Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides
Abstract
1. Introduction
2. Cyclic Tripeptides
3. Cyclic Tetrapeptides
4. Cyclic Pentapeptides
5. Cyclic Hexapeptides
6. Cyclic Heptapeptides
7. Cyclic Octapeptides
8. Cyclic Nonapeptides
9. Cyclic Decapeptides
10. Cyclic Undecapeptides
11. Cyclic Dodecapeptides
12. Cyclic Tetradecapeptides
13. Cyclic Octadecapeptides
14. Cyclic Peptides Containing Ether Bonds in the Core Ring
15. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tan, N.-H.; Zhou, J. Plant cyclopeptides. Chem. Rev. 2006, 106, 840–895. [Google Scholar] [CrossRef] [PubMed]
- Taevernier, L.; Wynendaele, E.; De Vreese, L.; Burvenich, C.; De Spiegeleer, B. The mycotoxin definition reconsidered towards fungal cyclic depsipeptides. J. Environ. Sci. Health Part C 2016, 34, 114–135. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, X.; Kim, S.J.; Zhang, W. Antimycin-type depsipeptides: Discovery, biosynthesis, chemical synthesis, and bioactivities. Nat. Prod. Rep. 2016, 33, 1146–1165. [Google Scholar] [CrossRef] [PubMed]
- Andavan, G.S.B.; Lemmens-Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, X.; Wang, B.-G. Secondary metabolites from the marine algal-derived endophtic fungi: Chemical diversity and biological activity. Planta Med. 2016, 82, 832–842. [Google Scholar] [PubMed]
- Falanga, A.; Nigro, E.; De biasi, M.G.; Daniele, A.; Morelli, G.; Galdiero, S.; Scudiero, O. Cyclic peptides as novel therapeutic microbicides engineering of human defensing mimetics. Molecules 2017, 22, 1217. [Google Scholar] [CrossRef] [PubMed]
- Brase, S.; Encinas, A.; Keck, J.; Nising, C. Chemistry and biology of mycotoxins and related fungal metabolites. Chem. Rev. 2009, 109, 3903–3990. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, A.; Deyle, K.; Heinis, C. Cyclic peptide therapeutics: Past, present and future. Curr. Opin. Chem. Biol. 2017, 38, 24–29. [Google Scholar] [CrossRef] [PubMed]
- De Leon Rodriguez, L.M.; Weidkamp, A.J.; Brimble, M.A. An update on new methods to synthesize cyclotetrapeptides. Org. Biomol. Chem. 2015, 13, 6906–6921. [Google Scholar] [CrossRef] [PubMed]
- Sussmuth, R.; Muller, J.; von Dohren, H.; Molnar, I. Fungal cyclooligomer depsipeptides; from classical biochemistry to combinatorial biosynthesis. Nat. Prod. Rep. 2011, 28, 99–124. [Google Scholar] [CrossRef] [PubMed]
- Kries, H. Biosynthetic engineering of nonribosomal peptide synthases. J. Pept. Sci. 2016, 22, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Thorstholm, L.; Craik, D.J. Discovery and applications of naturally occurring cyclic peptides. Drug Discov. Today Technol. 2012, 9, e13–e21. [Google Scholar] [CrossRef] [PubMed]
- Balkovec, J.M.; Hughes, D.L.; Masurekar, P.S.; Sable, C.A.; Schwartz, R.E.; Singh, S.B. Discovery and development of first in class antifungal caspofungin (CANCIDAS®)—A case study. Nat. Prod. Rep. 2014, 31, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Y.; Zhang, X.; Lai, D.; Zhou, L. Structural diversity and biological activities of the cyclodipeptides from fungi. Molecules 2017, 22, 2026. [Google Scholar] [CrossRef] [PubMed]
- Taevernier, L.; Wynendaele, E.; Gevaert, B.; De Spiegeleer, B. Chemical classification of cyclic depsipeptides. Curr. Protein Pept. Sci. 2017, 18, 425–452. [Google Scholar] [CrossRef] [PubMed]
- Myokei, R.; Sakurai, A.; Chang, C.-F.; Kodaira, Y.; Takahashi, N.; Tamura, S. Structure of aspochracin, an insecticidal metabolite of Aspergillus ochraceus. Tetrahedron Lett. 1969, 10, 695–698. [Google Scholar] [CrossRef]
