Hydrogen Sulfide (H2S) Releasing Capacity of Isothiocyanates from Moringa oleifera Lam.
Abstract
1. Introduction
2. Results and Discussion
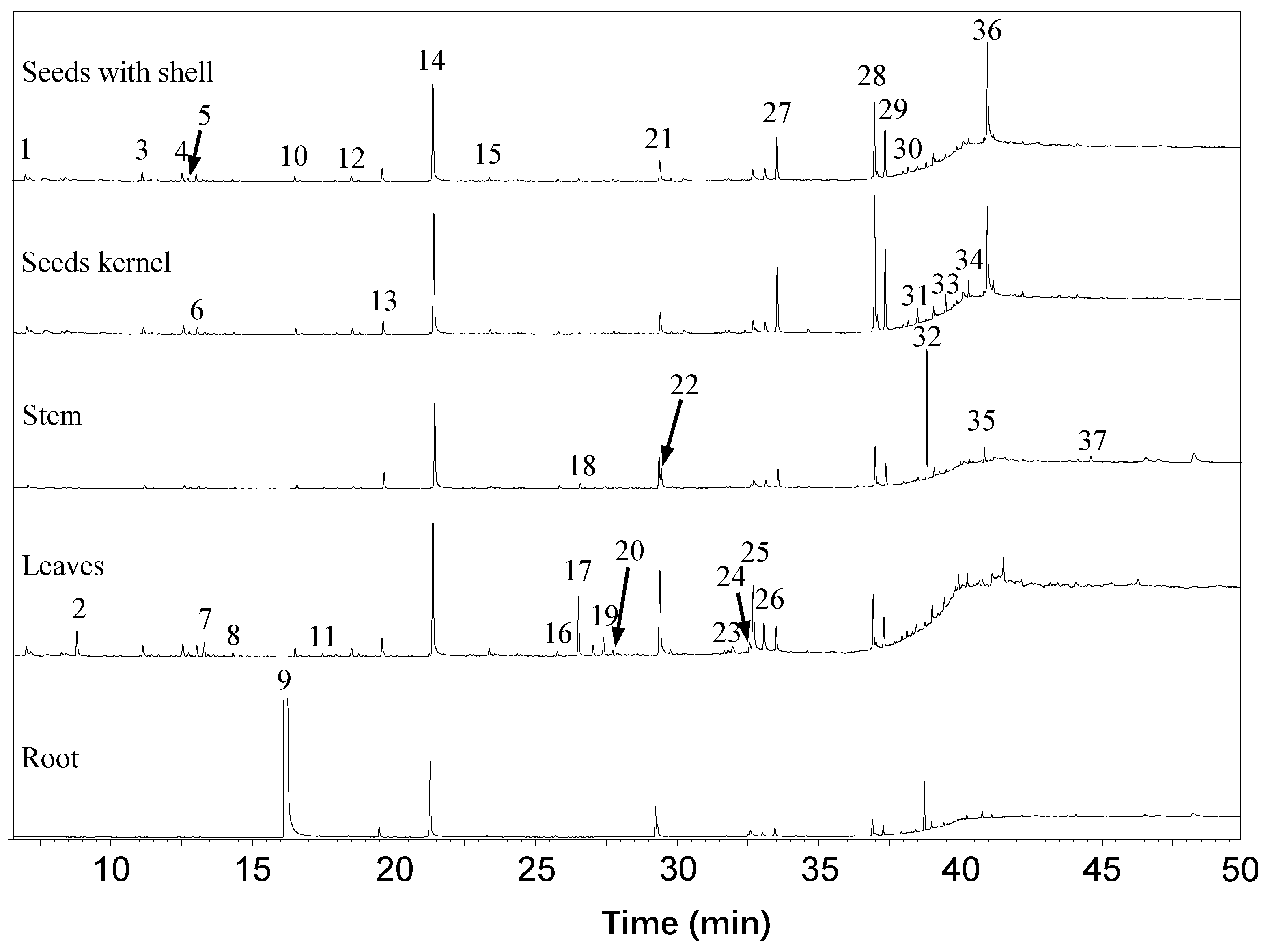
2.1. Extraction and Characterization of GSs and ITCs in Different Moringa Tissues
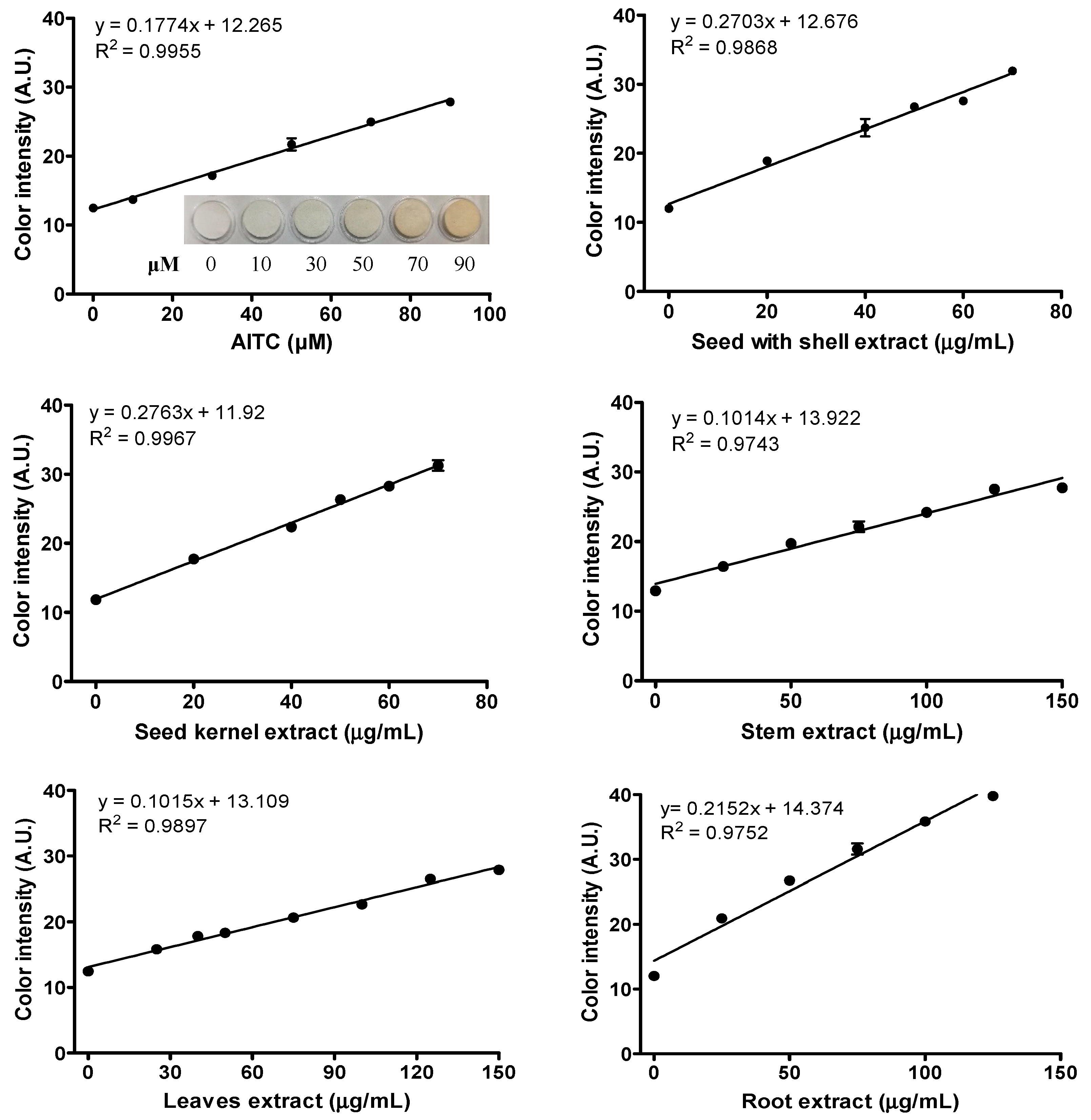
2.2. H2S Releasing Capacity of ITC Extracts from Different Moringa Tissues
3. Materials and Methods
3.1. Plant Material and Chemicals
3.2. Extraction and Myrosinase Hydrolysis of GSs
3.3. Extraction of Total ITCs from Different Moringa Tissues
3.4. HPLC Method for Separation of GSs and ITCs
3.5. Identification of GSs and ITCs Using LC-MS2
3.6. Identification of Volatile Compounds from Different Moringa Tissues
3.7. H2S Releasing Capacity of Total ITCs from Moringa Samples Using Lead (II) Acetate Paper
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Calvert, J.W.; Jha, S.; Gundewar, S.; Elrod, J.W.; Ramachandran, A.; Pattillo, C.B.; Kevil, C.G.; Lefer, D.J. Hydrogen sulfide mediates cardioprotection through Nrf2 signaling. Circ. Res. 2009, 105, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Goto, Y.I.; Kimura, H. Hydrogen sulfide increases glutathione production and suppresses oxidative stress in mitochondria. Antioxid. Redox Sign. 2010, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Lee, Z.W.; Teo, X.Y.; Tay, E.W.; Tan, C.H.; Hagen, T.; Moore, P.; Deng, L.W. Utilizing hydrogen sulfide as a novel anti-cancer agent by targeting cancer glycolysis and pH imbalance. Brit. J. Pharmacol. 2014, 171, 4322–4336. [Google Scholar] [CrossRef] [PubMed]
- Whiteman, M.; Li, L.; Rose, P.; Tan, C.H.; Parkinson, D.B.; Moore, P.K. The effect of hydrogen sulfide donors on lipopolysaccharide-induced formation of inflammatory mediators in macrophages. Antioxid. Redox Sign. 2010, 12, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ndisang, J.F.; Tang, G.; Cao, K.; Wang, R. Hydrogen sulfide induced relaxation of resistance mesenteric artery beds of rats. Am. J. Physiol. Heart C 2004, 287, 2316–2323. