Novel Transforming Growth Factor-Beta Receptor 1 Antagonists through a Pharmacophore-Based Virtual Screening Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Structure-Based Pharmacophore Model Construction
2.2. Ligand-Based Pharmacophore Model Construction
2.3. Evaluation and Validation of Pharmacophore Models
2.4. Virtual Screening of Databases
3. Results and Discussion
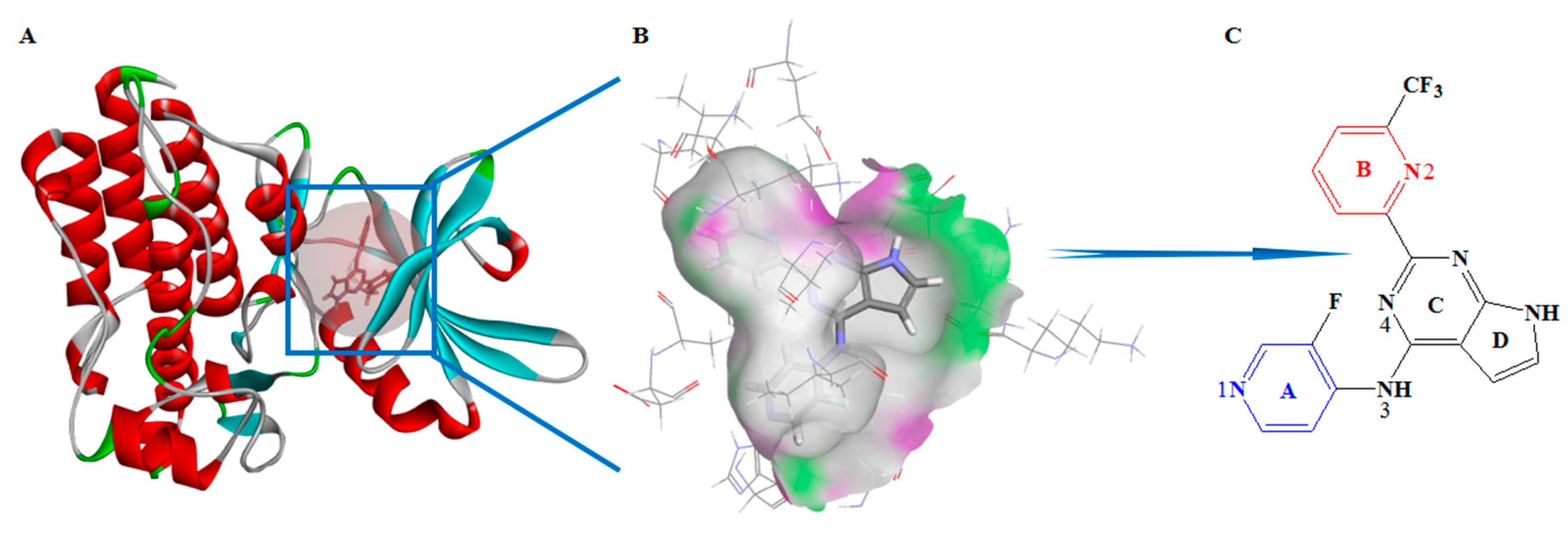
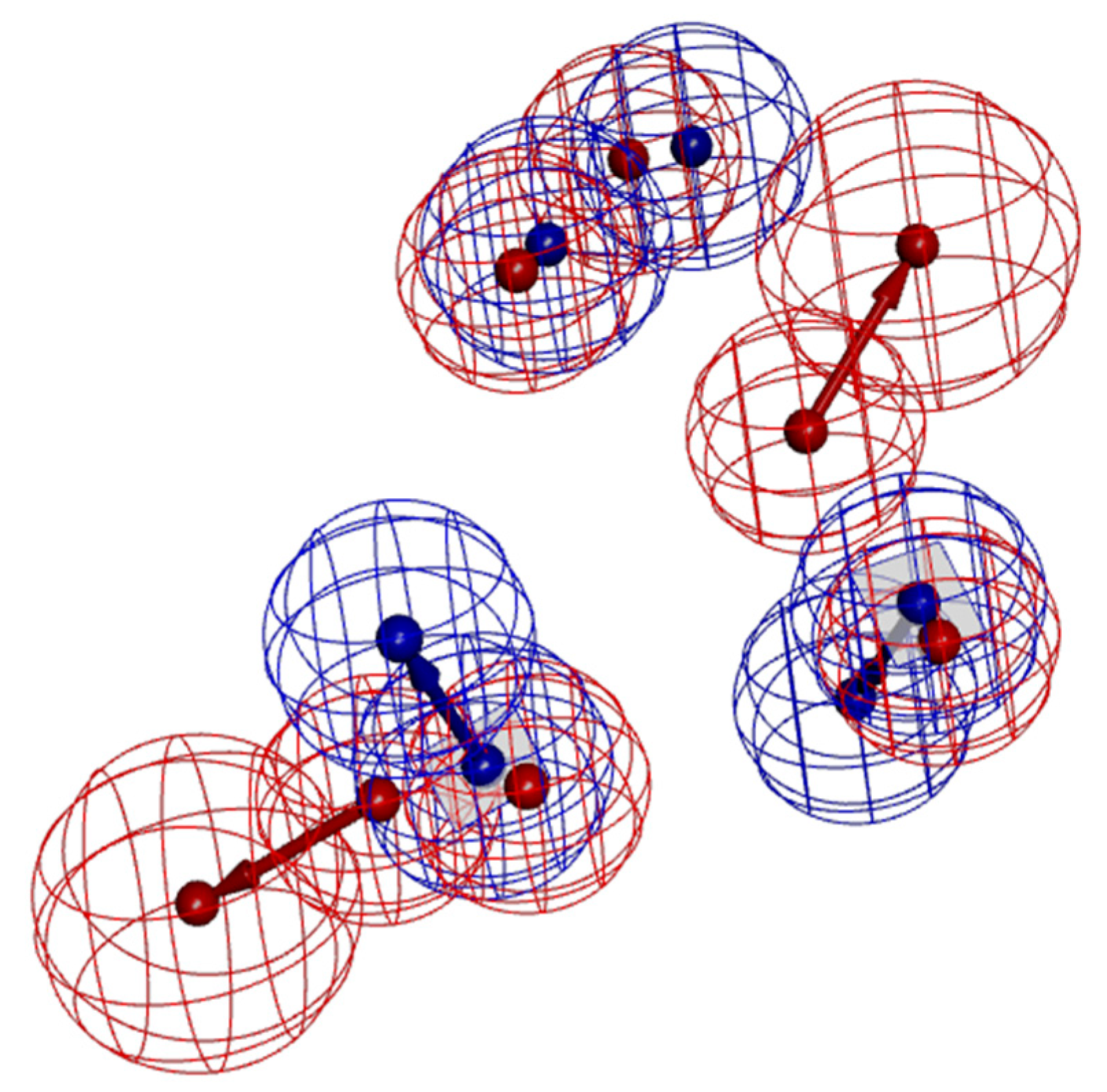
3.1. Characteristics and Reliability Verification of Ligand–Receptor Complex Pharmacophore Model
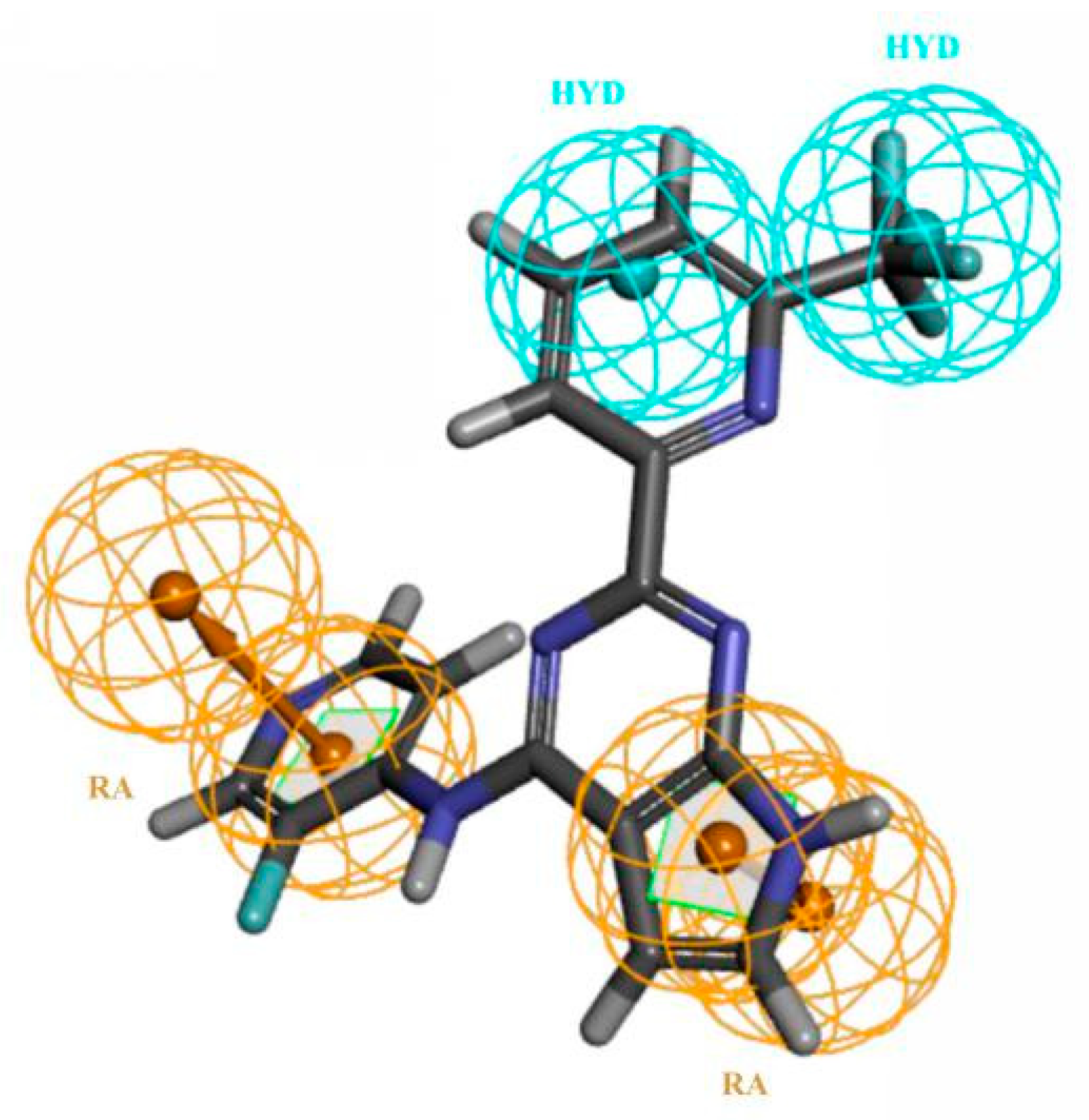
3.2. Characteristics and Reliability Verification of Ligand-Based Pharmacophore Model
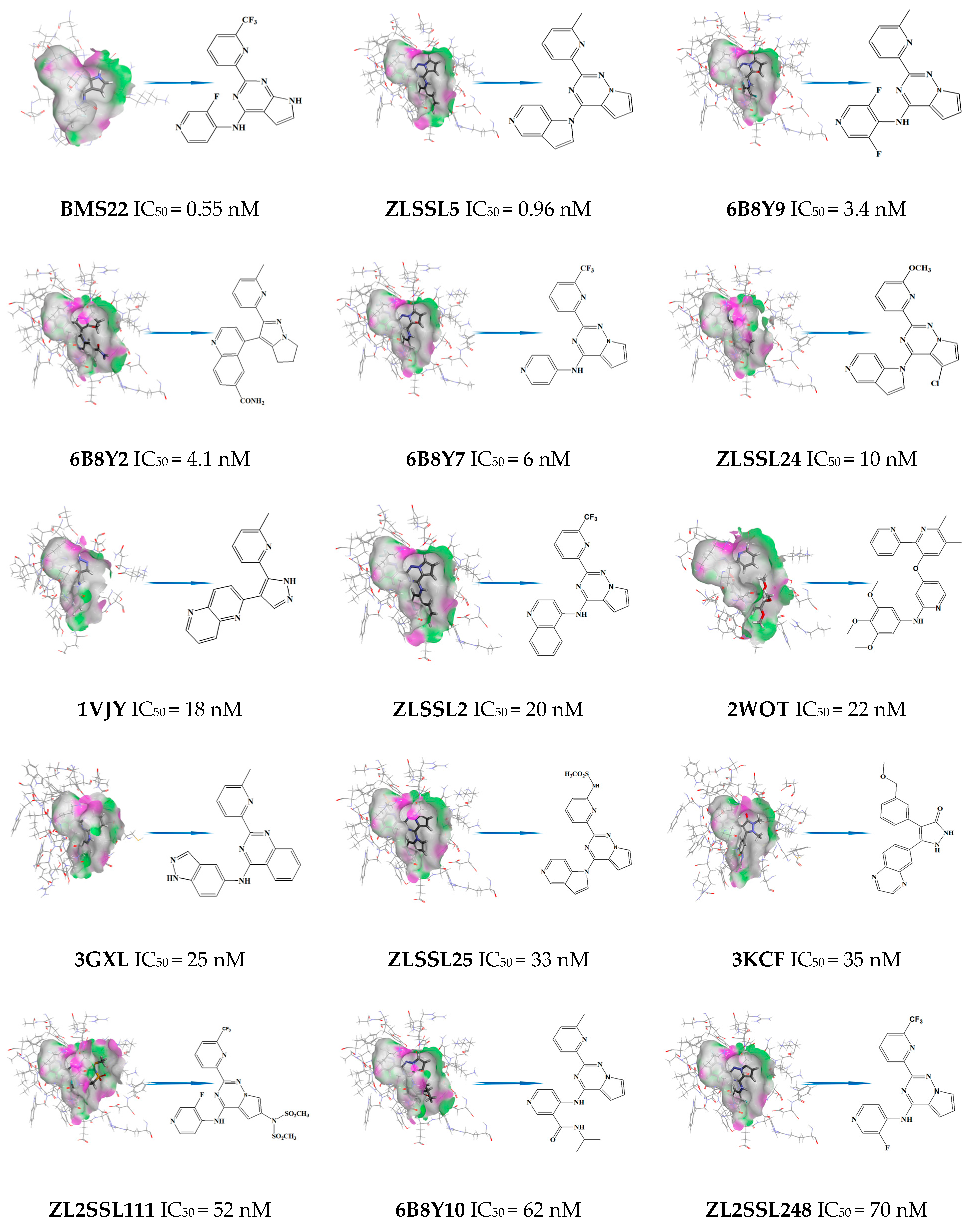
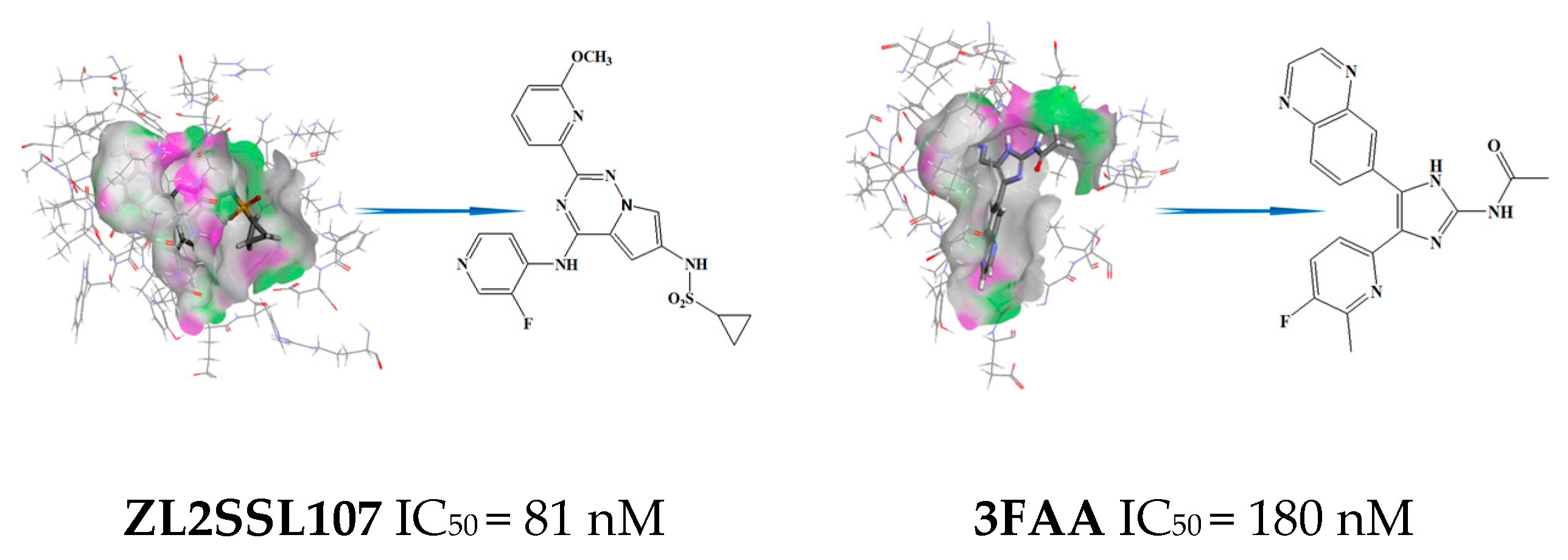
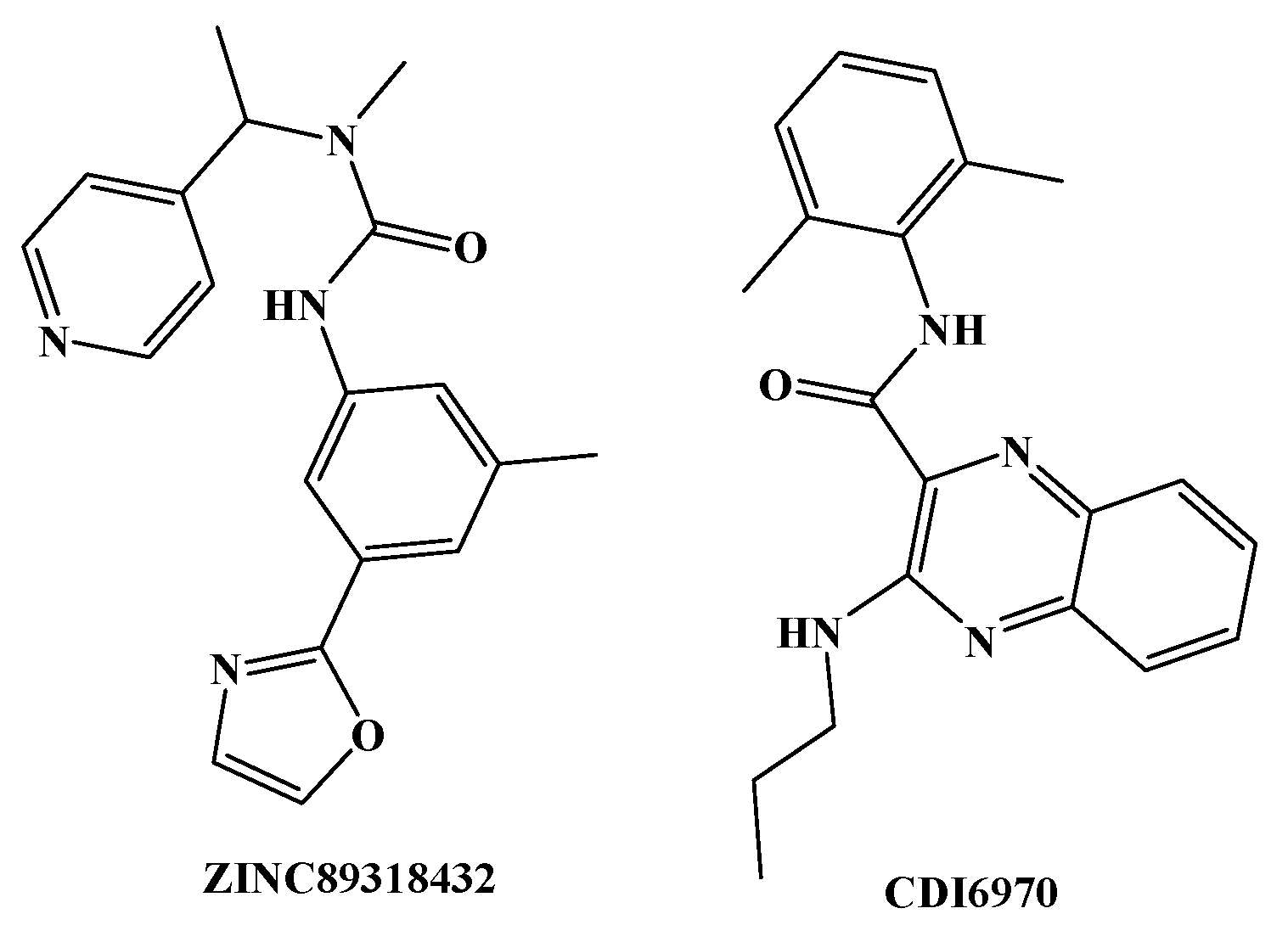
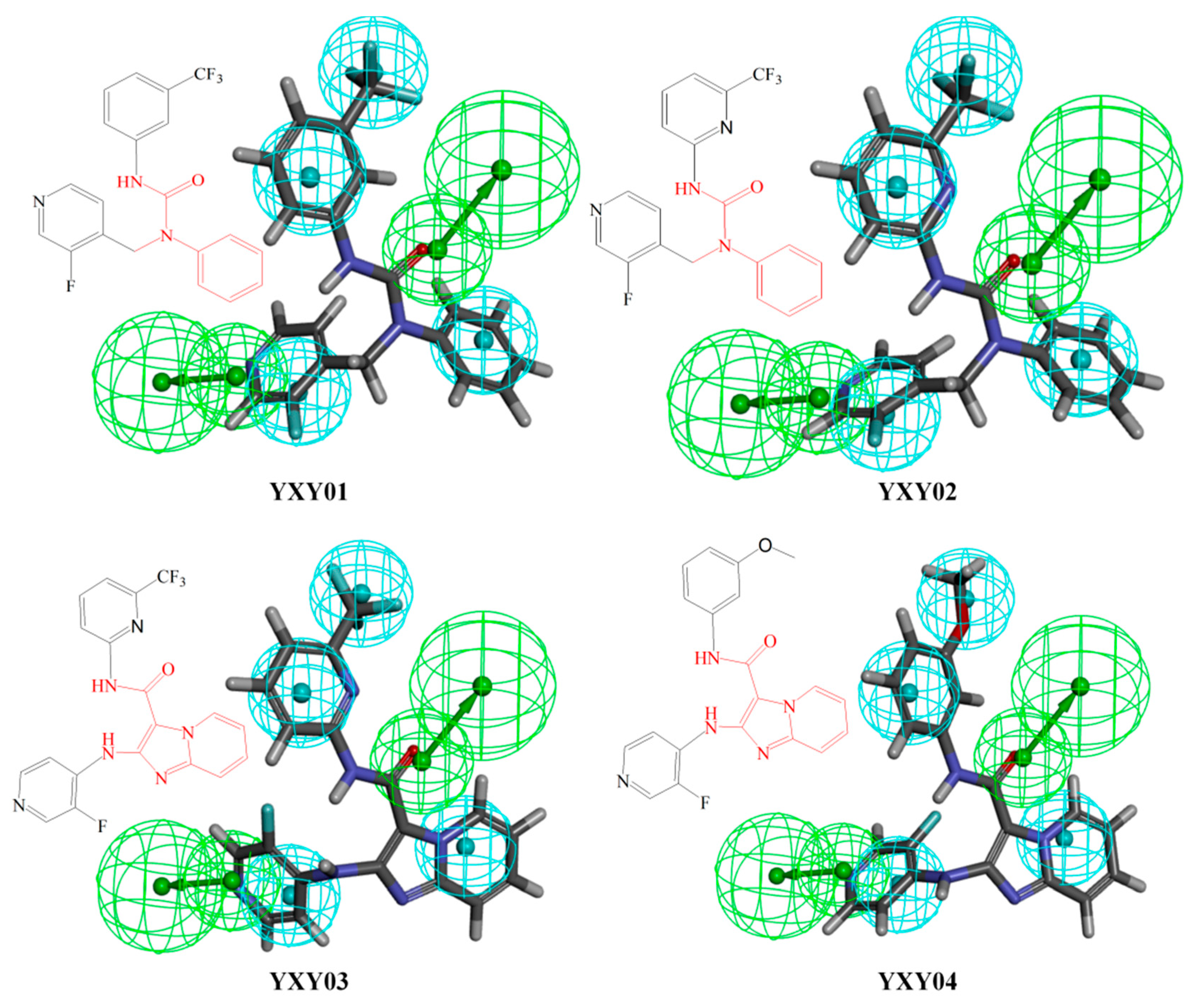
3.3. Virtual Screening and Structural Transformation of Lead Compounds
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papageorgis, P.; Stylianopoulos, T. Role of TGFβ in regulation of the tumor microenvironment and drug delivery. Int. J. Oncol. 