- Dalsgaard, P.W.; Larsen, T.O.; Christophersen, C. Bioactive cyclic peptides from the psychrotolerant fungus Penicillium algidum. J. Antibiot. 2005, 58, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Xu, Z.; Wang, Y.; Hong, K.; Liu, P.; Zhu, W. Cyclic tripeptides from the halotolerant fungus Aspergillus sclerotiorum PT06-1. J. Nat. Prod. 2010, 73, 1133–1137. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, P.W.; Larsen, T.O.; Frydenvang, K.; Christophersen, C. Psychrophilin A and cycloaspeptide D, novel cyclic peptides from the psychrotolerant fungus Penicillium ribeum. J. Nat. Prod. 2004, 67, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Dalsgaard, P.W.; Blunt, J.W.; Munro, M.H.G.; Larsen, T.O.; Christophersen, C. Psychrophilin B and C: Cyclic nitropeptides from the psychrotolerant fungus Penicillium rivulum. J. Nat. Prod. 2004, 67, 1950–1952. [Google Scholar] [CrossRef] [PubMed]
- Ebada, S.S.; Fischer, T.; Hamacher, A.; Du, F.-Y.; Roth, Y.O.; Kassack, M.U.; Wang, B.-G.; Roth, E.H. Psychrophilin E, a new cyclotripeptide, from co-fermentation of two marine alga-derived fungi of the genus Apsergillus. Nat. Prod. Res. 2014, 28, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Gao, H.; Zhang, X.; Wang, S.; Wu, C.; Gu, Q.; Guo, P.; Zhu, T.; Li, D. Psychrophilins E–H and versicotide C, cyclic peptides from the marine-derived fungus Aspergillus versicolor ZLN-60. J. Nat. Prod. 2014, 77, 2218–2223. [Google Scholar] [CrossRef] [PubMed]
- Lacey, H.J.; Vuong, D.; Pitt, J.I.; Lacey, E.; Piggott, A.M. Kumbicins A–D: Bis-indolyl benzenoids and benzoquinones form an Australian soil fungus, Aspergillus kumbius. Aust. J. Chem. 2016, 69, 152–160. [Google Scholar] [CrossRef]
- Motohashi, K.; Inaba, S.; Takagi, M.; Shin-ya, K. JBIR-15, a new aspochracin derivative, isolated from a sponge-derived fungus, Aspergillus sclerotiorum Huber Sp080903f04. Biosci. Biotechnol. Biochem. 2009, 73, 1898–1900. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Q.-X.; Jin, X.-J.; Draskovic, M.; Crews, M.S.; Tenney, K.; Valeriote, F.A.; Yao, X.-J.; Crews, P. Unraveling the numerous biosynthetic products of the marine sediment-derived fungus, Aspergillus insulicola. Phytochem. Lett. 2012, 5, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, X.; Feng, S.; Jiang, G.; Zhou, S.; Vrijmoed, L.L.P.; Jones, E.B.G. A novel N-cinnamoylcyclopeptide containing an allenic ether from the fungus Xylaria sp. (strain #2508) from the South China Sea. Tetrahedron Lett. 2001, 42, 449–451. [Google Scholar]
- Taunton, J.; Hassig, C.A.; Schreiber, S.L. A mammalian histone deacetylase related to the yeast transcriptional regulator Rd3p. Science 1996, 272, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Barret, M.-O.; Boyd, K.G.; Adams, D.R.; Boyd, A.S.F.; Burgess, J.G. JM47, a cyclic tetrapeptide HC-toxin analogue from a marine Fusarium species. Phytochemistry 2002, 60, 33–38. [Google Scholar] [CrossRef]
