One-Pot Lipase-Catalyzed Enantioselective Synthesis of (R)-(−)-N-Benzyl-3-(benzylamino)butanamide: The Effect of Solvent Polarity on Enantioselectivity
Abstract
:1. Introduction
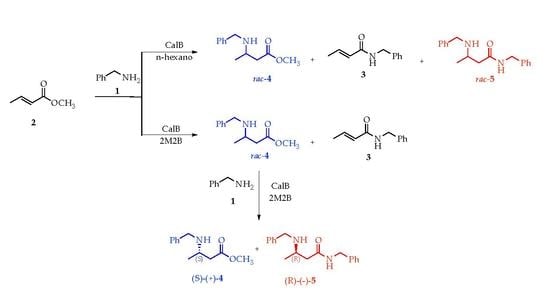
2. Results and Discussions
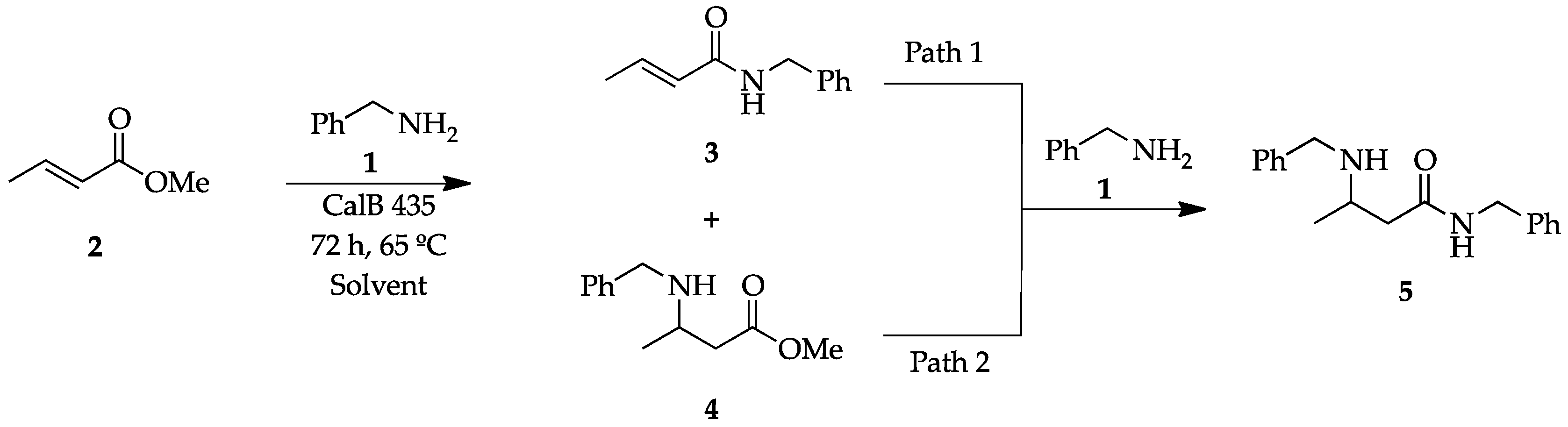
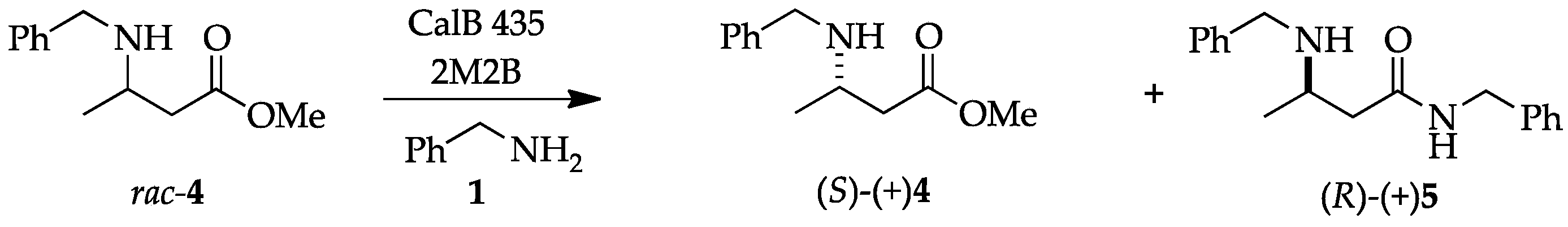
2.1. Profile of Products Obtained in Solvents of Opposite Polarity: n-Hexane and 2M2B
2.2. Enantioselectivity
2.3. Optimization of Enantiomeric Excess
3. Materials and Methods
3.1. Analytical Methods
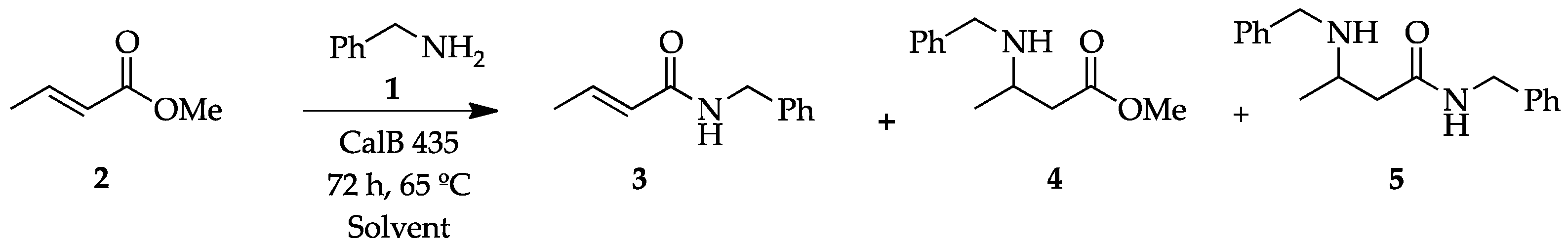
3.2. Chemistry
3.2.1. Enzymatic Reactions
3.2.2. Synthesis of rac-Methyl 3-(benzylamino)butanoate, rac-4 was Prepared According to the Literature
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Spiteller, P.; von Nussbaum, F. Enantioselective Synthesis of β-Amino Acids, 2nd ed.; Juaristi, E., Soloshonok, V.A., Eds.; Wiley-VCH: New York, NY, USA, 2005; pp. 19–91. ISBN 978-0-471-46738-0. [Google Scholar]
- Ballard, C.E.; Yu, H.; Wang, B. Recent Developments in Depsipeptide Research. Curr. Med. Chem. 2002, 9, 471–498. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Sibi, M.P. Recent advances in the stereoselective synthesis of β-amino acids. Tetrahedron 2002, 58, 7991–8035. [Google Scholar] [CrossRef]
- Li, X.G.; Kanerva, L.T. Lipases in β-Dipeptide Synthesis in Organic Solvents. Org. Lett. 2006, 8, 5593–5596. [Google Scholar] [CrossRef] [PubMed]
- Steer, D.L.; Lew, R.A.; Perlmutter, P.; Smith, A.I.; Aguilar, M.I. β-Amino Acids: Versatile Peptidomimetics. Curr. Med. Chem. 2002, 9, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Liljeblad, A.; Kanerva, L.T. Biocatalysis as a profound tool in the preparation of highly enantiopure β-amino acids. Tetrahedron 2006, 62, 5831–5854. [Google Scholar] [CrossRef]