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L.; Wang, R. Hydrogen sulfide-based therapeutics: Exploiting a unique but ubiquitous gasotransmitter. Nat. Rev. Drug Discov. 2015, 14, 329–345. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, N.; Tanaka, M.; Yoshida, M.; Ogasawara, Y.; Togawa, T.; Ishii, K.; Kimura, H. 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid. Redox Sign. 2009, 11, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Cerda, M.M.; Hammers, M.D.; Earp, M.S.; Zakharov, L.N.; Pluth, M.D. Applications of synthetic organic tetrasulfides as H2S donors. Org. Lett. 2017, 19, 2314–2317. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Biggs, T.D.; Xian, M. Hydrogen sulfide (H2S) releasing agents: Chemistry and biological applications. Chem. Commun. 2014, 5, 11788–11805. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Wang, C.; Tocmo, R.; Wu, H.; Deng, L.W.; Huang, D. Hydrogen sulfide (H2S) releasing capacity of essential oils isolated from organosulfur rich fruits and vegetables. J. Funct. Foods 2015, 14, 634–640. [Google Scholar] [CrossRef]
- Liang, D.; Bian, J.; Deng, L.W.; Huang, D. Cyclic polysulphide 1,2,4-trithiolane from stinky bean (Parkia speciosa seeds) is a slow releasing hydrogen sulfide (H2S) donor. J. Funct. Foods 2017, 35, 197–204. [Google Scholar] [CrossRef]
- Citi, V.; Martelli, A.; Testai, L.; Marino, A.; Breschi, M.C.; Calderone, V. Hydrogen sulfide releasing capacity of natural isothiocyanates: Is it a reliable explanation for the multiple biological effects of Brassicaceae? Planta Med. 2014, 80, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, L.D.C.; Lucarini, E.; Micheli, L.; Mosca, I.; Ambrosino, P.; Soldovieri, M.V.; Martelli, A.; Testai, L.; Taglialatela, M.; Calderone, V.; et al. Effects of natural and synthetic isothiocyanate-based H2S-releasers