Combinative Scouring, Bleaching, and Cationization Pretreatment of Greige Knitted Cotton Fabrics for Facilely Achieving Salt-Free Reactive Dyeing
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Scouring and Bleaching Pretreatment
2.2. Optimization of the Cationization Conditions in Combinative Pretreatment
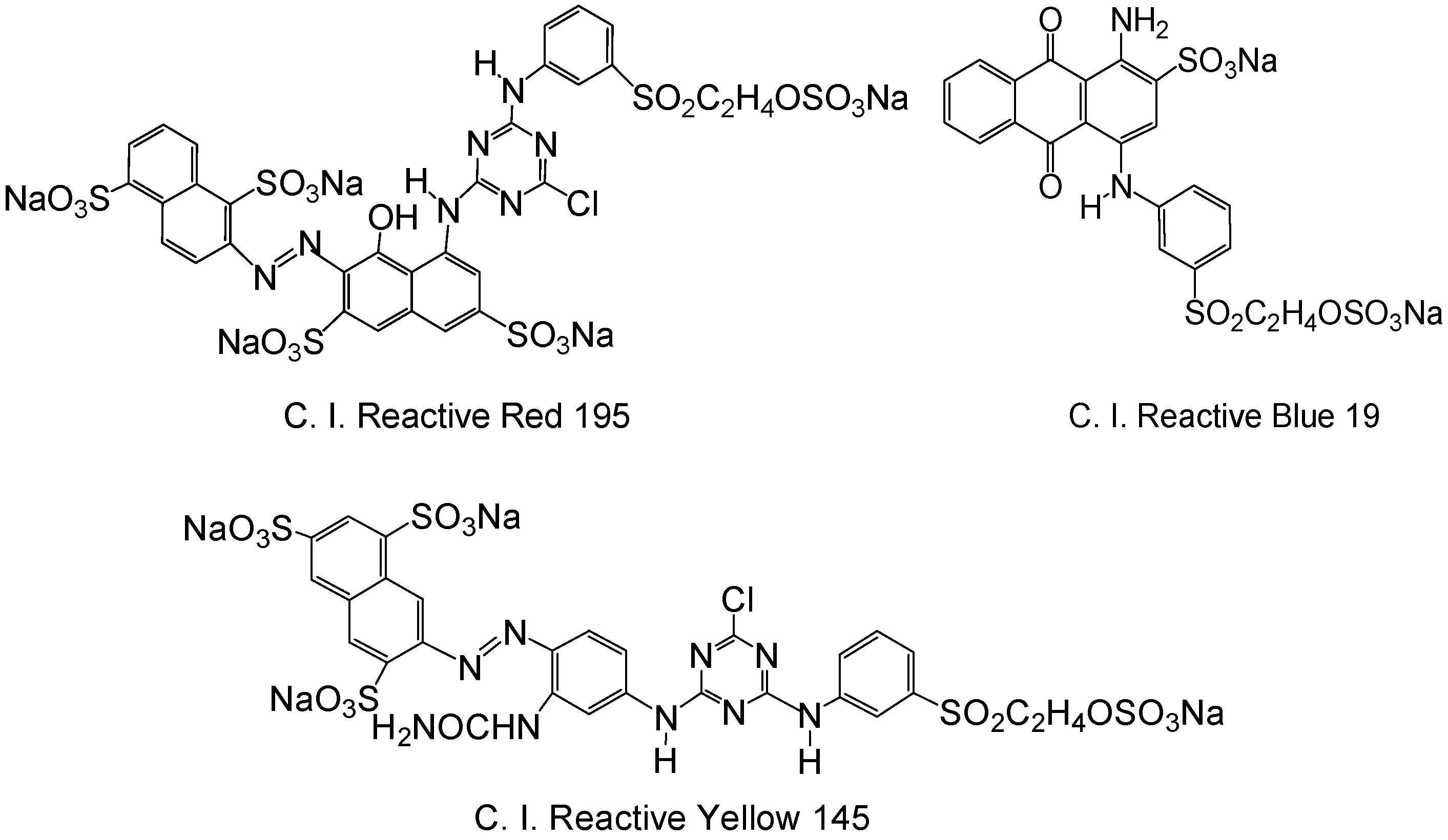
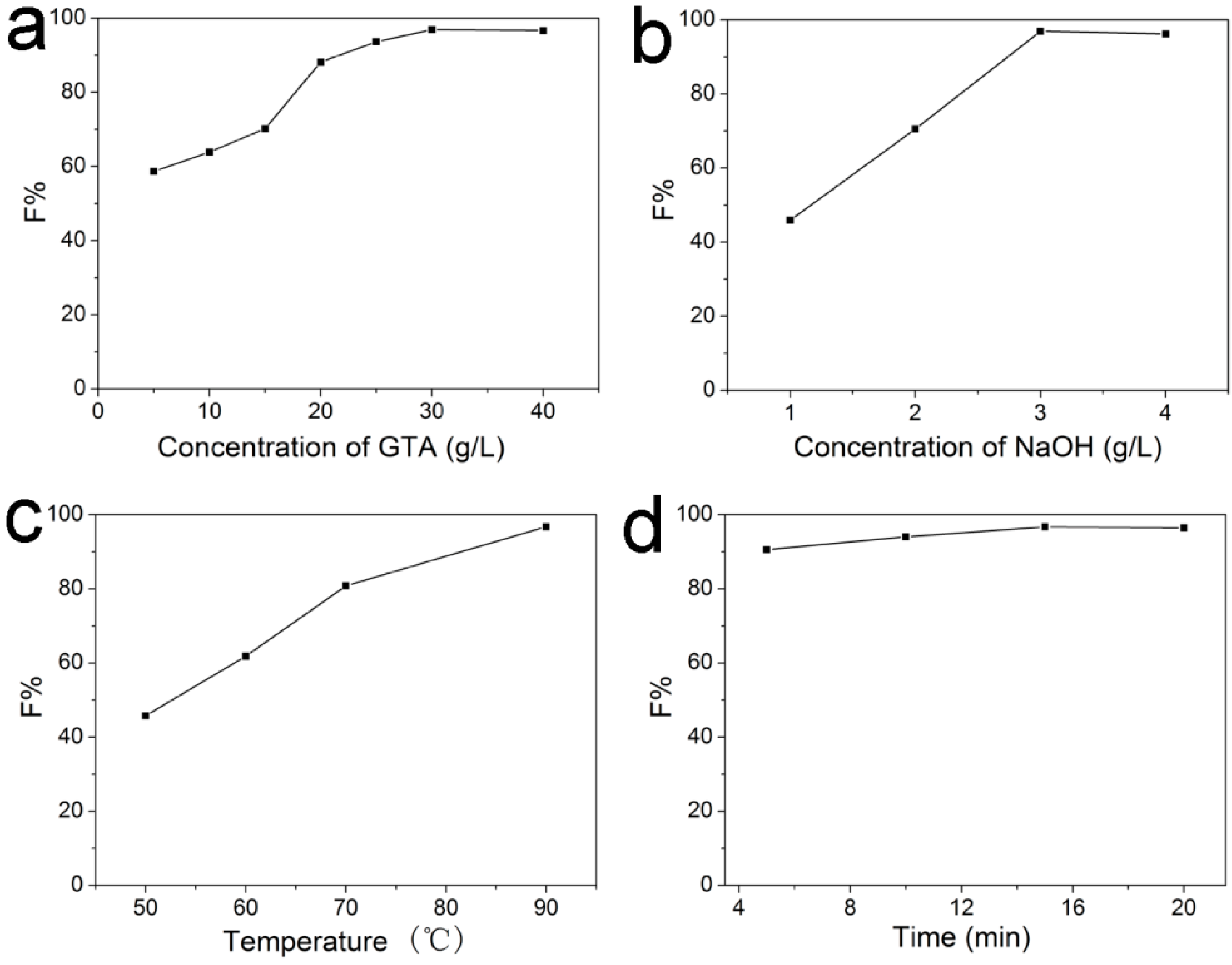
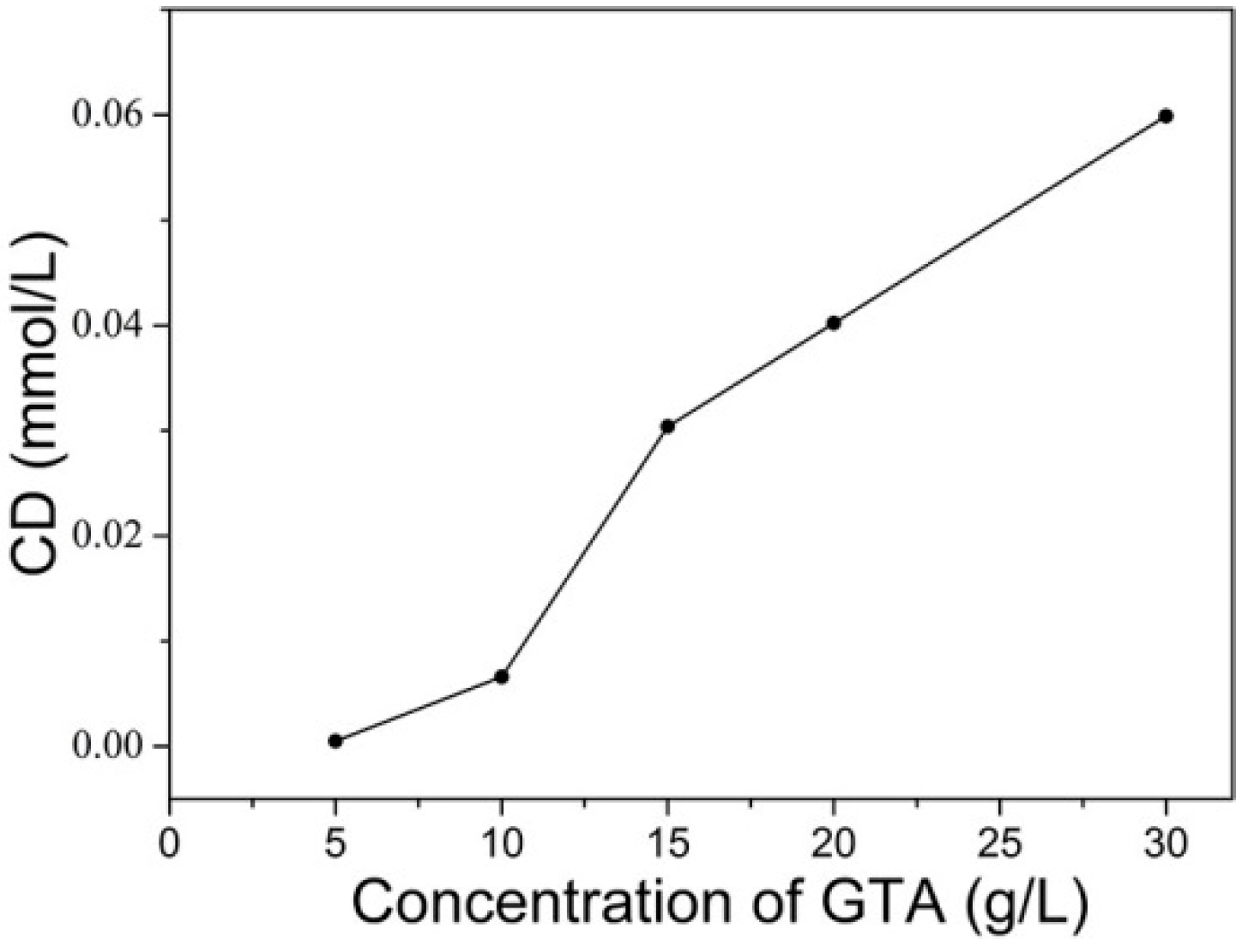
2.2.1. Effect of GTA Concentration on F% of C. I. Reactive Red 195 on Cationic Cotton
2.2.2. Effect of NaOH Concentration on F% of C. I. Reactive Red 195 on Cationic Cotton
2.2.3. Effect of Cationization Temperature on F% of C. I. Reactive Red 195 on Cationic Cotton
2.2.4. Effect of Cationization Time on F% of C. I. Reactive Red 195 on Cationic Cotton
2.3. Evaluation of Fiber Properties after Pretreatment
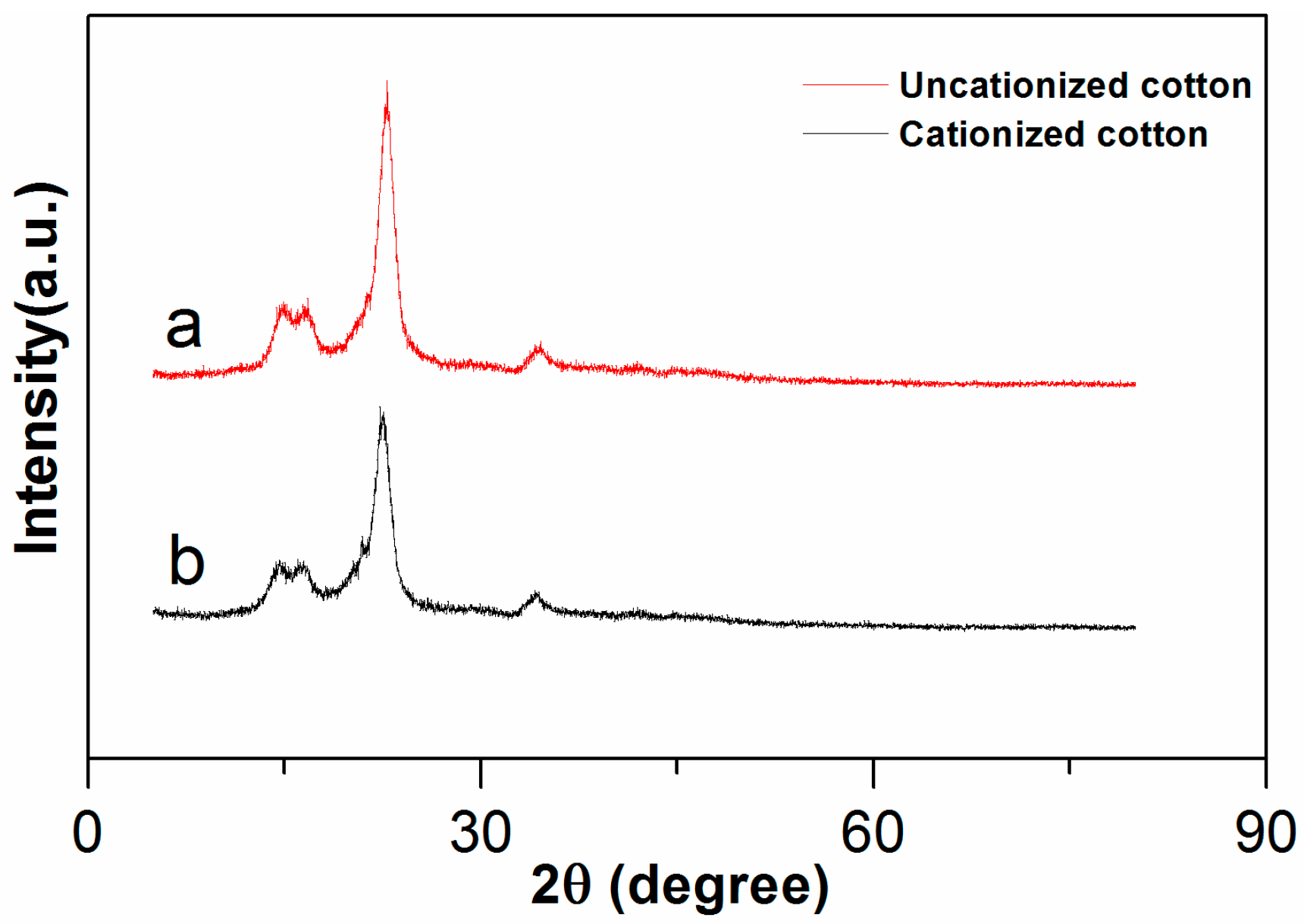
2.3.1. X-ray Diffraction Analysis (XRD)
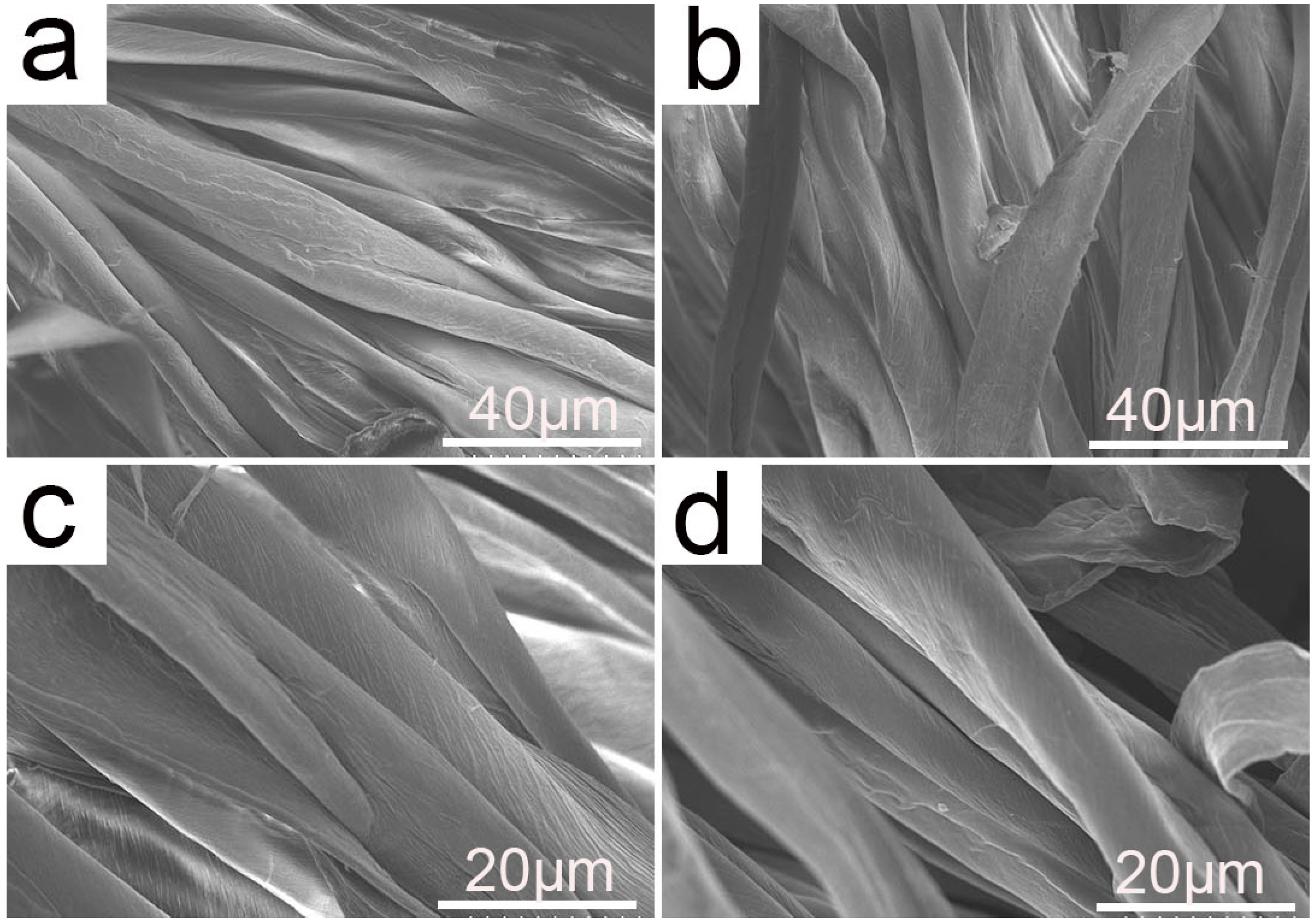
2.3.2. Surface Morphology
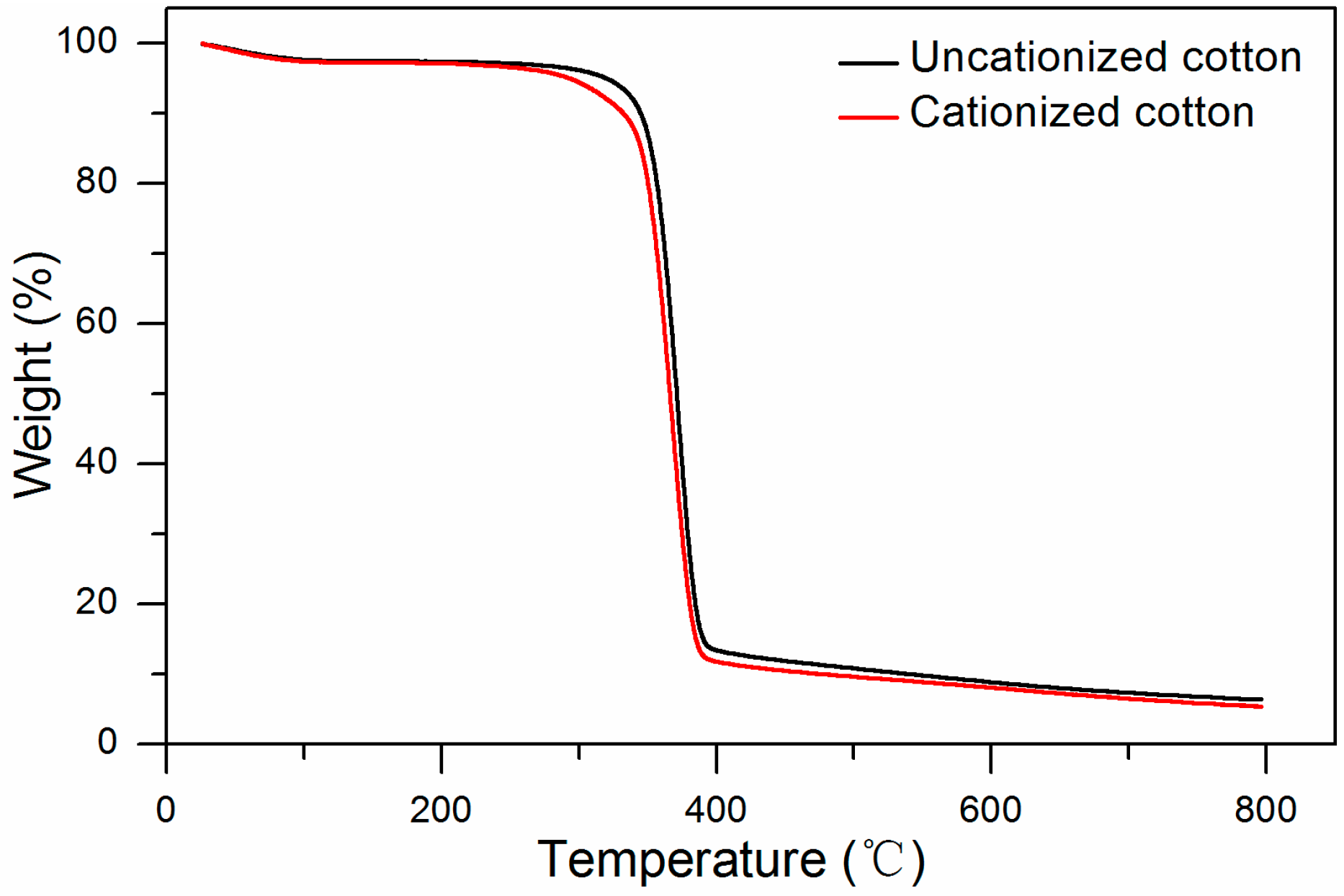
2.3.3. Determination of Thermal Properties
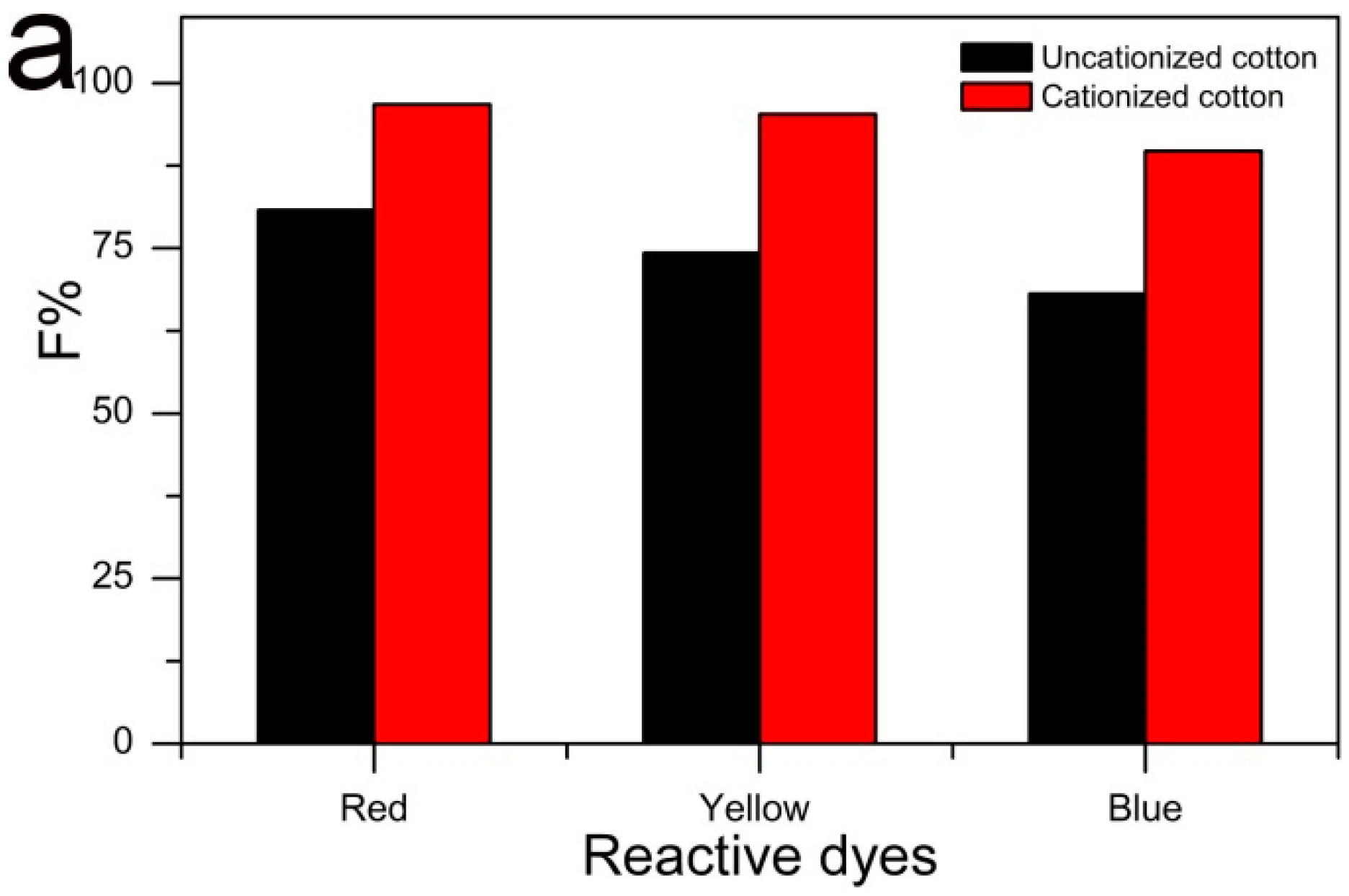
2.4. Comparison of F% on Cationized and Uncationized Cotton
2.5. Color Strength and Colorfastness Properties
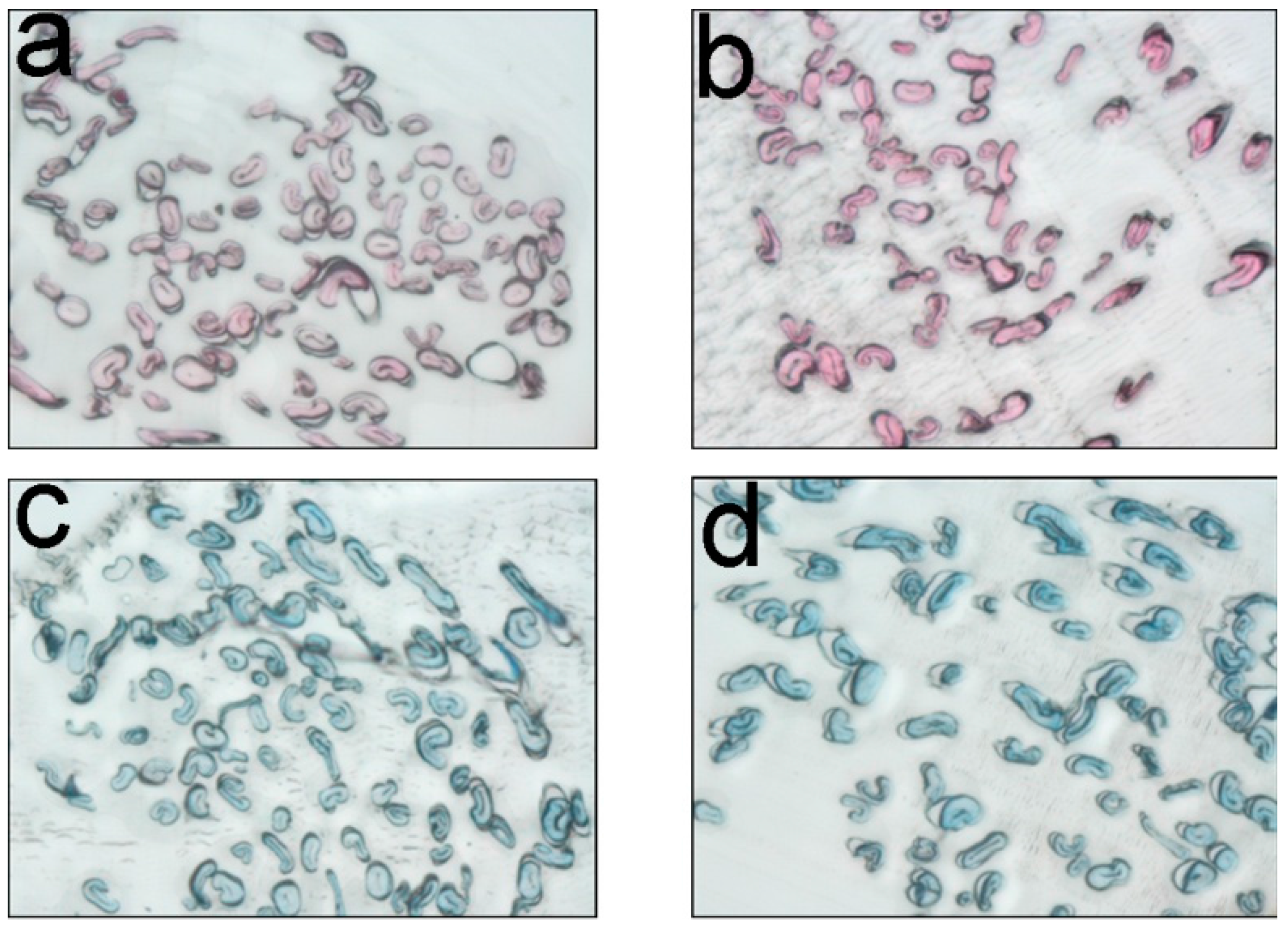