- Ghanem, A.; Aboul-Enein, H.Y. Lipase-mediated chiral resolution of racemates in organic solvents. Tetrahedron Asymmetry 2004, 15, 3331–3351. [Google Scholar] [CrossRef]
- Zaks, A. New enzymatic properties in organic media. In Enzymatic Reactions in Organic Media, 2nd ed.; Koskinen, A.M.P., Klibanov, A.M., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 70–93. ISBN 978-94-011-0611-5. [Google Scholar]
- Priego, J.; Ortíz-Nava, C.; Carrillo-Morales, M.; López-Munguía, A.; Escalante, J.; Castillo, E. Solvent engineering: An effective tool to direct chemoselectivity in a lipase-catalyzed Michael addition. Tetrahedron 2009, 65, 536–539. [Google Scholar] [CrossRef]
- Steunenberg, P.; Sijm, M.; Zuilhof, H.; Sanders, J.P.M.; Scott, E.L.; Franssen, M.C.R. Lipase-Catalyzed Aza-Michael Reaction on Acrylate Derivatives. J. Org. Chem. 2013, 78, 3802–3813. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Ramírez, J.D.; Escalante, J.; Lopez-Murguía, A.; Marty, A.; Castillo, E. Thermodynamically controlled chemoselectivity in lipase-catalyzed aza-Michael additions. Mol. Catal. B: Enzym. 2015, 112, 76–82. [Google Scholar] [CrossRef]
- Dhake, K.P.; Tambade, P.J.; Singhal, R.S.; Bhanage, B.M. Promiscuous Candida antarctica lipase B-catalyzed synthesis of β-amino esters via aza-Michael addition of amines to acrylates. Tetrahedron Lett. 2010, 51, 4455–4458. [Google Scholar] [CrossRef]