against chemotherapy-induced neuropathic pain: Role of Kv7 potassium channels. Neuropharmacology 2017, 121, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Higdon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.; Mithen, R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev. 2009, 8, 269–282. [Google Scholar] [CrossRef]
- Tumer, T.B.; Rojas-Silva, P.; Poulev, A.; Raskin, I.; Waterman, C. Direct and indirect antioxidant activity of polyphenol- and isothiocyanate-enriched fractions from Moringa oleifera. J. Agric. Food Chem. 2015, 63, 1505–1513. [Google Scholar] [CrossRef] [PubMed]
- Busserolles, J.; Tsantoulas, C.; Eschalier, A.; García, J.A.L. Potassium channels in neuropathic pain: Advances, challenges, and emerging ideas. Pain 2016, 157, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Martelli, A.; Testai, L.; Citi, V.; Marino, A.; Bellagambi, F.G.; Ghimenti, S.; Breschi, M.C.; Calderone, V. Pharmacological characterization of the vascular effects of aryl isothiocyanates: Is hydrogen sulfide the real player? Vasc. Pharmacol. 2014, 60, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Barrese, V.; Soldovieri, M.V.; Ambrosino, P.; Martelli, A.; Vinciguerra, I.; Miceli, F.; Greenwood, I.A.; Curtis, M.J.; Breschi, M.C. Expression and function of Kv7. 4 channels in rat cardiac mitochondria: Possible targets for cardioprotection. Cardiovas. Res. 2015, 110, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Kawakishi, S.; Kaneko, T. Interaction of proteins with allyl isothiocyanate. J. Agric. Food Chem. 1987, 35, 85–88. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Amaglo, N.K.; Bennett, R.N.; Curto, R.B.L.; Rosa, E.A.; Turco, V.L.; Giuffrida, A.; Curto, A.L.; Crea, F.; Timpo, G.M. Profiling selected phytochemicals and nutrients in different tissues of the multipurpose tree Moringa oleifera L., grown in Ghana. Food Chem. 2010, 122, 1047–1054. [Google Scholar] [CrossRef]