2.6. Determination of Dye Penetrability
3. Materials and Methods
3.1. Materials
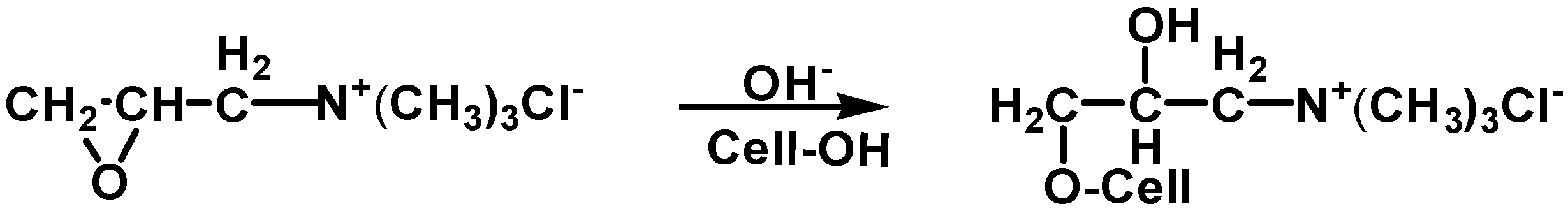
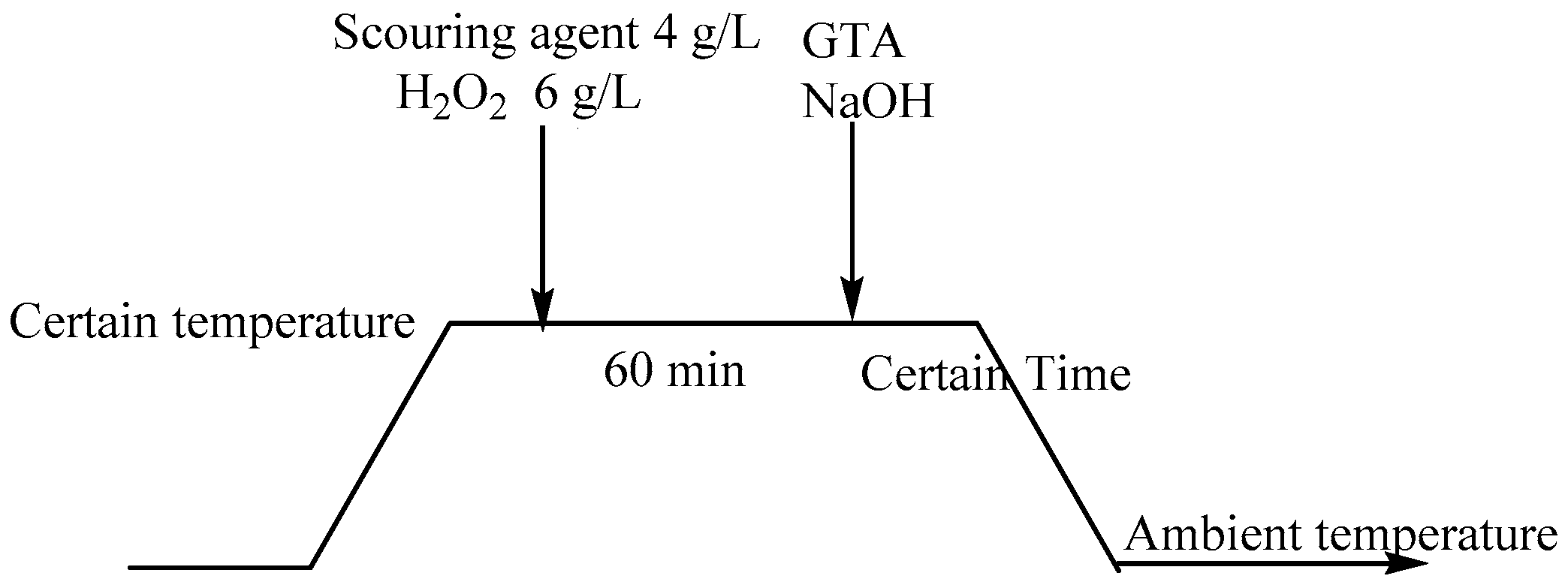
3.2. Combinative Pretreatment of Scouring, Bleaching, and Cationization
3.3. Dyeing Procedures
3.4. Whiteness Test
3.5. Water Absorptivity and Diffusion Time
3.6. Capillarity Effect Test
3.7. Determination of Nitrogen Content and Cationization Degree
3.8. X-ray Diffraction
3.9. Scanning Electronic Microscope
3.10. Thermogravimetric Analysis
3.10.1. Color Strength (K/S) and Dye Fixation (F%) Measurement
3.10.2. Colorfastness Testing
3.10.3. Microscopic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Patino, A.; Canal, C.; Rodriguez, C.; Caballero, G.; Navarro, A.; Canal, J.M. Surface and bulk cotton fibre modifications: Plasma and cationization. Influence on dyeing with reactive dye. Cellulose 2011, 18, 1073–1083. [Google Scholar] [CrossRef]
- Soleimani-Gorgani, A.; Karami, Z. The effect of biodegradable organic acids on the improvement of cotton ink-jet printing and antibacterial activity. Fibers Polym. 2016, 17, 512–520. [Google Scholar] [CrossRef]
- Teng, X.X.; Zhang, S.F.; Ma, W. Application of a hydrolyzable cationic agent, poly(acryloxyethyl trimethylammonium chloride), in salt-free reactive dyeing for good dyeing properties. J. Appl. Polym. Sci. 2011, 122, 2741–2748. [Google Scholar] [CrossRef]