- Du, L.; Risinger, A.L.; King, J.B.; Powell, D.R.; Cichewicz, R.H. A potent HDAC inhibitor, 1-alaninechlamydocin, from a Tolypocladium sp. induces G2/M cell cycle arrest and apoptosis in MIA PaCa-2 cells. J. Nat. Prod. 2014, 77, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Porter, N.J.; Christianson, D.W. Binding of the microbial cyclic tetrapeptide trapoxin A to the class I histone deacetylase HDAC8. ACS Chem. Biol. 2017, 12, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Zink, D.L.; Polishook, J.D.; Dombrowski, A.W.; Darkin-Rattray, S.J.; Schmatz, D.M.; Goetz, M.A. Apicidins: Novel cyclic tetrapeptides as coccidiostats and antimalarial agents from Fusarium pallidoroseum. Tetrahedron Lett. 1996, 37, 8077–8080. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Liesch, J.M.; Dombrowski, A.W.; Darkin-Rattray, S.J.; Schmatz, D.M.; Goetz, M.A. Structure, histone deacetylase, and antiprotozoal activities of apicidins B and C, congeners of apicidin with proline and valine substitutions. Org. Lett. 2001, 3, 2815–2818. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.B.; Zink, D.L.; Liesch, J.M.; Mosley, R.T.; Dombrowski, A.W.; Bills, G.F.; Darkin-Rattray, S.J.; Schmatz, D.M.; Goetz, M.A. Structure and chemistry of apicidins, a class of novel cyclic tetrapeptides without a terminal α-keto epoxide as inhibitors of histone deacetylase with potent antiprotozoal activities. J. Org. Chem. 2002, 67, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Darkin-Rattray, S.J.; Gurnett, A.M.; Myers, R.W.; Dulski, P.M.; Crumley, T.M.; Allocco, J.J.; Cannova, C.; Meinke, P.T.; Colletti, S.L.; Bendnarek, M.A.; et al. Apicidin: A novel antiprotozoal agent that inhibits parasite histone deacetylase. Proc. Natl. Acad. Sci. USA 1996, 93, 13143–13147. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Leman, L.J.; Ku, S.; Vickers, C.J.; Olsen, C.A.; Montero, A.; Ghadiri, M.R.; Gottesfeld, J.M. Cyclic tetrapeptide HDAC inhibitors as potential therapeutics for spinal muscular atrophy: Screending with iPSC-derived neuronal cells. Bioorg. Med. Chem. Lett. 2017, 27, 3289–3293. [Google Scholar] [CrossRef] [PubMed]
- Sasamura, S.; Sakamoto, K.; Takagaki, S.; Yamada, T.; Takase, S.; Mori, H.; Fujii, T.; Hino, M.; Hashimoto, M. AS1387392, a novel immunosuppressive cyclic tetrapeptide compound with inhibitory activity against mammalian histone deacetylase. J. Antibiot. 2010, 63, 633–636. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Bao, J.; Zhang, X.-Y.; Tu, Z.-C.; Shi, Y.-M.; Qi, S.-H. Asperterrestide A, a cyctotoxic cyclic tetrapeptide from the marine-derived fungus Aspergillus terreus SCSGAF0162. J. Nat. Prod. 2013, 76, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Close, A.; Huguenin, R. Isolation and structure elucidation of chlamydocin. Helv. Chim. Acta 1974, 57, 533–545. [Google Scholar]
- Bunbamrung, N.; Intaraudom, C.; Boonyuen, N.; Rachtawee, P.; Laksanacharoen, P.; Pittayakhajonwut, P. Penicisochromans from the endophytic fungus Penicillium sp. BCC18034. Phytochem. Lett. 2014, 10, 13–18. [Google Scholar] [CrossRef]
- Tani, H.; Fujii, Y.; Nakajima, H. Chlamydocin analogues from the soil fungus Peniophora sp.: Structures and plant growth-retardant activity. Phytochemistry 2001, 58, 305–310. [Google Scholar] [CrossRef]
- Huang, S.; Ding, W.; Li, C.; Cox, D.G. Two new cyclopeptides from the co-culture broth of two marine mangrove fungi and their antifungal activity. Pharmacogn. Mag. 2014, 10, 410–414. [Google Scholar] [PubMed]
- Li, C.; Wang, J.; Luo, C.; Ding, W.; Cox, D.G. A new cyclopepitde with antifungal activity from the co-culture broth of two marine mangrove fungi. Nat. Prod. Res. 2014, 28, 616–621. [Google Scholar] [CrossRef] [PubMed]