2015, 46, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Poniatowski, Ł.A.; Wojdasiewicz, P.; Gasik, R.; Szukiewicz, D. Transforming growth factor beta family: Insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediat. Inflamm. 2015, 2015, 137823. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Canaff, L.; Rajadurai, C.V.; Fils-Aimé, N.; Tian, J.; Dai, M.; Korah, J.; Villatoro, M.; Park, M.; Ali, S.; et al. Breast cancer anti-estrogen resistance 3 inhibits transforming growth factor β/Smad signaling and associates with favorable breast cancer disease outcomes. Breast Cancer Res. 2014, 16, 476. [Google Scholar] [CrossRef] [PubMed]
- Haque, S.; Morris, J.C. Transforming growth factor-β: A therapeutic target for cancer. Hum. Vaccin. Immunother. 2017, 13, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Bellomo, C.; Caja, L.; Moustakas, A. Transforming growth factor β as regulator of cancer stemness and metastasis. Br. J. Cancer 2016, 115, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Gudey, S.K.; Marcusson, A.; Fu, J.Y.; Bergh, A.; Heldin, C.H.; Landström, M. TGFβ-induced invasion of prostate cancer cells is promoted by c-Jun-dependent transcriptional activation of Snail1. Cell Cycle 2014, 13, 2400–2414. [Google Scholar] [CrossRef] [PubMed]