- Wang, L.L.; Ma, W.; Zhang, S.F.; Teng, X.X.; Yang, J.Z. Preparation of cationic cotton with two-bath pad-bake process and its application in salt-free dyeing. Carbohydr. Polym. 2009, 78, 602–608. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Wang, C.X.; Chen, K.L.; Yin, Y.J. Improvement of ink-jet printing performances using beta-cyclodextrin forming inclusion complex on cotton fabric. Fibers Polym. 2017, 18, 619–624. [Google Scholar] [CrossRef]
- Rana, M.; Chen, J.-T.; Yang, S.; Ma, P.-C. Biomimetic superoleophobicity of cotton fabrics for efficient oil-water separation. Adv. Mater. Interfaces 2016, 3, 1600128. [Google Scholar] [CrossRef]
- Raza, Z.A.; Rehman, A.; Hussain, M.T.; Masood, R.; Ul Haq, A.; Saddique, M.T.; Javid, A.; Ahmad, N. Production of rhamnolipid surfactant and its application in bioscouring of cotton fabric. Carbohydr. Res. 2014, 391, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Liu, J.; Gao, W. Effects of snailase treatment on wettability of raw cotton yarns in pre-wetting process of foam sizing. Appl. Biochem. Biotechnol. 2017, 182, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S.R. Chemical Technology in the Pre-Treatment Processes of Textiles; Elsevier: Amsterdam, The Netherlands, 1999; Volume 12. [Google Scholar]
