Metalloporphyrin Dimers Bridged by a Peptoid Helix: Host-Guest Interaction and Chiral Recognition
Abstract
1. Introduction
2. Results and Discussion
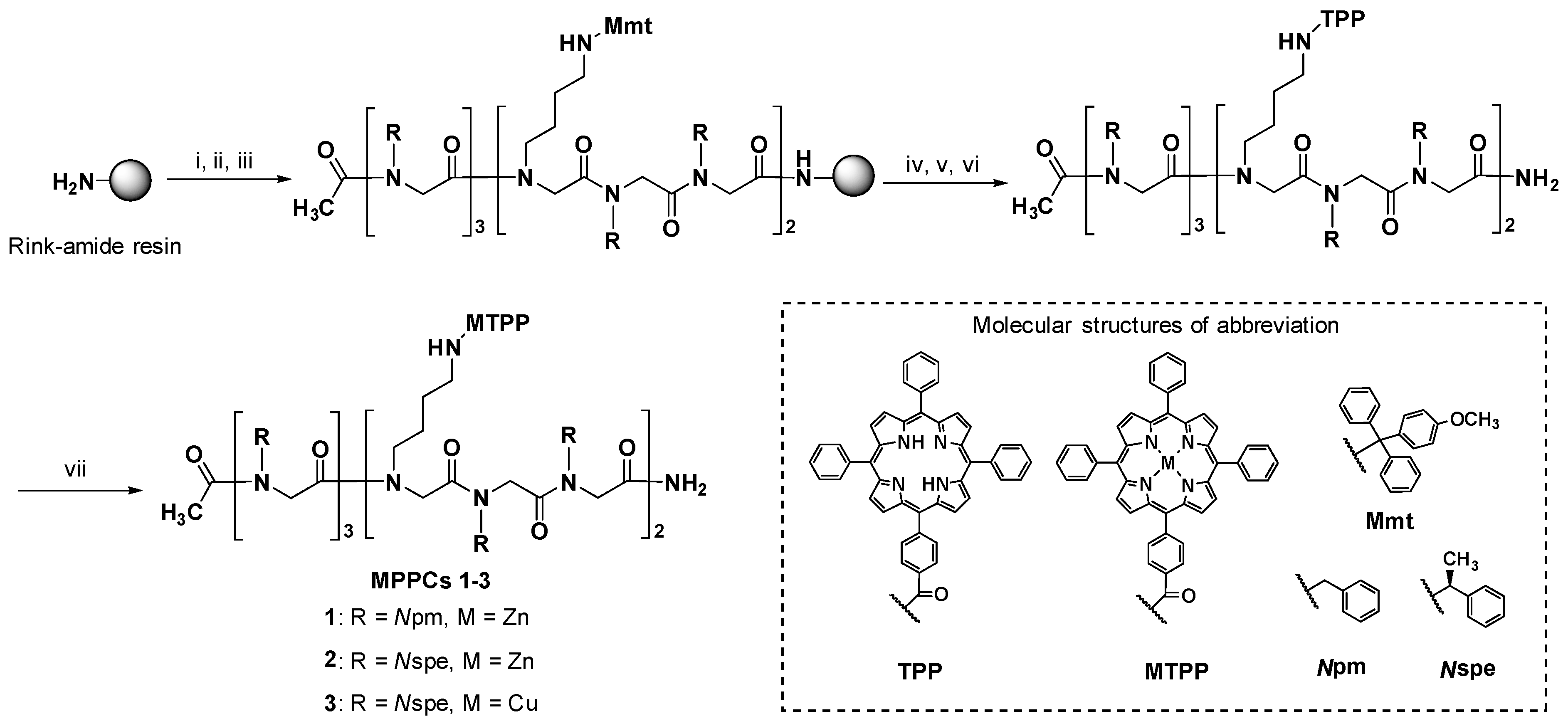
2.1. Synthesis of MPPCs
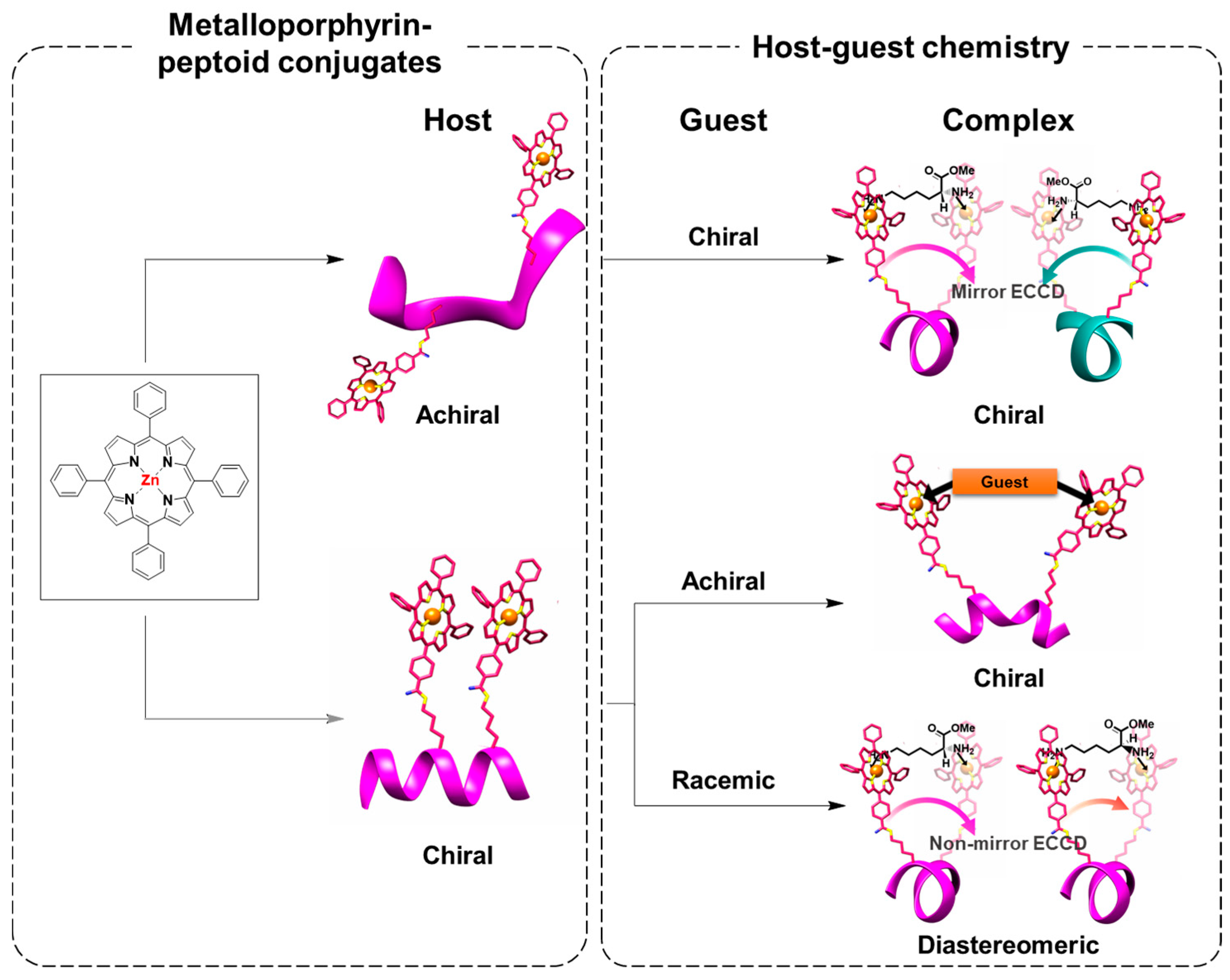
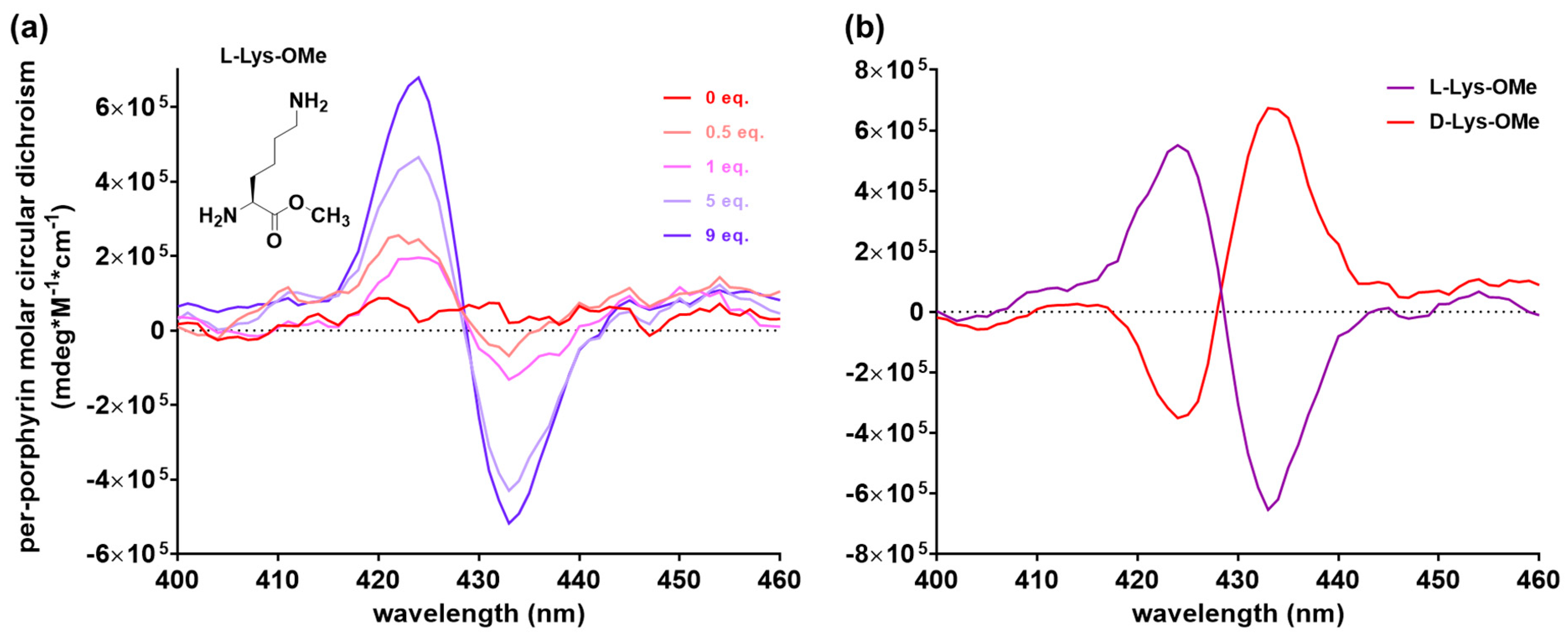
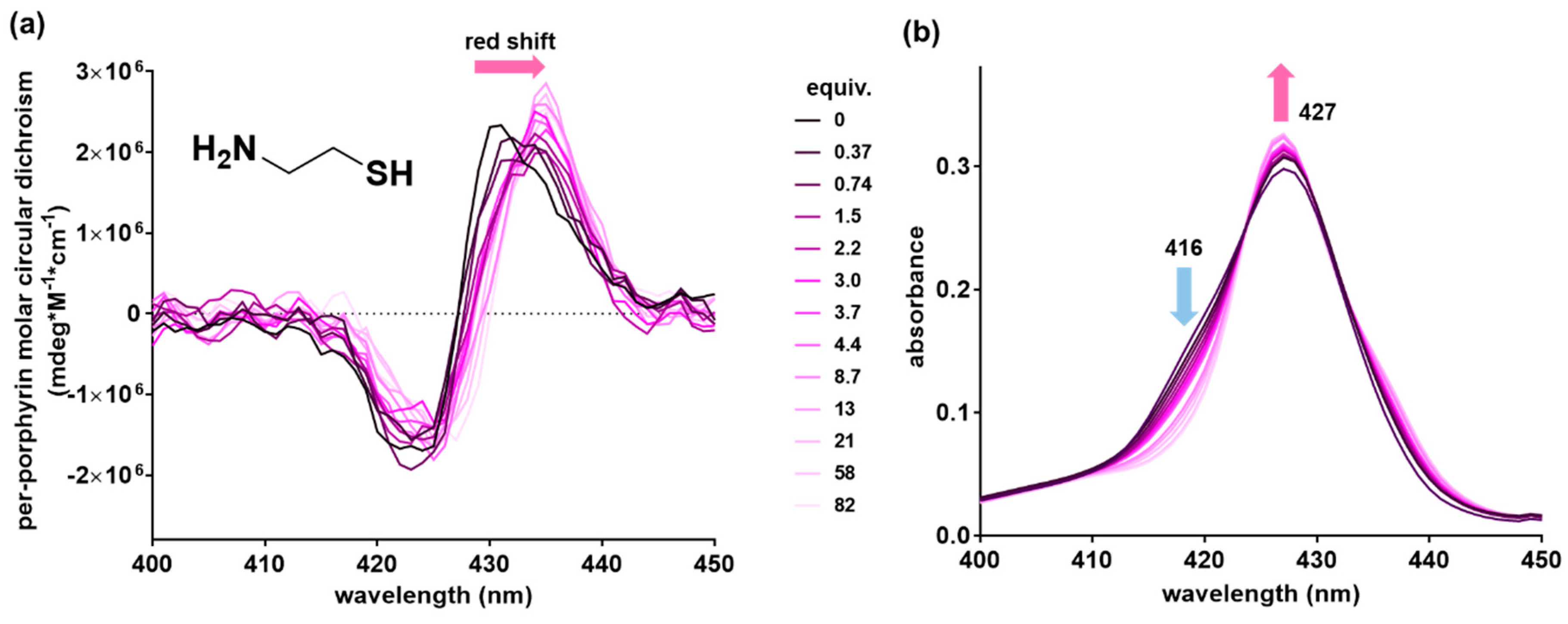
2.2. Achiral Host-Chiral Guest Complexation: Intermolecular Chirality Induction
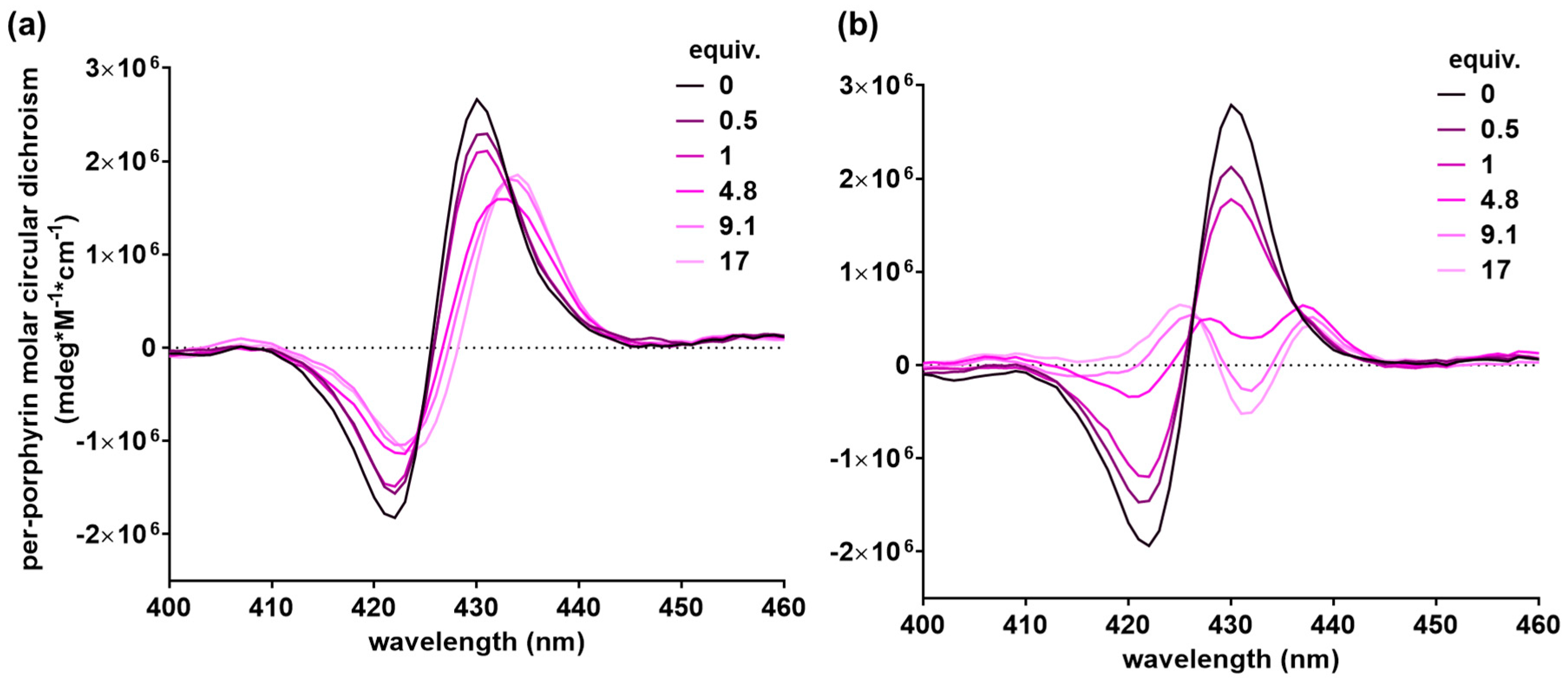
2.3. Chiral Host–Achiral Guest Complexation
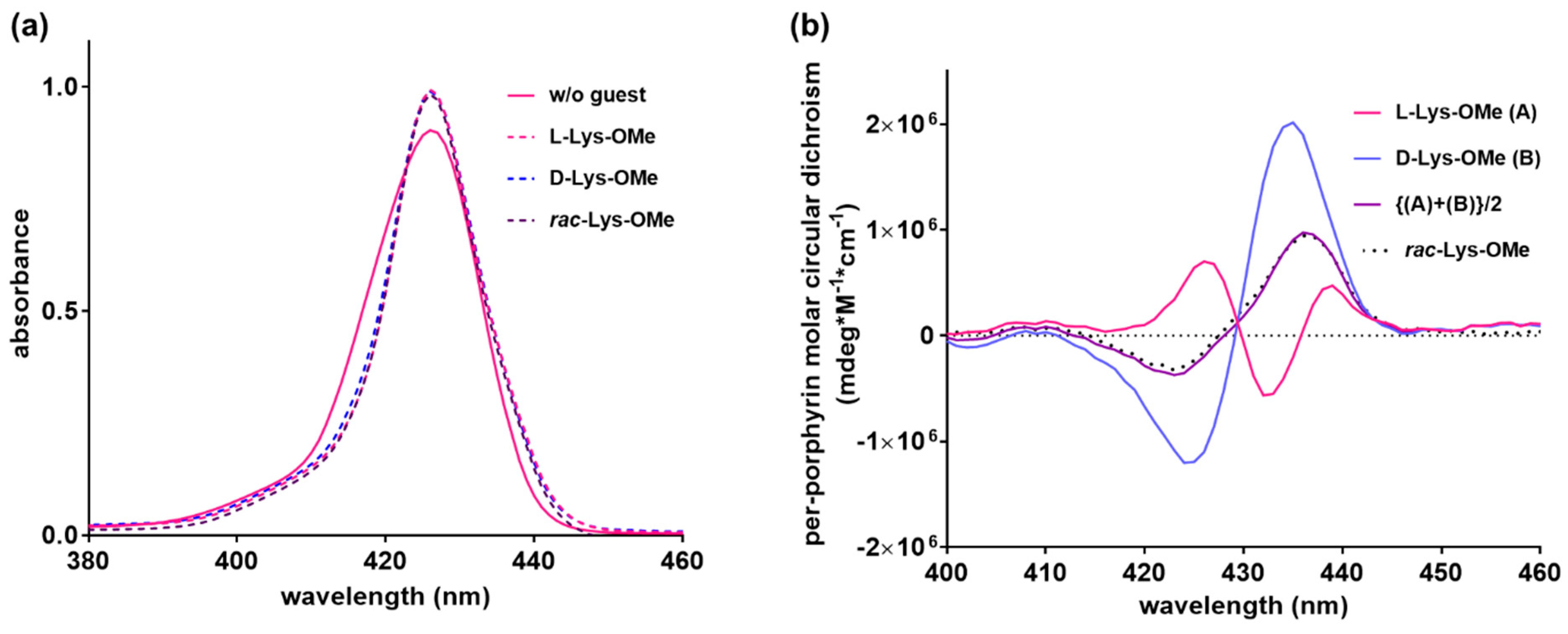
2.4. Chiral Host–Chiral Guest Complexation
3. Materials and Methods
3.1. Abbreviations
3.2. Materials and Reagents
3.3. Synthesis of MPPCs
3.4. Liquid Chromatography-Mass Spectrometry (LC-MS)
3.5. Desalting of d/l-Lys-OMe∙2HCl
3.6. Spectroscopic Titration
3.7. Calculation of Dissociation Constant
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McDermott, G.; Prince, S.M.; Freer, A.A.; Hawthornthwaite-Lawless, A.M.; Papiz, M.Z.; Cogdell, R.J.; Isaacs, N.W. Crystal structure of an intergal membrane light-harvesting complex from photosynthetic bacteria. Nature 1995, 374, 517–521. [Google Scholar] [CrossRef]
- Pullerits, T.; Sundström, V. Photosynthetic light-harvesting pigment-protein complexes: Toward understanding how and why. Acc. Chem. Res. 1996, 29, 381–389. [Google Scholar] [CrossRef]
- Prathapan, S.; Johnson, T.E.; Lindsey, J.S. Building-block synthesis of porphyrin light-harvesting arrays. J. Am. Chem. Soc. 1993, 115, 7519–7520. [Google Scholar] [CrossRef]
- Mak, C.C.; Pomeranc, D.; Montalti, M.; Prodi, L.; Sanders, J.K.M. A versatile synthetic strategy for sonstruction of large oligomers: Binding and photophysical properties of a nine-porphyrin array. Chem. Commun. 1999, 1083–1084. [Google Scholar] [CrossRef]
- Ravikanth, M. Synthesis and photophysical study of unsymmetrical porphyrin pentamers. Tetrahedron Lett. 2000, 41, 3709–3712. [Google Scholar] [CrossRef]
- Kim, D.; Osuka, A. Directly linked porphyrin arrays with tunable excitonic interactions. Acc. Chem. Res. 2004, 37, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Seth, J.; Palaniappan, V.; Johnson, T.E.; Prathapan, S.; Lindsey, J.S.; Bocian, D.F. Investigation of electronic communication in multi-porphyrin light-harvesting arrays. J. Am. Chem. Soc. 1994, 116, 10578–10592. [Google Scholar] [CrossRef]