- Carlqvist, P.; Svedendahl, M.; Branneby, C.; Hult, K.; Brinck, T.; Berglund, P. Exploring the Active-Site of a Rationally Redesigned Lipase for Catalysis of Michael-Type Additions. ChemBioChem 2005, 6, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Nagy, B.; Galla, Z.; Bencze, L.C.; Toșa, M.I.; Paizs, C.; Forró, E.; Fülöp, F. Covalently Immobilized Lipases are Efficient Stereoselective Catalysts for the Kinetic Resolution of rac-(5-Phenylfuran-2-yl)-β-alanine Ethyl Ester Hydrochlorides. Eur. J. Org. Chem. 2017, 20, 2878–2882. [Google Scholar] [CrossRef]
- Sanchez, V.M.; Rebolledo, F.; Gotor, V. Candida antarctica lipase catalyzed resolution of ethyl (±)-3- aminobutyrate. Tetrahedron Asymmetry 1997, 8, 37–40. [Google Scholar] [CrossRef]
- Fitz, M.; Forró, E.; Vigóczki, E.; Lázár, L.; Fülöp, F. Lipase-catalysed N-acylation of β2-amino esters. Tetrahedron Asymmetry 2008, 19, 1114–1119. [Google Scholar] [CrossRef]
- Rangel, H.; Carrillo-Morales, M.; Galindo, J.M.; Obregón-Zúñiga, A.; Juaristi, E.; Escalante, J. Structural features of N-benzylated-β-amino acid methyl esters essential for enantiodifferentiation by lipase B from Candida antarctica in hydrolytic reactions. Tetrahedron Asymmetry 2015, 26, 325–332. [Google Scholar] [CrossRef]
- Weiβ, M.; Gröger, H. Practical, Highly Enantioselective Chemoenzymatic One-Pot Synthesis of Short-Chain Aliphatic β-Amino Acid Esters. Synlett 2009, 8, 1251–1254. [Google Scholar] [CrossRef]
- Flores-Sánchez, P.; Escalante, J.; Castillo, E. Enzymatic resolution of N-protected-β3-amino methyl esters, using lipase B from Candida antarctica. Tetrahedron Asymmetry 2005, 16, 629–634. [Google Scholar] [CrossRef]