- Kolářová, K.; Vosmanská, V.; Rimpelová, S.; Švorčík, V. Effect of plasma treatment on cellulose fiber. Cellulose 2013, 20, 953–961. [Google Scholar] [CrossRef]
- Bashar, M.M.; Siddiquee, M.A.; Khan, M.A. Preparation of cotton knitted fabric by gamma radiation: A new approach. Carbohydr. Polym. 2015, 120, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, E.S. Simple and economic bleaching process for cotton fabric. Carbohydr. Polym. 2012, 88, 1233–1238. [Google Scholar] [CrossRef]
- Yin, C.; Huang, Y.; Zhang, L.; Xu, H.; Zhong, Y.; Mao, Z. Low-temperature bleaching of cotton fabric using a copper-based catalyst for hydrogen peroxide. Color. Technol. 2015, 131, 66–71. [Google Scholar] [CrossRef]
- Lēwîn, M. Chemical Processing of Fibers and Fabrics: Fundamentals and Preparation Part A; Marcel Dekker: New York, NY, USA, 1983. [Google Scholar]
- Lewin, M. Handbook of Fiber science and Technology Volume 2: Chemical Processing of Fibers and Fabrics—Functional Finishes Part B; Marcel Dekker: New York, NY, USA, 1984; Volume 2. [Google Scholar]
- Wakelyn, P.; Bertoniere, N.; French, A.; Zeronian, S.; Nevell, T.; Thibodeaux, D.; Blanchard, E.; Calamari, T.; Triplett, B.; Bragg, C. Cotton fibers. In Handbook of Fiber Chemistry; Menachem, L., Pearce, E.M., Eds.; Marcel Dekker: New York, NY, USA, 1998. [Google Scholar]