- Perez-Victoria, I.; Martin, J.; Gonzalez-Menendez, V.; De Pedro, N.; Aouad, N.E.; Ortiz-Lopez, F.J.; Tormo, J.R.; Platas, G.; Vicente, F.; Bills, G.F.; et al. Isolation and structural eluciation of cyclic tetrapeptides from Onychocola sclerotica. J. Nat. Prod. 2012, 75, 1210–1214. [Google Scholar] [CrossRef] [PubMed]
- Takayama, S.; Lsogai, A.; Nakata, M.; Suzuki, H.; Susuki, A. Structure of Cyl-1, a novel cyclic tetrapetide from Cylindrocladium scoparium. Agric. Biol. Chem. 1984, 48, 839–842. [Google Scholar]
- Isogai, A.; Takayama, S.; Hirota, A.; Suzuki, A. Determination of the absolute stereochemistry of the epoxide of aoe (2-amino-8-oxa-9,10-epoxy-decanoic acid) in Cyl-1 and Cyl-2 by CD spectra. Agric. Biol. Chem. 1986, 50, 517–518. [Google Scholar]
- Gu, W.; Cueto, M.; Jensen, P.R.; Fenical, W.; Silverman, R.B. Microsporins A and B: New histone deacetylase inhibitors from the marine-derived fungus Microsporum cf. gypseum and the solid-phase synthesis of microsporin A. Tetrahedron 2007, 63, 6535–6541. [Google Scholar] [CrossRef]
- Sun, W.; Chen, X.; Tong, Q.; Zhu, H.; He, Y.; Lei, L.; Xue, Y.; Yao, G.; Luo, Z.; Wang, J.; et al. Novel small molecule 11β-HSD1 inhibitor from the endophytic fungus Penicillium commune. Sci. Rep. 2016, 6, 26418. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Kreuzenbeck, N.B.; Otani, S.; Garcia-Altares, M.; Dahse, H.-M.; Christiane, W.; Aanen, D.K.; Hertweck, C.; Poulsen, M.; Beemelmanns, C. Pseudoxylallemycins A–F, cyclic tetrapeptides with rare allenyl modifications isolated from Pseudoxylaria sp. X802: A competor of fungus-growing termite cultivars. Org. Lett. 2016, 18, 3338–3341. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.; Fu, L.; Peng, Y.; Zhou, L. Metabolites form Alternaria fungi and their bioactivities. Molecules 2013, 18, 5891–5935. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.L.; Li, J.L.; Xiao, B.; Hong, J.; Yoo, E.S.; Yoon, W.D.; Jung, J.H. A new tetrapeptide from the jellyfish-derived fungus Phoma sp. Chem. Pharm. Bull. 2012, 60, 1590–1593. [Google Scholar] [PubMed]
- Itazaki, H.; Nagashima, K.; Sugita, K.; Yoshida, H.; Kawamura, Y.; Yasuda, Y.; Matsumoto, K.; Ishii, K.; Uotani, N.; Nakai, H.; et al. Isolation and structural elucidation of new cyclotetrapeptides, trapoxins A and B, having detransformation activities as antitumor agents. J. Antibiot. 1990, 43, 1524–1532. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Baek, S.-R.; Lee, K.-R.; Lee, J.; Yun, S.-H.; Kang, S.; Lee, Y.-W. Purification and phytotoxicity of apicidins produced by the Fusarium semitectum KCTC16676. Plant Pathol. J. 2008, 24, 417–422. [Google Scholar] [CrossRef]
- Jin, J.-M.; Lee, S.; Lee, J.; Baek, S.-R.; Kim, J.-C.; Yun, S.-H.; Park, S.-Y.; Kang, S.; Lee, Y.-W. Functional characterization and manipulation of the apicidin biosynthetic pathway in Fusarium semitectum. Mol. Microbiol. 2010, 76, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Von Bargen, K.W.; Niehaus, E.-M.; Bergander, K.; Brun, R.; Tudzynski, B.; Humpf, H.-U. Structure elucidation and antimalarial activity of apicidin F: An apicidin-like compound produced by Fusarium fujikuroi. J. Nat. Prod. 2013, 76, 2136–2140. [Google Scholar] [CrossRef] [PubMed]
- Garson, M.J. Isolation of the tetrapeptide apcidins G, H and I from the fungus Fusarium semitectum. Nat. Prod. Commun. 2014, 9, 233–236. [Google Scholar]
- Aucamp, P.J.; Holzapfel, C.W. Toxic substances from Aspergillus versicolor. J. S. Afr. Chem. Inst. 1969, 22, 35–40. [Google Scholar]