- Lovett, J.E.; Hoffmann, M.; Cnossen, A.; Shutter, A.T.J.; Hogben, H.J.; Warren, J.E.; Pascu, S.I.; Kay, C.W.M.; Timmel, C.R.; Anderson, H.L. Probing flexibility in porphyrin-based molecular wires using double electron electron resonance. J. Am. Chem. Soc. 2009, 131, 13852–13859. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef] [PubMed]
- Solladié, N.; Bouatra, S.; Merkas, S.; Rein, R.; Roeser, J. Influence of the spacer in bis-porphyrinic tweezers on the association constant for host/guest complexes. J. Porphyr. Phthalocyanines 2005, 9, 779–787. [Google Scholar] [CrossRef]
- Flamigni, L.; Talarico, A.M.; Ventura, B.; Rein, R.; Solladié, N. A versatile bis-porphyrin tweezer host for the assembly of noncovalent photoactive architectures: A photophysical characterization of the tweezers and their association with porphyrins and other guests. Chem. A Eur. J. 2006, 12, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Dunetz, J.R.; Sandstorm, C.; Young, E.R.; Baker, P.; Van Name, S.A.; Cathopolous, T.; Fairman, R.; de Padula, J.C.; Akerfeldt, K.S. Self-assembling porphyrin-modified peptides. Org. Lett. 2005, 7, 2559–2561. [Google Scholar] [CrossRef] [PubMed]
- Biscaglia, F.; Frezza, E.; Zurlo, E.; Gobbo, M. Linker dependent chirality of solvent induced self-assembled structures of porphyrin–α-helical peptide conjugates. Org. Biomol. Chem. 2016, 14, 9568–9577. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Chung, S.; Ahn, Y.D.; Lee, J.; Seo, J. Porphyrin-peptoid conjugates: Face-to-face display of porphyrins on peptoid helices. Org. Lett. 2013, 15, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.; Yang, W.; Lee, S.; Mukherjee, S.; Forstater, J.; Kim, H.; Goh, B.; Kim, T.-Y.; Voelz, V.A.; Pang, Y.; et al. Precisely tuneable energy transfer system using peptoid helix-based molecular scaffold. Sci. Rep. 2017, 7, 4786. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Kang, B.; Voelz, V.A.; Seo, J. Control of porphyrin interactions via structural changes of a peptoid scaffold. Org. Biomol. Chem. 2017, 15, 9670–9679. [Google Scholar] [CrossRef] [PubMed]
- Berova, N.; Pescitelli, G.; Petrovic, A.G.; Proni, G. Probing molecular chirality by CD-sensitive dimeric metalloporphyrin hosts. Chem. Commun. 2009, 5958–5980. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Rickman, B.H.; Borhan, B.; Berova, N.; Nakanishi, K. Zinc porphyrin tweezer in host-guest complexation: Determination of absolute configurations of primary monoamines by circular dichroism. J. Am. Chem. Soc. 1998, 120, 6185–6186. [Google Scholar] [CrossRef]