- Müller, C.; Van Loon, J.; Ruschioni, S.; De Nicola, G.R.; Olsen, C.E.; Iori, R.; Agerbirk, N. Taste detection of the non-volatile isothiocyanate moringin results in deterrence to glucosinolate-adapted insect larvae. Phytochemistry 2015, 118, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Waterman, C.; Cheng, D.M.; Rojas-Silva, P.; Poulev, A.; Dreifus, J.; Lila, M.A.; Raskin, I. Stable, water extractable isothiocyanates from Moringa oleifera leaves attenuate inflammation in vitro. Phytochemistry 2014, 103, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Imohiosen, O.; Gurama, H.H.; Lamidi, T.B. Phytochemical and antimicrobial studies on Moringa oleifera leaves extracts. J. Environ. Sci. Toxicol. Food Technol. 2014, 8, 39–45. [Google Scholar] [CrossRef]
- Biswas, S.K.; Chowdhury, A.; Das, J.; Roy, A.; Hosen, S.Z. Pharmacological potentials of Moringa oleifera Lam.: A review. Int. J. Pharm. Sci. Res. 2012, 3, 305–310. [Google Scholar]
- Giacoppo, S.; Rajan, T.S.; Iori, R.; Rollin, P.; Bramanti, P.; Mazzon, E. The α-cyclodextrin complex of the Moringa isothiocyanate suppresses lipopolysaccharide-induced inflammation in RAW 264.7 macrophage cells through Akt and p38 inhibition. Inflamm. Res. 2017, 66, 487–503. [Google Scholar] [CrossRef] [PubMed]
- Förster, N.; Ulrichs, C.; Schreiner, M.; Müller, C.T.; Mewis, I. Development of a reliable extraction and quantification method for glucosinolates in Moringa oleifera. Food Chem. 2015, 166, 456–464. [Google Scholar] [CrossRef] [PubMed]
- Aja, P.; Nwachukwu, N.; Ibiam, U.; Igwenyi, I.; Offor, C.; Orji, U. Chemical constituents of Moringa oleifera leaves and seeds from Abakaliki, Nigeria. Am. J. Phytomed. Clin. Therap. 2014, 2, 310–321. [Google Scholar]