- Li, Y.; Yue, Q.; Jayanetti, D.R.; Swenson, D.C.; Bartholomeusz, G.A.; An, Z.; Gloer, J.B.; Bills, G.F. Anti-Cryptococcus phenalenones and cyclic tetrapeptides from Auxarthron pseudauxarthron. J. Nat. Prod. 2017, 80, 2101–2109. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Peiser, G.; Nkajima, T.; Hwang, Y.-S. Characterization of a phytotoxic cyclotetrapeptide, a novel chlamydocin analogue, from Verticillium coccosporum. Tetrahedron Lett. 1994, 35, 6009–6012. [Google Scholar] [CrossRef]
- Hirota, A.; Suzuki, A.; Aizawa, K.; Tamura, S. Structure of Cyl-2, a novel cyclopeptide from Cylindrocladium scoparium. Agric. Biol. Chem. 1973, 37, 955–956. [Google Scholar] [CrossRef][Green Version]
- Masuoka, Y.; Shin-ya, K.; Furihata, K.; Hayakawa, Y.; Seto, H. A novel substance with TGF-β like activity, diheteropeptin, produced by a fungus, Diheterospora sp. J. Antibiot. 1997, 50, 1058–1060. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, Y.; Shin-ya, K.; Kim, Y.; Yoshida, M.; Nagai, K.; Suzuki, K. Diheteropeptin, a new substance with TGF-β-like activity, produced by a fungus, Diheterospora chlamydosporia. J. Antibiot. 2000, 53, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Akimoto, N.; Ohnishi, K.; Yamashita, M.; Maoka, T. Rapid and simultaneous determination of tetra cyclic peptide phytotoxins, tentoxin, isotentoxin and dihydrotentoxin, from Alternaria porri by LC/MS. Chromatography 2003, 24, 109–116. [Google Scholar]
- Almeida, C.; Maddah, F.E.; Kehraus, S.; Schnakenburg, G.; Konig, G.M. Endolides A and B, vasopressin and serotonin-receptor interacting N-methylated peptides from the sponge-derived fungus Stachylidium sp. Org. Lett. 2016, 18, 528–531. [Google Scholar] [CrossRef] [PubMed]
- Maddah, F.E.; Kehraus, S.; Nazir, M.; Almeida, C.; Konig, G.M. Insights into the biosynthetic origin of 3-(3-furyl)alanine in Stachylidium sp. 293 K04 tetrapeptides. J. Nat. Prod. 2016, 79, 2838–2845. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-F.; Yang, X.-H.; Liu, H.-L.; Gu, Y.-C.; Ye, B.-P.; Guo, Y.-W. A new cyclic peptide and a new steroid from the fungus Penicillium sp. GD6 isolated from the mangrove Bruguiera gymnorrhiza. Helv. Chim. Acta 2014, 97, 1564–1570. [Google Scholar] [CrossRef]
- Mori, H.; Urano, Y.; Abe, F.; Furukawa, S.; Furukawa, S.; Tsurumi, Y.; Sakamoto, K.; Hashimoto, M.; Takase, S.; Hino, M.; et al. FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase (HDAC). I. Taxonomy, fermentation, isolation and biological activities. J. Antibiot. 2003, 56, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Abe, F.; Furukawa, S.; Furukawa, S.; Sakai, F.; Hino, M.; Fujii, T. FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase (HDAC). II. Biological activities in animal models. J. Antibiot. 2003, 56, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Urano, Y.; Kinoshita, T.; Yoshimura, S.; Takase, S.; Hino, M. FR235222, a fungal metabolite, is a novel immunosuppressant that inhibits mammalian histone deacetylase III. Structure determination. J. Antibiot. 2003, 56, 181–185. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ali, H.; Ries, M.I.; Lankhorst, P.P.; Hoeven, R.A.M.; Schouten, O.L.; Noga, M.; Hankemeier, T.; Peij, N.N.M.E.; Bovenberg, R.A.L.; Vreeken, R.J.; et al. A non-canonical NPRS is involved in the synthesis of fungisporin and related hydrophobic cyclic tetrapeptides in Penicillium chrysogenum. PLoS ONE 2014, 9, e98212. [Google Scholar] [CrossRef] [PubMed]