- Yingling, J.M.; McMillen, W.T.; Yan, L.; Huang, H.; Sawyer, J.S.; Graff, J.; Clawson, D.K.; Britt, K.S.; Anderson, B.D.; Beight, D.W.; et al. Preclinical assessment of galunisertib (LY2157299 monohydrate), a first-in-class transforming growth factor-β receptor type I inhibitor. Oncotarget 2017, 9, 6659–6677. [Google Scholar] [CrossRef] [PubMed]
- Bristol-Myers Squibb. Transforming Growth Factor-Beta Receptor Antagonists. China Patent 201680049890.2, 17 April 2018. [Google Scholar]
- Bristol-Myers Squibb. Transforming Growth Factor-Beta Receptor Antagonists. China Patent 201680055202.3, 11 May 2018. [Google Scholar]
- Harikrishnan, L.S.; Warrier, J.; Tebben, A.J.; Tonukunuru, G.; Madduri, S.R.; Baligar, V.; Mannoori, R.; Seshadri, B.; Rahaman, H.; Arunachalam, P.N.; et al. Heterobicyclic inhibitors of transforming growth factor beta receptor I (TGFβRI). Bioorg. Med. Chem. 2018, 26, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Arooj, M.; Sakkiah, S.; Kim, S.; Arulalapperumal, V.; Lee, K.W. A