- Huang, X.; Nakanishi, K.; Berova, N. Porphyrins and metalloporphyrins: Versatile circular dichroic reporter groups for structural studies. Chirality 2000, 12, 237–255. [Google Scholar] [CrossRef]
- Huang, X.; Fujioka, N.; Pescitelli, G.; Koehn, F.E.; Williamson, R.T.; Nakanishi, K.; Berova, N. Absolute configurational assignments of secondary amines by CD-sensitive dimeric zinc porphyrin host. J. Am. Chem. Soc. 2002, 124, 10320–10335. [Google Scholar] [CrossRef] [PubMed]
- Proni, G.; Pescitelli, G.; Huang, X.; Nakanishi, K.; Berova, N. Magnesium tetraarylporphyrin tweezer: A CD-sensitive host for absolute configurational assignments of α-chiral carboxylic acids. J. Am. Chem. Soc. 2003, 125, 12914–12927. [Google Scholar] [CrossRef] [PubMed]
- Ishii, H.; Chen, Y.; Miller, R.A.; Karady, S.; Nakanishi, K.; Berova, N. Chiral recognition of cyclic α-hydroxyketones by CD-sensitive zinc tetraphenylporphyrin tweezer. Chirality 2005, 17, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, L.; Yang, H.; Zhao, L.; Qi, D.; Bian, Y.; Jiang, J. Intramolecular chirality induction and intermolecular chirality modulation in BINOL bridged bisporphyrin hosts. Dyes Pigment. 2017, 137, 608–614. [Google Scholar] [CrossRef]
- Saha, B.; Ikbal, S.A.; Petrovic, A.G.; Berova, N.; Rath, S.P. Complexation of chiral zinc-porphyrin tweezer with achiral diamines: Induction and two-step inversion of interporphyrin helicity monitored by ECD. Inorg. Chem. 2017, 56, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
- Ema, T.; Ouchi, N.; Doi, T.; Korenaga, T.; Sakai, T. Highly sensitive chiral shift reagent bearing two zinc porphyrins. Org. Lett. 2005, 7, 3985–3988. [Google Scholar] [CrossRef] [PubMed]
- Crossley, M.J.; Mackay, L.G.; Try, A.C. Enantioselective recognition of histidine and lysine esters by porphyrin chiral clefts and detection of amino acid conformations in the bound state. J. Chem. Soc. Chem. Commun. 1995, 1925–1927. [Google Scholar] [CrossRef]
- Hayashi, T.; Aya, T.; Nonoguchi, M.; Mizutani, T.; Hisaeda, Y.; Kitagawa, S.; Ogoshi, H. Chiral recognition and chiral sensing using zinc porphyrin dimers. Tetrahedron 2002, 58, 2803–2811. [Google Scholar] [CrossRef]
- Gunter, M.J.; Johnston, M.R. Porphyrin-based molecular tweezers as a receptor for bipyridinium guests. Tetrahedron Lett. 1992, 33, 1771–1774. [Google Scholar] [CrossRef]