- Preša, P.; Tavčer, P.F. Bioscouring and bleaching of cotton with pectinase enzyme and peracetic acid in one bath. Color. Technol. 2008, 124, 36–42. [Google Scholar] [CrossRef]
- Zeronian, S.; Inglesby, M. Bleaching of cellulose by hydrogen peroxide. Cellulose 1995, 2, 265–272. [Google Scholar] [CrossRef]
- Xu, C.; Hinks, D.; El-Shafei, A.; Hauser, P.; Li, M.; Ankeny, M.; Lee, K. Review of bleach activators for environmentally efficient bleaching of textiles. J. Fiber Bioeng. Inf. 2011, 4, 209–219. [Google Scholar] [CrossRef]
- Blackburn, R.S.; Burkinshaw, S.M. A greener approach to cotton dyeings with excellent wash fastness. Green Chem. 2002, 4, 47–52. [Google Scholar] [CrossRef]
- Allegre, C.; Moulin, P.; Maisseu, M.; Charbit, F. Treatment and reuse of reactive dyeing effluents. J. Membr. Sci. 2006, 269, 15–34. [Google Scholar] [CrossRef]
- Xie, K.; Cheng, F.; Zhao, W.; Xu, L. Micelle dyeing with low liquor ratio for reactive dyes using dialkyl maleic acid ester surfactants. J. Clean. Prod. 2011, 19, 332–336. [Google Scholar] [CrossRef]
- Wang, H.; Lewis, D. Chemical modification of cotton to improve fibre dyeability. Color. Technol. 2002, 118, 159–168. [Google Scholar] [CrossRef]