combination of receptor-based pharmacophore modeling & QM techniques for identification of human chymase inhibitors. PLoS ONE 2013, 8, e63030. [Google Scholar]
- Yang, K.; Nong, K.; Gu, Q.; Dong, J.; Wang, J. Discovery of N-hydroxy-3-alkoxybenzamides as direct acid sphingomyelinase inhibitors using a ligand-based pharmacophore model. Eur. J. Med. Chem. 2018, 151, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.; Kim, S.; Lee, S.J.; Heo, P.K.; Kim, S.; Kwon, Y.J.; Lee, K.W. Identification of novel human HDAC8 inhibitors by pharmacophore-based virtual screening and density functional theory approaches. Bull. Korean. Chem. Soc. 2018, 39, 197–206. [Google Scholar] [CrossRef]
- Modi, P.; Patel, S.; Chhabria, M.T. Identification of some novel pyrazolo[1,5-a]pyrimidine derivatives as InhA inhibitors through pharmacophore-based virtual screening and molecular docking. J. Biomol. Struct. Dyn. 2018, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Huse, M.; Chen, Y.G.; Massagué, J.; Kuriyan, J. Crystal structure of the cytoplasmic domain of the type I TGF beta receptor in complex with FKBP12. Cell 1999, 96, 425–436. [Google Scholar] [CrossRef]