- Wu, Z.; Shao, X.; Li, C.; Hou, J.; Wang, K.; Jiang, X.; Li, Z. Hydrogen-bonding-driven preorganized zinc porphyrin receptors for efficient complexation of C60, C70 and C60 derivatives. J. Am. Chem. Soc. 2005, 127, 17460–17468. [Google Scholar] [CrossRef] [PubMed]
- Pintre, I.C.; Pierrefixe, S.; Hamilton, A.; Valderrey, V.; Bo, C.; Ballester, P. Influence of the solvent and metal center on supramolecular chirality induction with bisporphyrin tweezer receptors. Strong metal modulation of effective molarity values. Inorg. Chem. 2012, 51, 4620–4635. [Google Scholar] [CrossRef] [PubMed]
- Kirshenbaum, K.; Barron, A.E.; Goldsmith, R.A.; Aramd, P.; Bradley, E.K.; Troung, K.T.V.; Dill, K.A.; Cohen, F.E.; Zuckermann, R.N. Sequence-specific polypeptoids: A diverse family of heteropolymers with stable secondary structure. Proc. Natl. Acad. Sci. USA 1998, 95, 4303–4308. [Google Scholar] [CrossRef] [PubMed]
- Crapster, J.A.; Guzei, I.A.; Blackwell, H.E. A peptoid ribbon secondary structure. Angew. Chemie. Int. Ed. 2013, 52, 5079–5084. [Google Scholar] [CrossRef] [PubMed]
- Gorske, B.C.; Mumford, E.M.; Gerrity, C.G.; Ko, I. A peptoid square helix via synergistic control of backbone dihedral angles. J. Am. Chem. Soc. 2017, 139, 8070–8073. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.M.; Kang, C.M.; Yoon, M.H.; Seo, J. Peptoid helicity modulation: Precise control of peptoid secondary structures via position-specific placement of chiral monomers. Chem. Commun. 2014, 50, 4465–4468. [Google Scholar] [CrossRef] [PubMed]
- Zuckermann, R.N.; Kerr, J.M.; Kent, S.B.H.; Moos, W.H. Efficient method for the preparation of peptoids [oligo(N-substituted glycines)] by submonomer solid-phase synthesis. J. Am. Chem. Soc. 1992, 114, 10646–10647. [Google Scholar] [CrossRef]
- Jang, H.; Fafarman, A.; Holub, J.M.; Kirshenbaum, K. Click to fit: Versatile polyvalent display on a peptidomimetic scaffold. Org. Lett. 2005, 7, 1951–1954. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.M.; Kirshenbaum, K. Tricks with clicks: Modification of peptidomimetic oligomers via copper-catalyzed azide-alkyne [3 + 2] cycloaddition. Chem. Soc. Rev. 2010, 39, 1325–1337. [Google Scholar] [CrossRef] [PubMed]
- Holub, J.M.; Garabedian, M.J.; Kirshenbaum, K. Peptoids on steroids: Precise multivalent estradiol-peptidomimetic conjugates generated via azide-alkyne [3 + 2] cycloaddition reactions. QSAR Comb. Sci. 2007, 26, 1175–1180. [Google Scholar] [CrossRef]