- Montazer, M.; Malek, R.M.A.; Rahimi, A. Salt free reactive dyeing of cationized cotton. Fiber Polym. 2007, 8, 608–612. [Google Scholar] [CrossRef]
- Ma, W.; Meng, M.; Yan, S.M.; Zhang, S.F. Salt-free reactive dyeing of betaine-modified cationic cotton fabrics with enhanced dye fixation. Chin. J. Chem. Eng. 2016, 24, 175–179. [Google Scholar] [CrossRef]
- Hauser, P.J.; Tabba, A.H. Dyeing cationic cotton with fiber reactive dyes: Effect of reactive chemistries. AATCC Rev. 2002, 2, 36–39. [Google Scholar]
- Acharya, S.; Abidi, N.; Rajbhandari, R.; Meulewaeter, F. Chemical cationization of cotton fabric for improved dye uptake. Cellulose 2014, 21, 4693–4706. [Google Scholar] [CrossRef]
- Hebeish, A.; Hashem, M.; Shaker, N.; Ramadan, M.; El-Sadek, B.; Hady, M.A. New development for combined bioscouring and bleaching of cotton-based fabrics. Carbohydr. Polym. 2009, 78, 961–972. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mokhtar, S.M. Chemically induced graft copolymerization of itaconic acid onto cellulose fibers. Polym. Test. 2002, 21, 337–343. [Google Scholar] [CrossRef]
- Alimohammadi, F.; Gashti, M.P.; Shamei, A.; Kiumarsi, A. Deposition of silver nanoparticles on carbon nanotube by chemical reduction method: Evaluation of surface, thermal and optical properties. Superlattice Microstruct. 2012, 52, 50–62. [Google Scholar] [CrossRef]