- Liesch, J.M.; Sweeley, C.C.; Staffeld, G.D.; Anderson, M.S.; Weber, D.J.; Scheffer, R.P. Structure of HC-toxin, a cyclic tetrapeptide from Helminthosporium carbonum. Tetrahedron 1982, 38, 45–48. [Google Scholar] [CrossRef]
- Kawai, M.; Jasensky, R.D.; Rich, D.H. Conformational analysis by NMR spectrometry of the highly substituted cyclic tetrapeptides, chlamydocin and Ala-chlamydocin. Evidence for a unique amide bond sequence in dimethyl-d sulfoxidy. J. Am. Chem. Soc. 1983, 105, 4456–4462. [Google Scholar] [CrossRef]
- Lang, G.; Blunt, J.W.; Cummings, N.J.; Cole, A.L.J.; Munro, M.H.G. Hirsutide, a cyclic tetrapeptide from a spider-derived entomopathogenic fungus, Hirsutella sp. J. Nat. Prod. 2005, 68, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Klitgaard, A.; Nielsen, J.B.; Frandsen, R.J.N.; Andersen, M.R.; Nielsen, K.F. Combining stable isotope labeling and molecular networking for biosynthetic pathway characterization. Anal. Chem. 2015, 87, 6520–6526. [Google Scholar] [CrossRef] [PubMed]
- Ochi, S.; Yoshida, M.; Nakagawa, A.; Natsume, M. Identification and activity of a phytotoxin produced by Calonectria ilicicola, the causal agent of soybean red crown rot. Can. J. Plant Pathol. 2011, 33, 347–354. [Google Scholar] [CrossRef]
- Yoshida, S.; Yoshioka, M.; Yaguchi, T.; Nagasawa, M.; Koyama, M. PF1070A and B, new antitumour antibiotics produced by Humicola sp. Sci. Rep. Meiji Seika Kaisha 1993, 32, 58–63. [Google Scholar]
- Masuoka, Y.; Shin-Ya, K.; Furihata, K.; Nagai, K.; Suzuki, K.-I.; Hayakawa, Y.; Seto, H. Phoenistatin, a new gene expression-enhancing substance produced by Acremonium fusigerum. J. Antibiot. 2001, 54, 187–190. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zin, W.W.M.; Buttachon, S.; Dethoup, T.; Fernandes, C.; Cravo, S.; Pinoto, M.M.M.; Gales, L.; Pereira, J.A.; Silva, A.M.S.; Sekeroglu, N.; et al. New cyclotetrapeptides and a new diketopiperazine derivative from the marine sponge-associated fngus Neosartorya glabra KUFA 0702. Mar. Drugs 2016, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.; Mcroberts, N.; Hill, R.A.; Marshall, G. Phytotoxin production by Alternaria linicola and phytoalexin production by the linseed host. Ann. Appl. Biol. 1996, 129, 415–431. [Google Scholar] [CrossRef]
- Umehara, K.; Nakahara, K.; Kiyoto, S.; Iwami, M.; Okamoto, M.; Tanaka, H.; Hohsaka, M.; Aoki, H.; Imanaka, H. Studies on WF-3161, a new antitumor antibiotic. J. Antibiot. 1983, 36, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Shiomi, K.; Yamaguchi, Y.; Masuma, R.; Iwai, Y.; Turberg, A.; Kolbl, H.; Omura, S. Argadin, a new chitinase inhibitor, produced by Clonostachys sp. FO-7314. Chem. Pharm. Bull. 2000, 48, 1442–1446. [Google Scholar] [CrossRef] [PubMed]
- Arai, N.; Shiomi, K.; Iwai, Y.; Omura, S. Argifin, a new chitinase inhibitor, produced by Gliocladium sp. FTD-0668 II. Isolation, physicochemical properties, and structure elucidation. J. Antibiot. 2000, 53, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Rao, F.V.; Houston, D.R.; Boot, R.G.; Aerts, J.M.F.G.; Hodkinson, M.; Adam, D.J.; Shimoi, K.; Omura, D.J.; Shiomi, K.; Omura, S.; et al. Specificity and affinity of natural product cyclopentapeptide inhibitors against A. fumigatus, human, and bacterial chitinases. Chem. Biol. 2005, 12, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Nong, X.-H.; Ren, Z.; Wang, J.; liang, X.; Wang, L.; Qi, S.-H. Antiviral peptides from marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501. Tetrahedron Lett. 2017, 58, 1151–1155. [Google Scholar] [CrossRef]