- Bennett, R.N.; Mellon, F.A.; Foidl, N.; Pratt, J.H.; Dupont, M.S.; Perkins, L.; Kroon, P.A. Profiling glucosinolates and phenolics in vegetative and reproductive tissues of the multi-purpose trees Moringa oleifera L. (Horseradish tree) and Moringa stenopetala L. J. Agric. Food Chem. 2003, 51, 3546–3553. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Diaz, S. The release and purification of sialic acids from glycoconjugates: Methods to minimize the loss and migration of O-acetyl groups. Anal. Biochem. 1984, 137, 236–247. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and isothiocyanates in health and disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Juge, N.; Mithen, R.; Traka, M. Molecular basis for chemoprevention by sulforaphane: A comprehensive review. Cell. Mol. Life Sci. 2007, 64, 1105. [Google Scholar] [CrossRef] [PubMed]
- Cheenpracha, S.; Park, E.J.; Yoshida, W.Y.; Barit, C.; Wall, M.; Pezzuto, J.M.; Chang, L.C. Potential anti-inflammatory phenolic glycosides from the medicinal plant Moringa oleifera fruits. Bioorgan. Med. Chem. 2010, 18, 6598–6602. [Google Scholar] [CrossRef] [PubMed]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P. The isothiocyanate produced from glucomoringin inhibits NF-kB and reduces myeloma growth in nude mice in vivo. Biochem. Pharmacol. 2010, 79, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.A. Floral scent composition of Moringa oleifera Lam. J. Essential Oil Bear Plants 2013, 16, 315–317. [Google Scholar] [CrossRef]
- Afsharypuor, S.; Asghari, G.; Mohagheghzadeh, A.; Dehshahri, S. Volatile constituents of the seed kernel and leaf of Moringa peregrina (Forssk.) Fiori, Agricolt. Cultivated in Chabahar (Iran). Iran. J. Pharm. Sci. 2010, 6, 141–144. [Google Scholar]
- Rani, A.; Zahirah, N.; Husain, K.; Kumolosasi, E. Moringa genus: A review of phytochemistry and pharmacology. Front. Pharmacol. 2018, 9, 108. [Google Scholar] [CrossRef] [PubMed]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Treviño-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shen, Y.; Wu, X.; Zhu, Y.; Mupunga, J.; Bao, W.; Huang, J.; Mao, J.; Liu, S.; You, Y. Hydrolysis before Stir-Frying Increases the Isothiocyanate Content of Broccoli. J. Agric. Food Chem. 2018, 66, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