- Tebben, A.J.; Ruzanov, M.; Gao, M.; Xie, D.; Kiefer, S.E.; Yan, C.; Newitt, J.A.; Zhang, L.; Kim, K.; Lu, H.; et al. Crystal structures of apo and inhibitor-bound TGFβR2 kinase domain: Insights into TGFβR isoform selectivity. Acta. Crystallogr. D Struct. Biol. 2016, 72, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Meslamani, J.; Li, J.; Sutter, J.; Stevens, A.; Bertrand, H.O.; Rognan, D. Protein-ligand-based pharmacophores: Generation and utility assessment in computational ligand profiling. J. Chem. Inf. Model. 2012, 52, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Mysinger, M.M.; Carchia, M.; Irwin, J.J.; Shoichet, B.K. Directory of useful decoys, enhanced (DUD-E): Better ligands and decoys for better benchmarking. J. Med. Chem. 2012, 55, 6582–6594. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Model | Features | Selectivity Score | Total Actives | Total Inactives | TP | TN | FP | FN | SE | SP |
|---|---|---|---|---|---|---|---|---|---|---|
| A01 | AADHHH | 10.516 | 85 | 8397 | 13 | 7820 | 577 | 72 | 0.15294 | 0.93128 |
| A02 | AAHHHH | 9.6023 | 85 | 8397 | 69 | 6613 | 1784 | 16 | 0.81176 | 0.78754 |
| A03 | AADHH | 9.0011 | 85 | 8397 | 30 | 6542 | 1855 | 55 | 0.35294 | 0.77909 |
| A04 | ADHHH | 9.0011 | 85 | 8397 | 60 | 5688 | 2709 | 25 | 0.70588 | 0.67738 |
| A05 | AADHH | 9.0011 | 85 | 8397 | 18 | 6984 | 1413 | 67 | 0.21176 | 0.83173 |
| A06 | ADHHH | 9.0011 | 85 | 8397 | 20 | 7275 | 1122 | 65 | 0.23529 | 0.86638 |
| A07 | AADHH | 9.0011 | 85 | 8397 | 24 | 7059 | 1338 | 61 | 0.28235 | 0.84066 |
| A08 | AAHHH | 8.0875 | 85 | 8397 | 72 | 3865 | 4532 | 13 | 0.84706 | 0.46028 |
| A09 | AAHHH | 8.0875 | 85 | 8397 | 80 | 4677 | 3720 | 5 | 0.94118 | 0.55698 |
| A10 | AAHHH | 8.0875 | 85 | 8397 | 69 | 3497 | 4900 | 16 | 0.81176 | 0.41646 |
| Model | Features | Rank | Total Actives | Total Inactives | TP | TN | FP | FN | SE | SP |
|---|---|---|---|---|---|---|---|---|---|---|
| B01 | ARHH | 135.897 | 85 | 8397 | 81 | 2315 | 6082 | 4 | 0.95294 | 0.27569 |
| B02 | AHHH | 134.118 | 85 | 8397 | 83 | 4554 | 3843 | 2 | 0.97647 | 0.54243 |
| B03 | RRHH | 133.760 | 85 | 8397 | 79 | 6427 | 1970 | 6 | 0.92941 | 0.76539 |
| B04 | RRHH | 133.760 | 85 | 8397 | 79 | 6456 | 1941 | 6 | 0.92941 | 0.76885 |