- Kobayashi, R.; Samejima, Y.; Nakajima, S.; Kawai, K.; Udagawa, S. Studies on fungal products. XI. Isolation and structures of novel cyclic pentapeptides from Aspergillus sp. NE-45. Chem. Pharm. Bull. 1987, 35, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Lewer, P.; Graupner, P.R.; Hahn, D.R.; Karr, L.L.; Duebelbeis, D.O.; Lira, J.M.; Anzeveno, P.B.; Fields, S.C.; Gilbert, J.R.; Pearce, C. Discovery, synthesis, and insecticidal activity of cycloaspeptide E. J. Nat. Prod. 2006, 69, 1506–1510. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, S.; Liu, H.; Liu, X.; Che, Y. Cycloaspeptides F and G, cyclic pentapeptides from a cordyceps-colonizing isolate of Isaria farinosa. J. Nat. Prod. 2009, 72, 1364–1367. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-W.; Sugawara, F.; Uzawa, J.; Yoshida, S.; Murofushi, N.; Takahashi, N.; Curtis, R.W.; Kanai, M. Structure of malformin B, phytotoxic metabolites produced by Aspergillus niger. Tetrahedron Lett. 1991, 32, 6715–6718. [Google Scholar] [CrossRef]
- Zhan, J.; Gunaherath, G.M.K.B.; Wijeratne, E.M.K.; Gunatilaka, A.A.L. Asperpyrone D and other metabolites of the plant-associated fungal strain Aspergillus tubingensis. Phytochemistry 2007, 68, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.-W.; Gao, F.-L.; Wang, F.-R.; Chen, Q.-J. Anti-TMV activity of malformin A1, a cyclic penta-peptide produced by an endophytic fungus Aspergillus tubingensis FJBJ11. Int. J. Mol. Sci. 2015, 16, 5750–5761. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-M.; Liang, X.-A.; Zhang, H.-C.; Liu, R. Cytotoxic and antibiotic cyclic pentapeptide from an endiphytic Aspergillus tamarii of Ficus carica. J. Agric. Food Chem. 2016, 64, 3789–3793. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, F.; Kim, K.-W.; Uzawa, J.; Yoshida, S.; Takahashi, N.; Curtis, R.W. Structure of malformin A2, reinvestigation of phytotoxic metabolites produced by Aspergillus niger. Tetrahedron Lett. 1990, 31, 4337–4340. [Google Scholar] [CrossRef]
- Li, X.-B.; Xie, F.; Liu, S.-S.; Li, Y.; Zhou, J.-C.; Liu, Y.-Q.; Yuan, H.-Q.; Lou, H.-X. Naphtho-γ-pyrones from endophyte Aspergillus niger occurring in the liverwort Heteroscyphus tener (Steph.) Schiffn. Chem. Biodivers. 2013, 10, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Anderegg, R.J.; Biemann, K.; Buechi, G.; Cushman, M. Malformin C, a new metabolite of Aspergillus niger. J. Am. Chem. Soc. 1976, 98, 3365–3370. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, Z.; Lam, W.; Gullen, E.A.; Yu, Z.; Wei, Y.; Wang, L.; Zeiss, C.; Beck, A.; Cheng, E.-C.; et al. Study of malformin C, a fungal source cyclic pentapeptide, as an anti-cancer drug. PLoS ONE 2015, 10, e0140069. [Google Scholar] [CrossRef] [PubMed]
- Pavlaskova, K.; Nedved, J.; Kuzma, M.; Zabka, M.; Sulc, M.; Sklenar, J.; Novak, P.; Benada, O.; Kofronova, O.; Hajduch, M.; et al. Characterization of pseudacyclins A–E, a suite of cyclic peptides prduced by Pseudallescheria boydii. J. Nat. Prod. 2010, 73, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, K.; Arai, N.; Iwai, Y.; Turberg, A.; Kolbl, H.; Omura, S. Structure of argifin, a new chitinase inhibitor produced by Gliocladium sp. Tetrahedron Lett. 2000, 41, 2141–2143. [Google Scholar] [CrossRef]