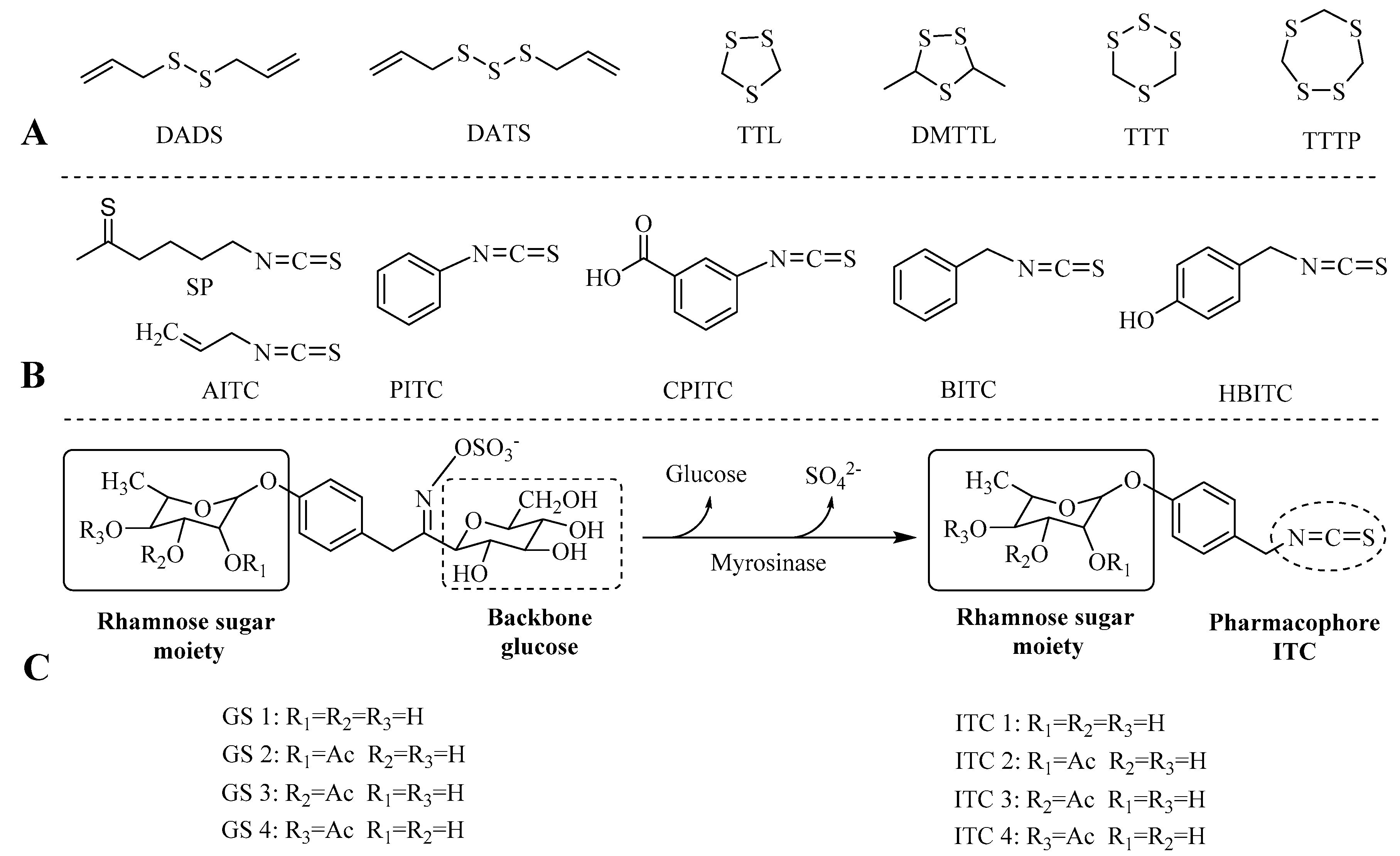
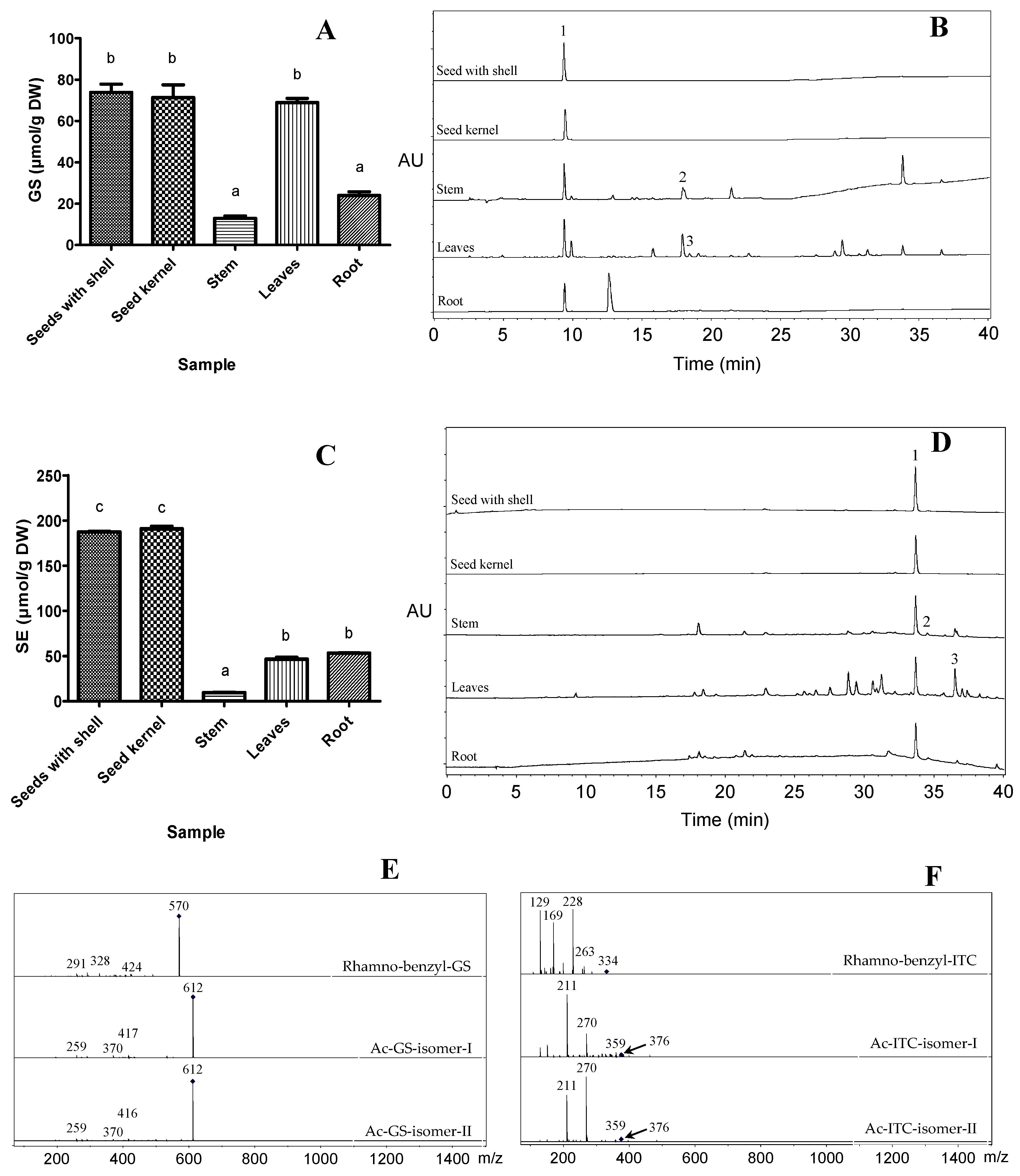
| Peak No. | Retention Time (min) | Compounds | m/z [M − H]− | MS2 |
|---|---|---|---|---|
| 1 | 9.5 | Rhamno-benzyl-GS | 570 | 424, 328, 291 |
| 2 | 18.1 | Ac-GS-isomer-I | 612 | 417, 370, 259 |
| 3 | 18.4 | Ac-GS-isomer-II | 612 | 416, 370, 259 |
| Peak No. | Retention Time (min) | Compound | m/z [M + Na]+ | MS2 |
|---|---|---|---|---|
| 1 | 33.7 | Rhamno-benzyl-ITC | 334 | 263, 228, 169, 129 |
| 2 | 34.5 | Ac-ITC isomer I | 376 | 359, 270, 211, 151 |
| 3 | 36.5 | Ac-ITC isomer II | 376 | 359, 270, 211 |
| No. | Compound Name | RT (min) $ | LRI & | Identification | Different Moringa Tissues Peak Area (%) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Seeds with Shell | Seed Kernels | Leaves | Stem | Root | |||||
| 1 | Undecane | 7.02 | 1053 | LRI, MS | 1.17 | 1.09 | 1.16 | 0.57 | 0.08 |
| 2 | Benzoic acid, 2,4-bis[(trimethylsilyl)oxy]- | 8.81 | -- | LRI, MS | 0.00 | 0.00 | 3.32 | 0.00 | 0.00 |
| 3 | Dodecane | 11.15 | 1200 | LRI, MS | 1.77 | 1.04 | 1.43 | 0.84 | 0.10 |
| 4 | Benzene,1,3-bis (1,1-dimethylethyl)- | 12.56 | 1251 | LRI, MS | 1.47 | 1.30 | 1.44 | 0.75 | 0.09 |
| 5 | 4-Ethylundecane | 12.77 | 1259 | LRI, MS | 0.43 | 0.31 | 0.41 | 0.31 | 0.02 |
| 6 | Tridecane | 13.05 | 1269 | LRI, MS | 1.28 | 1.06 | 1.40 | 0.62 | 0.07 |
| 7 | Cyclopentasiloxane, dodeca methyl | 13.32 | -- | MS | 0.43 | 0.49 | 1.92 | 0.42 | 0.06 |
| 8 | 2,3,5,8-Tetramethyldecane | 14.34 | 1317 | LRI, MS | 0.38 | 0.30 | 0.41 | 0.22 | 0.02 |
| 9 | m-Tolyl isothiocyanate | 16.38 | 1394 | LRI, MS | 0.00 | 0.00 | 0.00 | 0.00 | 79.48 |
| 10 | Tetradecane | 16.53 | 1400 | LRI, MS | 0.93 | 0.80 | 1.03 | 0.81 | 2.05 |
| 11 | Cycloheptasiloxane, tetradeca methyl | 17.51 | -- | MS | 0.14 | 0.29 | 0.35 | 0.29 | 0.05 |