- Holub, J.M.; Jang, H.; Kirshenbaum, K. Fit to be tied: Conformation-directed macrocyclization of peptoid foldamers. Org. Lett. 2007, 9, 3275–3278. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Seo, J. Postsynthetic modification of peptoids via the Suzuki-Miyaura cross-coupling reaction. Biopolymers 2016, 106, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, D.; Seo, J. Facile method for the synthesis of triazole- and tetrazole-containing peptoids on a solid support. Tetrahedron Lett. 2018, 59, 3311–3316. [Google Scholar] [CrossRef]
- Lee, J.; Huang, W.; Broering, J.M.; Barron, A.E.; Seo, J. Prostate tumor specific peptide-peptoid hybrid prodrugs. Bioorganic Med. Chem. Lett. 2015, 25, 2849–2852. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Patch, J.A.; Czyzewski, A.M.; Dohm, M.T.; Ivankin, A.; Gidalevitz, D.; Zuckermann, R.; Barron, A.E. Peptoids that mimic the structure, function, and mechanism of helical antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2008, 105, 2794–2799. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Wetzler, M.; Jardetzky, T.S.; Barron, A.E. A readily applicable strategy to convert peptides to peptoid-based therapeutics. PLoS ONE 2013, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chongsiriwatana, N.P.; Lin, J.S.; Kapoor, R.; Wetzler, M.; Rea, J.A.C.; Didwania, M.K.; Contag, C.H.; Barron, A.E. Intracellular biomass flocculation as a key mechanism of rapid bacterial killing by cationic, amphipathic antimicrobial peptides and peptoids. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.; Lee, J.H.; Moon, H.; Hyun, Y.J.; Lim, H.S. A chemical inhibitor of the Skp2/p300 interaction that promotes p53-mediated apoptosis. Angew. Chem. Int. Ed. 2016, 55, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Hyun, Y.J.; Lee, J.H.; Lim, H.S. Comparison of cell permeability of cyclic peptoids and linear peptoids. ACS Comb. Sci. 2018, 20, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Prathap, K.J.; Maayan, G. Metallopeptoids as efficient biomimetic catalysts. Chem. Commun. 2015, 51, 11096–11099. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Qi, J.; Zuckermann, R.N.; DeYoreo, J.J. Engineered biomimetic polymers as tunable agents for controlling CaCO3 mineralization. J. Am. Chem. Soc. 2011, 113, 5214–5217. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.C.; Chu, T.K.; Dill, K.A.; Zuckermann, R.N. Biomimetic nanostructures: Creating a high-affinity zinc-binding site in a folded nonbiological polymer. J. Am. Chem. Soc. 2008, 130, 8847–8855. [Google Scholar] [CrossRef] [PubMed]