- Strompen, S.; Weiss, M.; Gröger, H.; Hilterhaus, L.; Liese, A. Development of a Continuously Operating Process for the Enantioselective Synthesis of a β-Amino Acid Ester via a Solvent-Free Chemoenzymatic Reaction Sequence. Adv. Synth. Catal. 2013, 355, 2391–2399. [Google Scholar] [CrossRef]
- Strompen, S.; Weiβ, M.; Ingram, T.; Smirnova, I.; Gröger, H.; Hilterhaus, L.; Liese, A. Kinetic Investigation of a Solvent-Free, Chemoenzymatic Reaction Sequence Towards Enantioselective Synthesis of a β-Amino Acid Ester. Biotechnol. Bioeng. 2012, 109, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
- Escalante, J.; Carrillo-Morales, M.; Linzaga, I. Michael Additions of Amines to Methyl Acrylates Promoted by Microwave Irradiation. Molecules 2008, 13, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Puertas, S.; Brieva, R.; Rebolledo, F.; Gotor, V. Lipase Catalyzed Aminolysis of Ethyl Propiolate and Acrylic Esters. Synthesis of Chiral Acrylamides. Tetrahedron 1993, 49, 4007–4014. [Google Scholar] [CrossRef]
Sample Availability: Small samples (a few milligrams) of 3, 4 and 5 are available for the authors. |
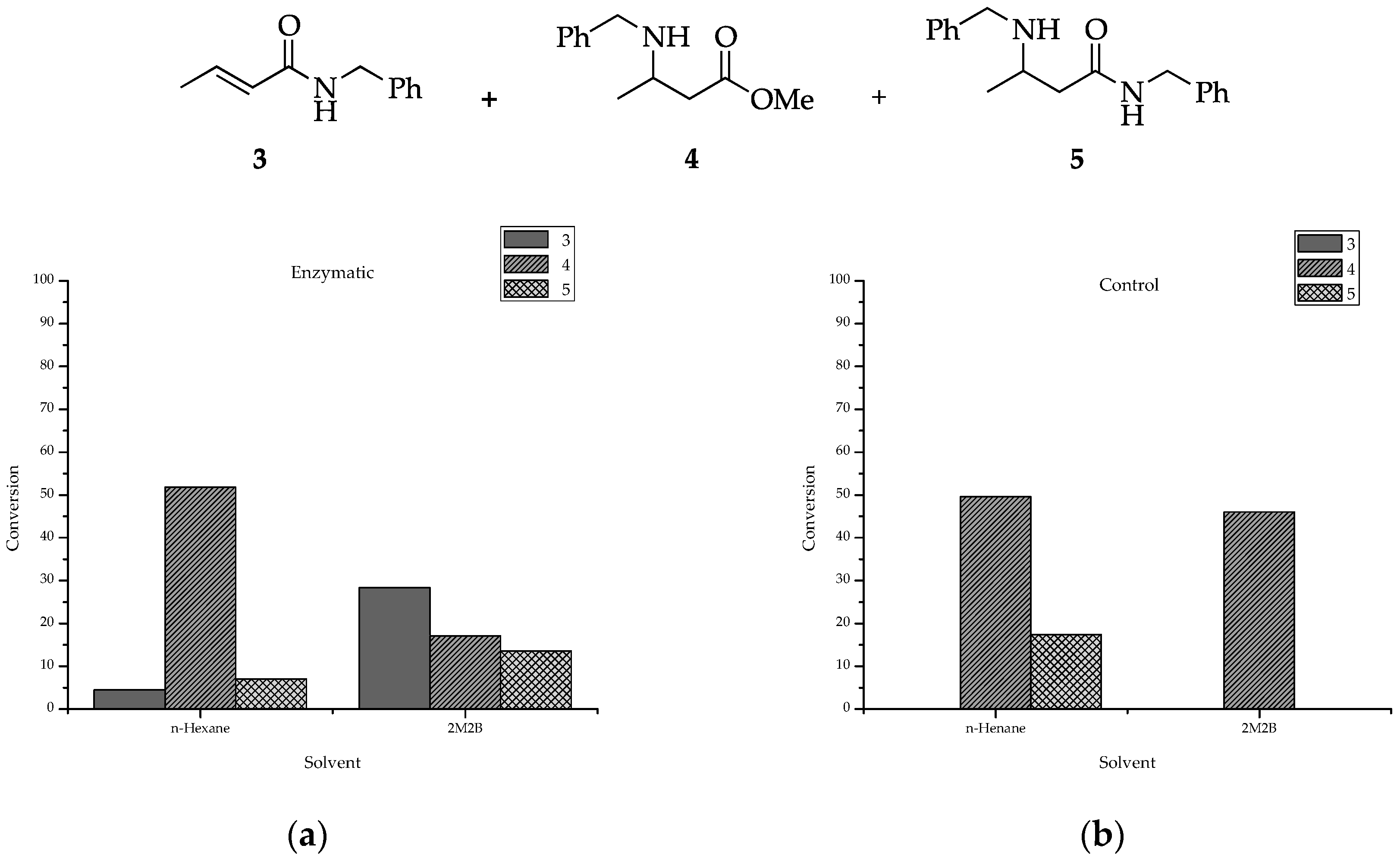
| Entry | Enzyme | Solvent | Conversion a | Proportion (%) b 3/4/5 | 1,4-Addition 4 | Double Add 5 | ||
|---|---|---|---|---|---|---|---|---|
| [α]D c | ee (%) d | [α]D c | ee (%) e | |||||
| 1 | CalB 435 | n-hexane, 100% | 64 | 7/81/12 | +0.3 | 1.1 | -1.4 | 3.4 |
| 2 | Control | n-hexane, 100% | 67 | ND/74/26 | 0 | 0 | 0 | 0 |
| 3 | CalB 435 | 2M2B, 100% | 59 | 48/29/23 | +15.6 | 96 | -19.2 | 72 |
| 4 | Control | 2M2B, 100% | 46 | ND/100/ND | 0 | 0 | 0 | 0 |
| 5 | CalB 435 | n-hexane/2M2B, 50:50 | 67 | 48/29/23 | +11.6 | 79.8 | -20.8 | 67.8 |
| 6 | Control | n-hexane/2M2B, 50:50 | 53 | ND/90/10 | 0 | 0 | 0 | 0 |