- Cabrales, L.; Abidi, N. On the thermal degradation of cellulose in cotton fibers. J. Therm. Anal. Calorim. 2010, 102, 485–491. [Google Scholar] [CrossRef]
- Tian, L.; Branford-White, C.; Wang, W.; Nie, H.; Zhu, L. Laccase-mediated system pretreatment to enhance the effect of hydrogen peroxide bleaching of cotton fabric. Int. J. Biol. Macromol. 2012, 50, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Forte Tavčer, P. Impregnation and exhaustion bleaching of cotton with peracetic acid. Text. Res. J. 2009, 80, 3–11. [Google Scholar] [CrossRef]
Sample Availability: All dyed fabrics of this paper are available from the authors. |

| Time (min) | Whiteness |
|---|---|
| 30 | 68.07 |
| 45 | 73.63 |
| 60 | 77.04 |
| 75 | 76.54 |
| Dye | Fabrics | K/S | Wash Fastness | Rub Fastness | Light Fastness | |||
|---|---|---|---|---|---|---|---|---|
| Change | Staining | Dry | Wet | |||||
| Cotton | Wool | |||||||
| C. I. Reactive Red 195 | Uncationized | 13.0 | 5 | 4–5 | 4–5 | 4–5 | 3–4 | 3–4 |
| Cationized | 14.7 | 4–5 | 4–5 | 4–5 | 4–5 | 3–4 | 4 | |
| C. I. Reactive Yellow 145 | Uncationized | 5.3 | 4–5 | 4–5 | 4–5 | 4–5 | 4–5 | 6–7 |