- Wetzler, M.; Barron, A.E. Progress in the de novo design of structured peptoid protein mimics. Biopolymers 2011, 96, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Fuller, A.A.; Tenorio, K.; Huber, J.; Hough, S.; Dowell, K.M. A peptoid supramolecular host for benzo[a]pyrene in water. Supramol. Chem. 2018, 30, 115–123. [Google Scholar] [CrossRef]
- Fuller, A.A.; Holmes, C.A.; Seidl, F.J. A fluorescent peptoid pH-sensor. Biopolymers 2013, 100, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.; Zhou, E. Selective chromium (VI) ligands identified using combinatorial peptoid libraries. J. Am. Chem. Soc. 2013, 135, 17488–17493. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.S.; Zhou, E.Y.; Francis, M.B. Development of peptoid-based ligands for the removal of cadmium from biological media. Chem. Sci. 2015, 4042–4048. [Google Scholar] [CrossRef] [PubMed]
- Knight, A.S.; Kulkarni, R.U.; Zhou, E.Y.; Franke, J.M.; Miller, E.W.; Francis, M.B. A modular platform to develop peptoid-based selective fluorescent metal sensors. Chem. Commun. 2017, 53, 3477–3480. [Google Scholar] [CrossRef] [PubMed]
- Chandra Mohan, D.; Sadhukha, A.; Maayan, G. A metallopeptoid as an efficient bioinspired cooperative catalyst for the aerobic oxidative synthesis of imines. J. Catal. 2017, 355, 139–144. [Google Scholar] [CrossRef]
- Zborovsky, L.; Smolyakova, A.; Baskin, M.; Maayan, G. A pure polyproline type I-like peptoid helix by metal coordination. Chem. A Eur. J. 2018, 24, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Baskin, M.; Maayan, G. A rationally designed metal-binding helical peptoid for selective recognition processes. Chem. Sci. 2016, 7, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.W.; Sanborn, T.J.; Huang, K.; Zuckermann, R.N.; Barron, A.E. Peptoid oligomers with α-chiral, aromatic side chains: Sequence requirements for the formation of stable peptoid helices. J. Am. Chem. Soc. 2001, 123, 6778–6784. [Google Scholar] [CrossRef] [PubMed]
- Person, R.V.; Monde, K.; Humpf, H.U.; Berova, N.; Nakanishi, K. A new approach in exciton-coupled circular dichroism (ECCD)-insertion of an auxiliary stereogenic center. Chirality 1995, 7, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Pubchem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/1049#section=Top (accessed on 19 October 2018).