| 7 | CalB 435 | n-hexane/2M2B, 90:10 | 84 | 25/28/47 | +7.7 | 75 | -21 | 67 |
| 8 | Control | n-hexane/2M2B, 90:10 | 74 | ND/80/20 | 0 | 0 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Rojas, M.A.; Rivera-Ramírez, J.D.; Ávila-Ortiz, C.G.; Juaristi, E.; González-Muñoz, F.; Castillo, E.; Escalante, J. One-Pot Lipase-Catalyzed Enantioselective Synthesis of (R)-(−)-N-Benzyl-3-(benzylamino)butanamide: The Effect of Solvent Polarity on Enantioselectivity. Molecules 2017, 22, 2189. https://doi.org/10.3390/molecules22122189
Ortega-Rojas MA, Rivera-Ramírez JD, Ávila-Ortiz CG, Juaristi E, González-Muñoz F, Castillo E, Escalante J. One-Pot Lipase-Catalyzed Enantioselective Synthesis of (R)-(−)-N-Benzyl-3-(benzylamino)butanamide: The Effect of Solvent Polarity on Enantioselectivity. Molecules. 2017; 22(12):2189. https://doi.org/10.3390/molecules22122189
Chicago/Turabian StyleOrtega-Rojas, Marina A., José Domingo Rivera-Ramírez, C. Gabriela Ávila-Ortiz, Eusebio Juaristi, Fernando González-Muñoz, Edmundo Castillo, and Jaime Escalante. 2017. "One-Pot Lipase-Catalyzed Enantioselective Synthesis of (R)-(−)-N-Benzyl-3-(benzylamino)butanamide: The Effect of Solvent Polarity on Enantioselectivity" Molecules 22, no. 12: 2189. https://doi.org/10.3390/molecules22122189
APA StyleOrtega-Rojas, M. A., Rivera-Ramírez, J. D., Ávila-Ortiz, C. G., Juaristi, E., González-Muñoz, F., Castillo, E., & Escalante, J. (2017). One-Pot Lipase-Catalyzed Enantioselective Synthesis of (R)-(−)-N-Benzyl-3-(benzylamino)butanamide: The Effect of Solvent Polarity on Enantioselectivity. Molecules, 22(12), 2189. https://doi.org/10.3390/molecules22122189