| 12 | Pentadecane | 18.54 | 1480 | LRI, MS | 1.03 | 1.00 | 1.10 | 0.51 | 0.13 |
| 13 | Phenol, 3,5-bis(1,1-dimethylethyl)- | 19.62 | 1524 | LRI, MS | 2.62 | 2.28 | 2.68 | 4.21 | 0.79 |
| 14 | Hexadecane | 21.42 | 1599 | LRI, MS | 21.91 | 21.47 | 19.06 | 24.86 | 6.54 |
| 15 | Heptadecane | 23.41 | 1688 | LRI, MS | 0.57 | 0.58 | 0.75 | 0.44 | 0.09 |
| 16 | Octadecane | 25.82 | 1784 | LRI, MS | 0.41 | 0.34 | 0.48 | 0.52 | 0.09 |
| 17 | Hexadecanal | 26.57 | 1836 | LRI, MS | 0.46 | 0.21 | 7.28 | 0.99 | 0.04 |
| 18 | Octadecanal | 27.09 | 1861 | LRI, MS | 0.17 | 0.20 | 1.33 | 0.24 | 0.02 |
| 19 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | 27.46 | 1880 | LRI, MS | 0.33 | 0.31 | 1.97 | 0.13 | 0.10 |
| 20 | Nonadecane | 27.78 | 1895 | LRI, MS | 0.39 | 0.37 | 0.49 | 0.30 | 0.05 |
| 21 | Dibutyl phthalate | 29.36 | -- | MS | 0.00 | 0.00 | 0.00 | 7.26 | 2.26 |
| 22 | Hexadecanoic acid | 29.45 | 1981 | LRI, MS | 4.04 | 3.32 | 13.62 | 5.34 | 1.00 |
| 23 | E-Phytol | 32.02 | 2136 | LRI, MS | 0.00 | 0.00 | 1.31 | 0.00 | 0.00 |
| 24 | (Z,Z)-9,12-Octadecadienoic acid | 32.62 | -- | MS | 0.00 | 0.00 | 1.43 | 0.59 | 0.16 |
| 25 | Linolenic acid | 32.76 | -- | MS | 1.62 | 1.49 | 11.09 | 1.36 | 0.32 |
| 26 | Eicosanoic acid | 33.13 | 2269 | LRI, MS | 2.25 | 1.67 | 4.25 | 2.06 | 0.28 |
| 27 | Hexadecanamide | 33.57 | 2310 | LRI, MS | 7.39 | 9.92 | 3.28 | 4.67 | 0.64 |
| 28 | 9-Octadecenamide | 37.00 | 2493 | LRI, MS | 13.21 | 18.72 | 6.21 | 8.95 | 1.16 |
| 29 | Octadecanamide | 37.38 | 2519 | LRI, MS | 7.72 | 10.41 | 3.16 | 4.85 | 0.65 |
| 30 | Octadecanoic acid, phenylmethyl ester | 38.02 | -- | MS | 0.41 | 0.39 | 0.42 | 0.40 | 0.12 |
| 31 | 1-Palmitoyl-1,3-propanediol, trimethylsilyl | 38.19 | -- | MS | 0.78 | 0.64 | 0.63 | 0.00 | 0.00 |
| 32 | Mono(2-ethylhexyl) phthalate | 38.83 | -- | MS | 0.72 | 0.28 | 0.47 | 21.50 | 2.42 |
| 33 | Heptacosane | 39.09 | 2677 | LRI, MS | 1.63 | 1.33 | 1.37 | 1.46 | 0.39 |
