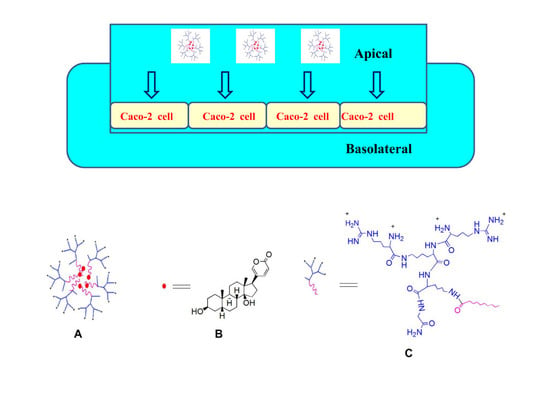
Enhanced Intestinal Permeability of Bufalin by a Novel Bufalin-Peptide-Dendrimer Inclusion through Caco-2 Cell Monolayer
Abstract
:1. Introduction
2. Results
2.1. Cytotoxicity of BPDI on Caco-2 Cells
2.2. Validation of Caco-2 Monolayer Model
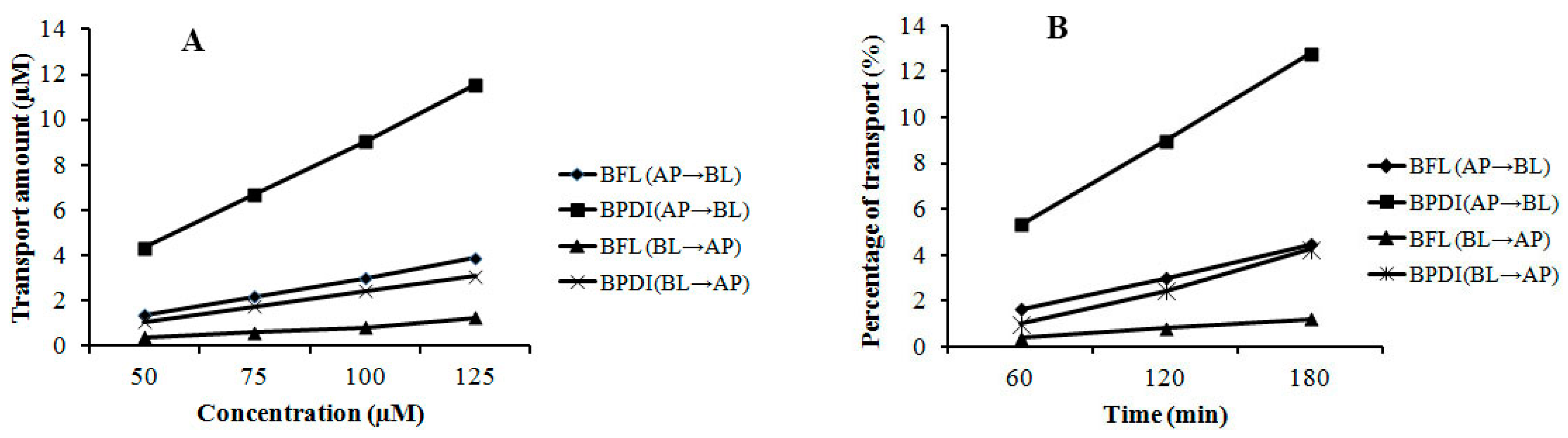
2.3. Transmembrane Transport of BFL and BPDI
2.4. The Validation of HPLC Analytical Methods
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cytotoxicity of BPDI in Caco-2 Cells by MTT Assay
4.3. Transport Experiment of BFL and BPDI through Caco-2 Monolayer
4.3.1. Establishment of Caco-2 Monolayer Model
4.3.2. Transport Experiment
4.4. HPLC Analysis
4.4.1. Chromatographic Conditions
4.4.2. Preparation of BFL Standard Solution and Test Solutions
4.4.3. Validation of HPLC Method
4.5. Data Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- State Pharmacopoeia Committee of People’s Republic of China. Pharmacopoeia of the People’s Republic of China, 10th ed.; China Medical Science and Technology Press: Beijing, China, 2015; p. 383. [Google Scholar]
- Xu, B.; Xia, H.; Zhao, X. Effect of concocting on bufadienolide in Bufonis Venenum. China J. Chin. Mater. Med. 1998, 23, 722–725. [Google Scholar]
- Zhang, H.; Yin, Z.; Sheng, J.; Jiang, Z.; Wu, B.; Su, Y. A comparison study of pharmacokinetics between bufalin-loaded bovine serum albumin nanoparticles and bufalin in rats. J. Chin. Integr. Med. 2012, 10, 674–680. [Google Scholar] [CrossRef]
- Su, Y.; Huang, X.; Zhang, D.; Zhang, Y.; Xie, J.; Linh, C. HPLC separation and determination of bufadienolide in cinobufacini injection. Chin. Tradit. Pat. Med. 2003, 25, 24–27. [Google Scholar]
- Liu, Y.; Wang, P.; Sun, C.; Zhao, J.; Du, Y.; Shi, F.; Feng, N. Bioadhesion and enhanced bioavailability by wheat germ agglutinin-grafted lipid nanoparticles for oral delivery of poorly water-soluble drug bufalin. Int. J. Pharm. 2011, 419, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Senthil, K.M.; Valarmathi, S.; Bhima, P.; Prudhvi, D.S.; Raja, A.; Vallabhaneni, S.D. Dendrimers: A novel drug delivery system. J. Pharm. Sci. Technol. 2012, 4, 972–984. [Google Scholar]
- Lombardo, D. Dendrimer-based host/guest systems for drug delivery. Mater. Sci. Res. J. 2014, 8, 403–431. [Google Scholar]
- Prusty, A. Dendrimer: The recent drug delivery system. Int. Res. J. Pharm. 2012, 3, 10–12. [Google Scholar]