- Bryantsev, V.S.; Diallo, M.S.; Goddard, W.A. pKa calculations of aliphatic amines, diamines, and aminoamides via density functional theory with a poisson−boltzmann continuum solvent model. J. Phys. Chem. A 2007, 111, 4422–4430. [Google Scholar] [CrossRef] [PubMed]
- Hall, H.K., Jr. Correlation of the base strength of amines. J. Am. Chem. 1957, 79, 5441–5444. [Google Scholar] [CrossRef]
- Lee, C.H.; Yoon, H.; Jang, W.D. Biindole-bridged porphyrin dimer as allosteric molecular tweezers. Chem. A Eur. J. 2009, 15, 9972–9976. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Tanasova, M.; Vasileiou, C.; Borhan, B. Fluorinated porphyrin tweezer: A powerful reporter of absolute configuration for erythro and threo diols, amino alcohols, and diamines. J. Am. Chem. Soc. 2008, 130, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Besouw, M.; Masereeuw, R.; Van Den Heuvel, L.; Levtchenko, E. Cysteamine: An old drug with new potential. Drug Discov. Today 2013, 18, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Kepp, K.P. A quantitative scale of oxophilicity and thiophilicity. Inorg. Chem. 2016, 55, 9461–9470. [Google Scholar] [CrossRef] [PubMed]
- Thordarson, P. Determining association constants from titration experiments in supramolecular chemistry. Chem. Soc. Rev. 2011, 40, 1305–1323. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds, MPPCs (1–3) are available from the authors. |
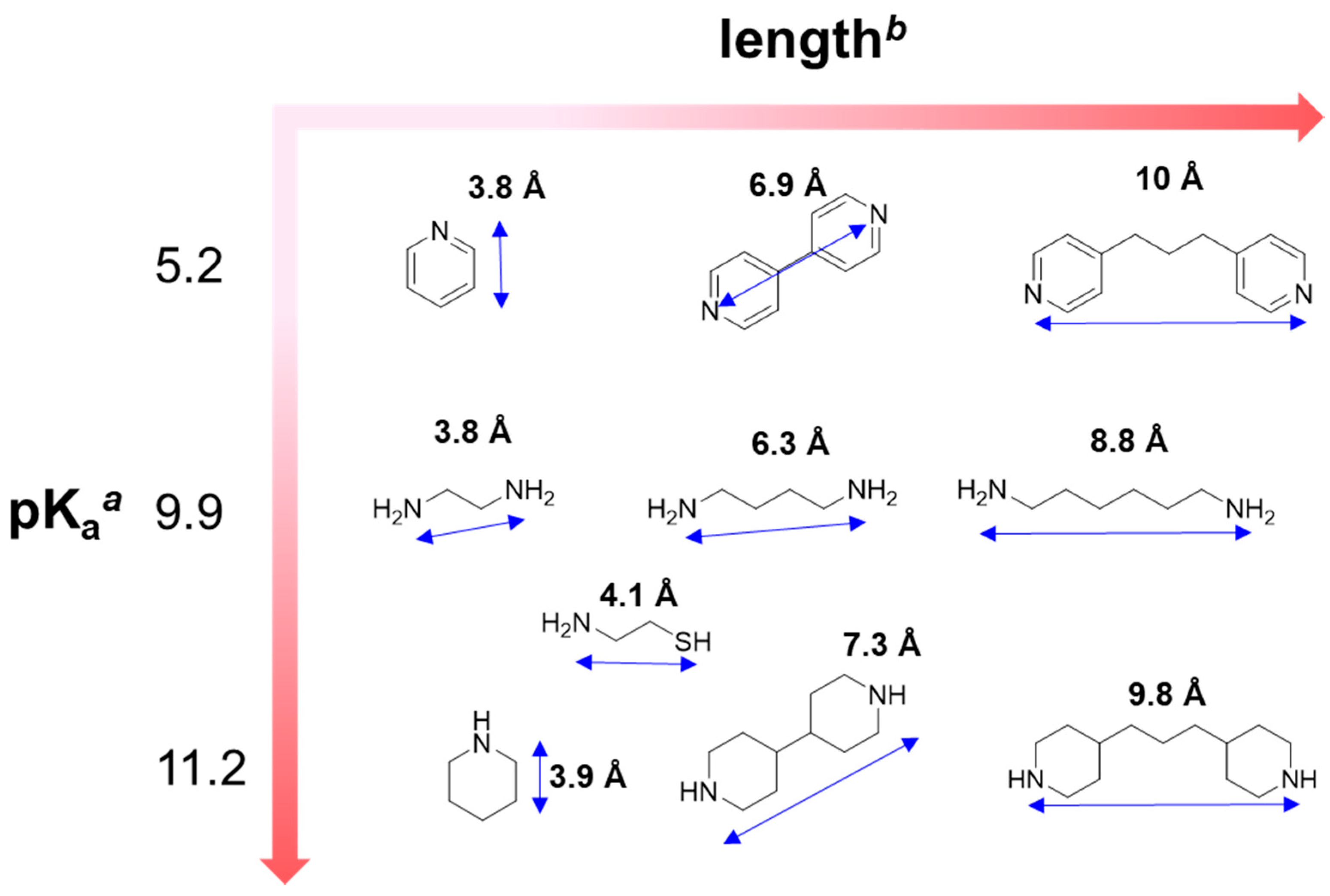
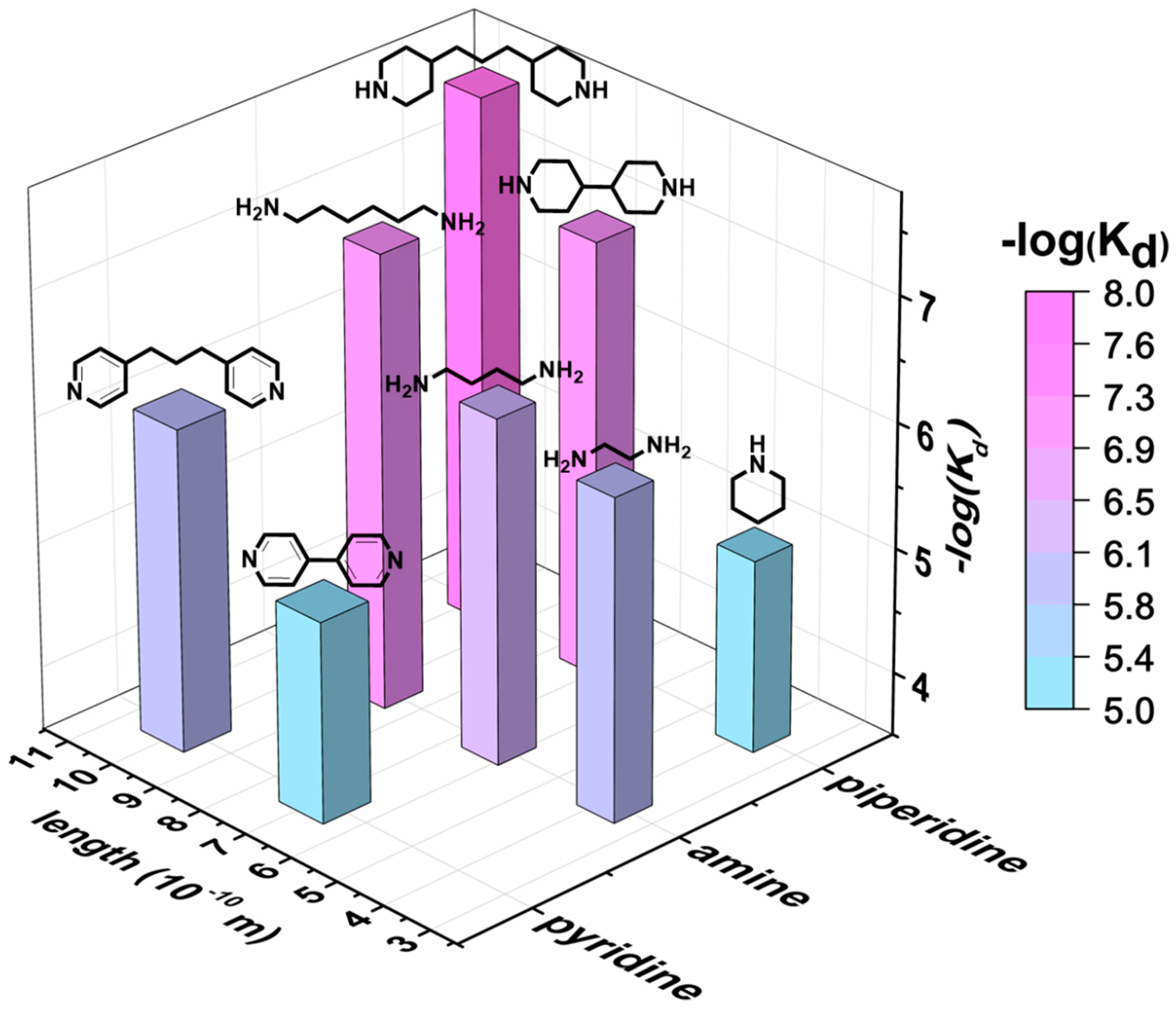
| Guests | Length (Å) | Kd (10−6 M) | R2 | ECCD λmax/λmin (nm) a | UV-vis λmax (nm) a |
