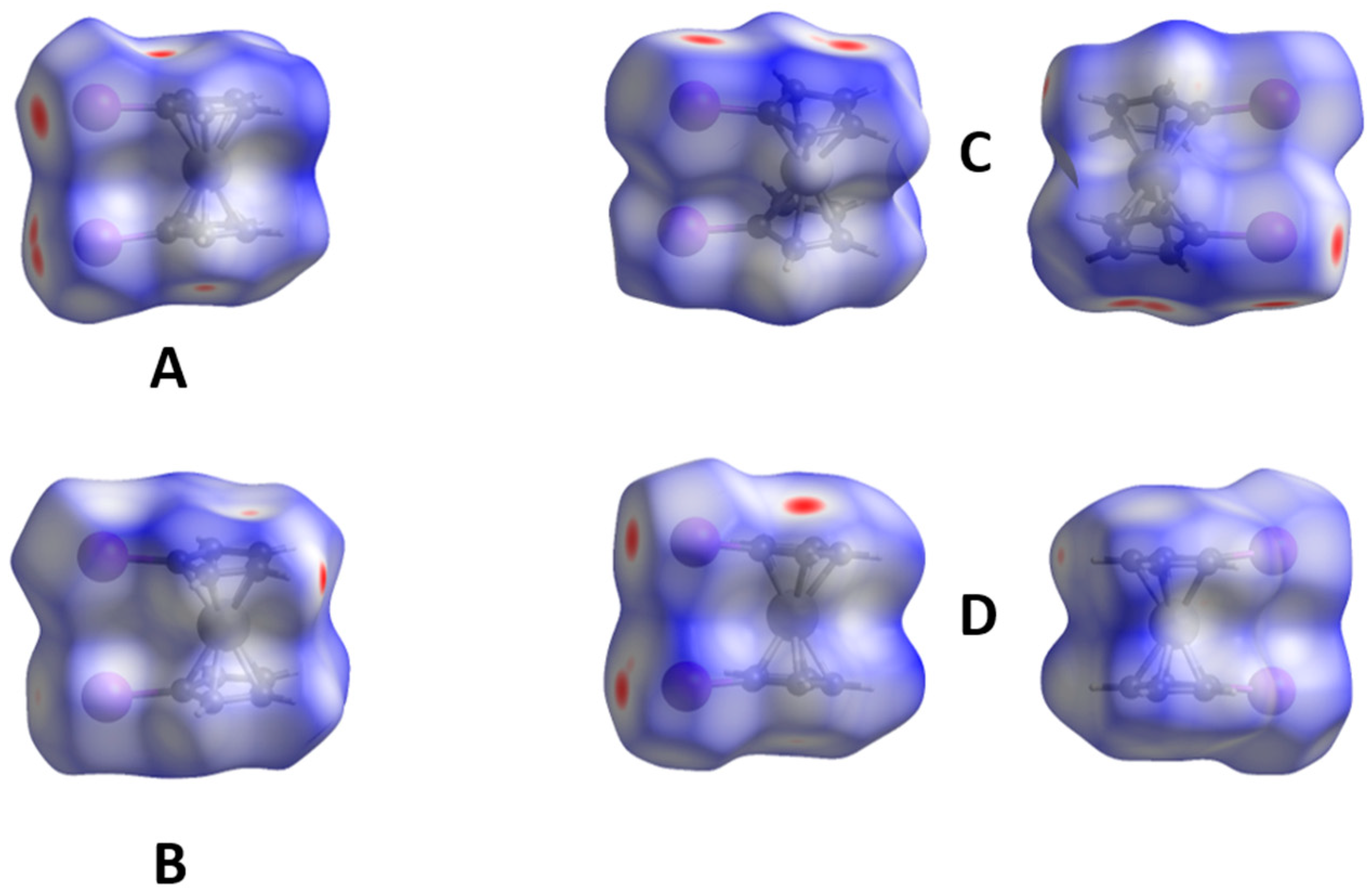
| Compound | Ref.-Year of Publication | CSD Refcode | Space Group | Z | Z′ | Temp. (K) | R-Factor (%) | |
|---|---|---|---|---|---|---|---|---|
| (CpF)2Fe | [85]-2015 | RACROF | Monoclinic | P21/n | 4 | 1 | 173 | 2.96 |
| (CpCl)2Fe | [84]-1986 | DUTSUH | Monoclinic | C2/c | 4 | 0 a | 295 | 4.60 a |
| (CpCl)2Fe | [85]-2015 | DUTSUH01 | Monoclinic | C2/c | 4 | 1 | 173 | 2.00 |
| (CpBr)2Fe | [86]-2004 | BIPDOU | Monoclinic | P21 | 2 | 1 | 100 | 3.67 |
| (CpI)2Fe | [87]-2014 | KOPFAY | Monoclinic | C2/c | 12 | 3 | 100 | 2.20 |
| (CpI)2Ru | [88]-2014 | JODWOQ | Triclinic | P-1 | 8 | 4 | 183 | 4.1 |
| Contact | (CpF)2Fe | (CpCl)2Fe | (CpBr)2Fe | (CpI)2Fe (A) | (CpI)2Fe (B) | (CpI)2Fe (C) | (CpI)2Ru (Average) |
|---|---|---|---|---|---|---|---|
| X-X | 1 | 0.6 | 2.3 | 11.3 | 13.7 | 8.9 | 7.2 |
| X-C | 0.2 | 1.9 | 3.7 | 0.8 | 1.1 | 0 | 8.7 |
| X-H | 36.8 | 40.6 | 39.6 | 36 | 33.3 | 29.3 | 33.7 |
| X-M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C-C | 9.1 | 8.5 | 0 | 2.9 | 2.9 | 0 | 0.9 |
| C-H | 3.6 | 3.2 | 17.1 | 25.5 | 8.6 | 13.7 | 13.4 |
| C-M | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-H | 49.3 | 45.2 | 37.3 | 23.5 | 40.4 | 48.1 | 35.8 |
| H-M | 0 | 0 | 0 | 0 | 0 | 0 | 0.2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, K.; Ferreira da Silva, J. Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes. Molecules 2018, 23, 2959. https://doi.org/10.3390/molecules23112959
Shimizu K, Ferreira da Silva J. Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes. Molecules. 2018; 23(11):2959. https://doi.org/10.3390/molecules23112959
Chicago/Turabian StyleShimizu, Karina, and João Ferreira da Silva. 2018. "Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes" Molecules 23, no. 11: 2959. https://doi.org/10.3390/molecules23112959
APA StyleShimizu, K., & Ferreira da Silva, J. (2018). Halogen and Hydrogen Bonding Interplay in the Crystal Packing of Halometallocenes. Molecules, 23(11), 2959. https://doi.org/10.3390/molecules23112959