- Rewatkar, P.V.; Parekh, H.S.; Parat, M.O. Molecular determinants of the cellular entry of asymmetric peptide dendrimers and role of caveolae. PLoS ONE 2016, 11, e0147491. [Google Scholar] [CrossRef] [PubMed]
- Bugno, J.; Hsu, H.J.; Hong, S. Recent advances in targeted drug delivery approaches using dendritic polymers. Biomater. Sci. 2015, 3, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, T.L.; Lu, Z.R. Targeted intracellular codelivery of chemotherapeutics and nucleic acid with a well-defined dendrimer-based nanoglobular carrier. Biomaterials 2009, 30, 5660–5666. [Google Scholar] [CrossRef] [PubMed]
- Bosman, A.W.; Janssen, H.M.; Meijer, E.W. About Dendrimers: Structure, Physical Properties, and Applications. Chem. Rev. 1999, 99, 1665–1699. [Google Scholar] [CrossRef] [PubMed]
- Vergara-Jaque, A.; Comer, J.; Monsalve, L.; Gonzalez-Nilo, F.D.; Sandoval, C. Computationally efcient methodology for atomic-level characterization of dendrimer-drug complexes: A comparison of amine- and acetyl-terminated PAMAM. J. Phys. Chem. B 2013, 117, 6801–6813. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Song, Y.; Hu, H.; Qiao, M.; Zhao, X.; Chen, W. Preparation and evaluation of doxorubicin loaded polyamide-amine dendrimer locked in liposome. J. Shenyang Pharm. Univ. 2013, 30, 663–668. [Google Scholar]
- Mutalik, S.; Shetty, P.K.; Kumar, A.; Kalra, R.; Parekh, H.S. Enhancement in deposition and permeation of 5-fluorouracil through human epidermis assisted by peptide dendrimers. Drug Deliv. 2014, 21, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.D.; Parekh, H.S.; Steptoe, R.J. Asymmetric peptide dendrimers are effective linkers for antibody-mediated delivery of diverse payloads to B cells in vitro and in vivo. Pharm. Res. 2014, 31, 3150–3160. [Google Scholar] [CrossRef] [PubMed]
- Van Breemen, R.B.; Li, Y. Caco-2 cell permeability assays to measure drug absorption. Expert Opin. Drug Metab. Toxicol. 2005, 1, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Yee, S. In vitro permeability across Caco-2 cells (colonic) can predict in vivo (small intestinal) absorption in man-fact or myth. Pharm. Res. 1997, 14, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.; Dando, S.A.; Morrison, R.A. Evaluation of Biocoat intestinal epithelium differentiation environment (3-day culture Caco-2 cells) as an absorption screening model with improved productivity. Pharm. Res. 1997, 14, 1835–1837. [Google Scholar] [CrossRef] [PubMed]