|---|---|---|---|---|---|
| Without guest | - | - | - | 430/423 | 427 |
| Pyridine b | 3.8 | 1.5 | 0.84 | 430/423 | 428 |
| 4,4′-Dipyridyl | 6.9 | 7.9 | 0.99 | Nd c | 427 |
| 4,4′-Trimethylenedipyridine | 10 | 0.83 | 0.98 | 433/427 | 427 |
| Ethylenediamine | 3.8 | 0.83 | 0.94 | Nd c | 424 |
| 1,4-Diaminobutane | 6.3 | 0.55 | 0.99 | 434/423 | 426 |
| 1,6-Diaminohexane | 8.8 | 0.074 | 0.99 | 435/427 | 427 |
| Piperidine | 3.9 | 9.2 | 0.99 | 434/425 | 430 |
| 4,4′-Bipiperidine | 7.3 | 0.10 | 0.99 | Nd c | 427 |
| 4,4′-Trimethylenedipiperidine | 9.8 | 0.019 | 0.99 | 435/428 | 429 |
| Cysteamine | 4.1 | 0.95 | 0.99 | 435/427 | 427 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.J.; Kang, B.; Seo, J. Metalloporphyrin Dimers Bridged by a Peptoid Helix: Host-Guest Interaction and Chiral Recognition. Molecules 2018, 23, 2741. https://doi.org/10.3390/molecules23112741
Lee YJ, Kang B, Seo J. Metalloporphyrin Dimers Bridged by a Peptoid Helix: Host-Guest Interaction and Chiral Recognition. Molecules. 2018; 23(11):2741. https://doi.org/10.3390/molecules23112741
Chicago/Turabian StyleLee, Yen Jea, Boyeong Kang, and Jiwon Seo. 2018. "Metalloporphyrin Dimers Bridged by a Peptoid Helix: Host-Guest Interaction and Chiral Recognition" Molecules 23, no. 11: 2741. https://doi.org/10.3390/molecules23112741
APA StyleLee, Y. J., Kang, B., & Seo, J. (2018). Metalloporphyrin Dimers Bridged by a Peptoid Helix: Host-Guest Interaction and Chiral Recognition. Molecules, 23(11), 2741. https://doi.org/10.3390/molecules23112741