| 34 | Octacosane | 40.02 | 2798 | LRI, MS | 0.00 | 0.00 | 1.12 | 0.50 | 0.04 |
| 35 | Nonacosane | 40.87 | 2911 | LRI, MS | 0.67 | 0.54 | 0.42 | 3.09 | 0.46 |
| 36 | 2-(2-Hexyloxyethoxy)ethanol | 41.00 | -- | MS | 23.17 | 17.36 | 0.00 | 0.00 | 0.00 |
| 37 | Dotriacontane | 44.17 | 3223 | LRI, MS | 0.50 | 0.47 | 0.67 | 0.64 | 0.19 |
| Ranking | Sample Name | Oil Yield (g/100 g Moringa Tissue) | ITCs Extract Yield (g/100 g Defatted Moringa Tissue) | ITCs Extract Yield (g/100 g Moringa Tissue) | AITC-E of Moringa ITC Extract (mmol AITC/g of ITC Extract) | AITC-E of Moringa Tissue (mmol AITC/100 g of Moringa Tissue) |
|---|---|---|---|---|---|---|
| 1 | Seed kernel | 37.28 | 10.18 | 6.56 | 1.56 ± 0.09 | 9.94 ± 0.58 |
| 2 | Seed with shell | 28.81 | 6.88 | 4.90 | 1.52 ± 0.03 | 7.49 ± 0.16 |
| 3 | Root | 2.43 | 3.66 | 3.57 | 1.21 ± 0.05 | 4.34 ± 0.17 |
| 4 | Leaves | 2.82 | 5.32 | 5.17 | 0.57 ± 0.03 | 2.96 ± 0.18 |
| 5 | Stem | 1.43 | 2.65 | 2.61 | 0.57 ± 0.04 | 1.49 ± 0.09 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, Y.; Liu, X.; Lin, Y.; Zheng, X.; Lu, Y. Hydrogen Sulfide (H2S) Releasing Capacity of Isothiocyanates from Moringa oleifera Lam. Molecules 2018, 23, 2809. https://doi.org/10.3390/molecules23112809
Wang X, Liu Y, Liu X, Lin Y, Zheng X, Lu Y. Hydrogen Sulfide (H2S) Releasing Capacity of Isothiocyanates from Moringa oleifera Lam. Molecules. 2018; 23(11):2809. https://doi.org/10.3390/molecules23112809
Chicago/Turabian StyleWang, Xiangshe, Yunjiao Liu, Xingdi Liu, Yi Lin, Xueqin Zheng, and Yuyun Lu. 2018. "Hydrogen Sulfide (H2S) Releasing Capacity of Isothiocyanates from Moringa oleifera Lam." Molecules 23, no. 11: 2809. https://doi.org/10.3390/molecules23112809
APA StyleWang, X., Liu, Y., Liu, X., Lin, Y., Zheng, X., & Lu, Y. (2018). Hydrogen Sulfide (H2S) Releasing Capacity of Isothiocyanates from Moringa oleifera Lam. Molecules, 23(11), 2809. https://doi.org/10.3390/molecules23112809