- Walle, U.K.; French, K.L.; Walgren, R.A.; Walle, T. Transport of genistein-7-glucoside by human intestinal Caco-2 cells: Potential role for MRP2. Res. Commun. Mol. Pathol. Pharmacol. 1999, 103, 45–56. [Google Scholar] [PubMed]
- Wen, Z.; Li, G.; Lin, D.; Wang, J.; Qin, L.; Guo, G. Transport of PLGA nanoparticles across Caco-2/HT29-MTX co-cultured cells. Acta Pharm. Sin. 2013, 48, 1829–1835. [Google Scholar]
- Lin, H.; Gebhardt, M.; Bian, S.; Kwon, K.A.; Shim, C.K.; Chung, S.J.; Kim, D.D. Enhancing effect of surfactants on fexofenadine. HCl transport across the human nasal epithelial cell monolayer. Int. J. Pharm. 2007, 330, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Alsenz, J.; Haenel, E. Development of a 7-day, 96-well Caco-2 permeability assay with high-throughput direct UV compound analysis. Pharm. Res. 2003, 20, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Kigen, G.; Edwards, G. Drug-transporter mediated interactions between anthelminthic and antiretroviral drugs across the Caco-2 cell monolayers. BMC Pharmacol. Toxicol. 2017, 18, 20. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Wu, L.; Zhao, H.; Liang, W.; Chen, W.; Han, S.; Qi, Q.; Cui, Y.; Li, S.; Yang, G.; et al. Transport of corilagin, gallic acid, and ellagic acid from fructus Phyllanthi tannin fraction in Caco-2 Cell Monolayers. Evid. Based Complement. Alternat. Med. 2016, 2016, 9205379. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Di, L.; Wang, J.; Shan, J.; Liu, S.; Ju, W.; Cai, B. Intestinal absorption of forsythoside A in in situ single-pass intestinal perfusion and in vitro Caco-2 cell models. Acta Pharmacol. Sin. 2012, 33, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, X.; Wang, Y.; Ma, L.; Zhang, Y.; Yang, X.; Wang, K. Establishment of Caco-2 cell monolayer model and standard operation procedure for assessing intestinal absorption of chemical components of traditional Chinese medicine. J. Chin. Integr. Med. 2007, 5, 634–641. [Google Scholar] [CrossRef]
- Artursson, P.; Karlsson, J. Correlation between oral drug absorption in humans and apparent drug permeability coefficients in human intestinal epithelial (Caco-2) cells. Biochem. Biophys. Res. Commun. 1991, 175, 880–885. [Google Scholar] [CrossRef]
- Lennernäs, H.; Palm, K.; Fagerholm, U.; Artursson, P. Comparison between active and passive drug transport in human intestinal epithelial (Caco-2) cells in vitro and human jejunum in vivo. Int. J. Pharm. 1996, 127, 103–107. [Google Scholar] [CrossRef]