| B05 | ARHH | 132.197 | 85 | 8397 | 80 | 2325 | 6072 | 5 | 0.94118 | 0.27688 |
| B06 | RHHH | 132.169 | 85 | 8397 | 79 | 6121 | 2276 | 6 | 0.92941 | 0.72895 |
| B07 | RHHH | 130.718 | 85 | 8397 | 82 | 4558 | 3839 | 3 | 0.96471 | 0.54281 |
| B08 | RHHH | 129.478 | 85 | 8397 | 79 | 5872 | 2525 | 6 | 0.92941 | 0.69930 |
| B09 | RRHH | 129.283 | 85 | 8397 | 79 | 6370 | 2027 | 6 | 0.92941 | 0.75860 |
| B10 | RRHH | 128.545 | 85 | 8397 | 79 | 6149 | 2248 | 6 | 0.92941 | 0.73229 |
| MW | LogP | ROTB | HBA | HBD | Mutagenicity | DTP | Carcinogenicity (Female) | LOAEL (g/kg) | MTD (Feed, g/kg) | LD50 (Oral, g/kg) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| BMS22 | 374 | 3.956 | 4 | 5 | 2 | Nonmutagen | Nontoxic | Noncarcinogen | 0.0064 | 0.137 | 0.0413 |
| YXY01 | 389 | 4.309 | 5 | 2 | 1 | Nonmutagen | Nontoxic | Noncarcinogen | 0.0037 | 0.069 | 0.863 |
| YXY02 | 390 | 4.126 | 5 | 3 | 1 | Nonmutagen | Nontoxic | Noncarcinogen | 0.0027 | 0.075 | 0.274 |
| YXY03 | 416 | 3.687 | 5 | 5 | 2 | Nonmutagen | Nontoxic | Noncarcinogen | 0.0101 | 0.143 | 0.42 |