| Cationized | 7.2 | 4–5 | 4–5 | 4–5 | 5 | 4–5 | 6–7 | |
| C. I. Reactive Blue 19 | Uncationized | 15.5 | 4–5 | 4–5 | 3 | 4–5 | 3–4 | 7 |
| Cationized | 18.5 | 4–5 | 4 | 3 | 4–5 | 4 | 7 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, W.; Shen, K.; Xiang, N.; Zhang, S. Combinative Scouring, Bleaching, and Cationization Pretreatment of Greige Knitted Cotton Fabrics for Facilely Achieving Salt-Free Reactive Dyeing. Molecules 2017, 22, 2235. https://doi.org/10.3390/molecules22122235
Ma W, Shen K, Xiang N, Zhang S. Combinative Scouring, Bleaching, and Cationization Pretreatment of Greige Knitted Cotton Fabrics for Facilely Achieving Salt-Free Reactive Dyeing. Molecules. 2017; 22(12):2235. https://doi.org/10.3390/molecules22122235
Chicago/Turabian StyleMa, Wei, Kezhan Shen, Nan Xiang, and Shufen Zhang. 2017. "Combinative Scouring, Bleaching, and Cationization Pretreatment of Greige Knitted Cotton Fabrics for Facilely Achieving Salt-Free Reactive Dyeing" Molecules 22, no. 12: 2235. https://doi.org/10.3390/molecules22122235
APA StyleMa, W., Shen, K., Xiang, N., & Zhang, S. (2017). Combinative Scouring, Bleaching, and Cationization Pretreatment of Greige Knitted Cotton Fabrics for Facilely Achieving Salt-Free Reactive Dyeing. Molecules, 22(12), 2235. https://doi.org/10.3390/molecules22122235