Sample Availability: Samples of bufalin-peptide-dendrimer inclusion (A), bufalin (B) and peptide-dendrimer (C) are available from the authors. |

| Compounds | Viability (%) | |||
|---|---|---|---|---|
| Paclitaxel | 25 μM | 50 μM | 75 μM | 100 μM |
| 65.3 ± 7.2 | 43.5 ± 5.4 | 34.8 ± 3.2 | 30.5 ± 2.6 | |
| BFL | 50 μM | 75 μM | 100 μM | 125 μM |
| 102.06 ± 7.2 | 100.10 ± 8.6 | 99.09 ± 8.5 | 98.90 ± 6.4 | |
| BPDI | 50 μM | 75 μM | 100 μM | 125 μM |
| 103.28 ± 10.2 | 98.60 ± 8.2 | 100.75 ± 4.8 | 97.20 ± 9.8 | |
| Concentration (μM) | Papp (10−6cm/s) | Papp AP→BL/Papp BL→AP | ||||
|---|---|---|---|---|---|---|
| AP→BL | BL→AP | |||||
| BFL | BPDI | BFL | BPDI | BFL | BPDI | |
| 50 | 1.58 ± 0.32 | 5.48 ± 0.42 | 1.37 ± 0.18 | 3.83 ± 0.70 | 1.15 | 1.43 |
| 75 | 1.47 ± 0.08 | 4.65 ± 0.48 | 1.32 ± 0.14 | 3.39 ± 0.53 | 1.11 | 1.37 |
| 100 | 1.46 ± 0.02 | 4.22 ± 0.39 | 1.29 ± 0.10 | 3.32 ± 0.12 | 1.13 | 1.27 |
| 125 | 1.64 ± 0.12 | 4.34 ± 0.48 | 1.35 ± 0.45 | 3.21 ± 0.82 | 1.21 | 1.35 |
| Time (min) | Papp (10−6cm/s) | Papp AP→BL/Papp BL→AP | ||||
|---|---|---|---|---|---|---|
| AP→BL | BL→AP | |||||
| BFL | BPDI | BFL | BPDI | BFL | BPDI | |
| 60 | 1.52 ± 0.22 | 5.24 ± 0.82 | 1.14 ± 0.28 | 3.91 ± 0.82 | 1.33 | 1.40 |
| 120 | 1.36 ± 0.08 | 4.40 ± 0.84 | 1.08 ± 0.17 | 3.20 ± 0.74 | 1.26 | 1.38 |
| 180 | 1.28 ± 0.02 | 4.18 ± 0.64 | 0.99 ± 0.12 | 2.97 ± 0.17 | 1.30 | 1.40 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, C.-o.; Jing, J.; Xiao, W.; Tan, Z.; Lv, Q.; Yang, J.; Chen, S. Enhanced Intestinal Permeability of Bufalin by a Novel Bufalin-Peptide-Dendrimer Inclusion through Caco-2 Cell Monolayer. Molecules 2017, 22, 2088. https://doi.org/10.3390/molecules22122088
Chan C-o, Jing J, Xiao W, Tan Z, Lv Q, Yang J, Chen S. Enhanced Intestinal Permeability of Bufalin by a Novel Bufalin-Peptide-Dendrimer Inclusion through Caco-2 Cell Monolayer. Molecules. 2017; 22(12):2088. https://doi.org/10.3390/molecules22122088
Chicago/Turabian StyleChan, Chi-on, Jing Jing, Wei Xiao, Zhexu Tan, Qiuyue Lv, Jingyu Yang, and Sibao Chen. 2017. "Enhanced Intestinal Permeability of Bufalin by a Novel Bufalin-Peptide-Dendrimer Inclusion through Caco-2 Cell Monolayer" Molecules 22, no. 12: 2088. https://doi.org/10.3390/molecules22122088
APA StyleChan, C.-o., Jing, J., Xiao, W., Tan, Z., Lv, Q., Yang, J., & Chen, S. (2017). Enhanced Intestinal Permeability of Bufalin by a Novel Bufalin-Peptide-Dendrimer Inclusion through Caco-2 Cell Monolayer. Molecules, 22(12), 2088. https://doi.org/10.3390/molecules22122088