| YXY04 | 377 | 2.911 | 5 | 5 | 2 | Mutagen | Nontoxic | Noncarcinogen | 0.0185 | 0.104 | 1.02 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Zhou, H.; Jiang, Q.; Sun, L.; Deng, P. Novel Transforming Growth Factor-Beta Receptor 1 Antagonists through a Pharmacophore-Based Virtual Screening Approach. Molecules 2018, 23, 2824. https://doi.org/10.3390/molecules23112824
Jiang J, Zhou H, Jiang Q, Sun L, Deng P. Novel Transforming Growth Factor-Beta Receptor 1 Antagonists through a Pharmacophore-Based Virtual Screening Approach. Molecules. 2018; 23(11):2824. https://doi.org/10.3390/molecules23112824
Chicago/Turabian StyleJiang, Junhao, Hui Zhou, Qihua Jiang, Lili Sun, and Ping Deng. 2018. "Novel Transforming Growth Factor-Beta Receptor 1 Antagonists through a Pharmacophore-Based Virtual Screening Approach" Molecules 23, no. 11: 2824. https://doi.org/10.3390/molecules23112824
APA StyleJiang, J., Zhou, H., Jiang, Q., Sun, L., & Deng, P. (2018). Novel Transforming Growth Factor-Beta Receptor 1 Antagonists through a Pharmacophore-Based Virtual Screening Approach. Molecules, 23(11), 2824. https://doi.org/10.3390/molecules23112824