- Chen, M.; Shao, C.-L.; Fu, X.-M.; Kong, C.-J.; She, Z.-G.; Wang, C.-Y. Lumazine peptides penilumandes B–D and the cyclic pentapeptide asperpeptide A from a gorgonian-derived Aspergillus sp. fungus. J. Nat. Prod. 2014, 77, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, Y.; Hanafusa, T.; Gohda, F.; Peterson, S.; Bills, G. Species-level assessment of secondary metabolite diversity among Hamigera species and a taxonomic note on the genus. Mycology 2014, 5, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Mitova, M.I.; Stuart, B.G.; Cao, G.H.; Blunt, J.W.; Cole, A.L.J.; Munro, M.H.G. Chrysosporide, a cyclic pentapeptide from a New Zealand sample of the fungus Sepedonium chrysospermum. J. Nat. Prod. 2006, 69, 1481–1484. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Shen, L.; Jiang, W.; Ye, Y.; Chen, C.-T.A.; Wu, X.; Wang, K.; Wu, B. Zn-driven discovery of a hydrothermal vent fungal metabolite clavatustide C, and an experimental study of the anti-cancer mechanism of clavatustide B. Mar. Drugs 2014, 12, 3203–3217. [Google Scholar] [CrossRef] [PubMed]
- Fremlin, L.J.; Piggott, A.M.; Lacey, E.; Capon, R.J. Cottoquinazoline A and cotteslosins A and B, metabolites from an Australian marine-derived strain of Aspergillus versicolor. J. Nat. Prod. 2009, 72, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Talontsi, F.M.; Facey, P.; Tagong, M.D.K.; Islam, M.T.; Frauendorf, H.; Draeger, S.; von Tiedemann, A.; Laatsch, H. Zoosporicidal metabolties from an endophytic fungus Cryptosporiospsis sp. of Zanthoxylum leprieurii. Phytochemistry 2012, 83, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kusari, S.; Golz, C.; Strohmann, C.; Spiteller, M. Three cyclic petapeptides and a cyclic lipopeptide produced by endophytic Fusarium decemcellulare LG53. RSC Adv. 2016, 6, 54092–54098. [Google Scholar] [CrossRef]
- Li, H.-J.; Lin, Y.-C.; Yao, J.-H.; Vrijmoed, L.L.P.; Jones, E.B.G. Two new metabolites from the mangrove endophytic fungus No. 2524. J. Asian Nat. Prod. Res. 2004, 6, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Frisvad, J.C.; Larsen, T.O.; Dalsgaard, P.W.; Seifert, K.A.; Louis-Seize, G.; Lyhne, E.K.; Jarvis, B.B.; Fettinger, J.C.; Overy, D.P. Four psychrotolerant species with high chemical diversity consistently producing cycloaspeptide A, Penicillium jamesonlandense sp. nov., Penicillium ribium sp. nov., Penicillium soppii and Penicillium lanosum. Int. J. Syst. Evol. Microbiol. 2006, 56, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- Schmeda-Hirschmann, G.; Hormazabal, E.; Rodriguez, J.A.; Theoduloz, C. Cycloaspeptide A and pseurotin A from the endophytic fungus Penicillium janczewskii. Z. Naturforsch. C 2008, 63c, 383–388. [Google Scholar] [CrossRef]
- Mizutani, K.; Hirasawa, Y.; Sugita-Konishi, Y.; Mochizuki, N.; Morita, H. Structural and conformational analysis of hydroxycyclochlorotine and cyclochlorotine, chlorinated cyclic peptides from Penicillium islandicum. J. Nat. Prod. 2008, 71, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Ito, E. Liver fibrosis induced in mice by cyclochlorotine. Mycotoxins 1983, 17, 59–61. [Google Scholar] [CrossRef][Green Version]
- Terao, K.; Ito, E.; Tatsuno, T. Liver injuries induced by cyclochlorotine isolated from Penicillium islandicum. Arch. Toxicol. 1984, 55, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.C.; Ramgopal, M. Cyclic peptides from Penicillium isolandicum. A review and a reevaluation of the structure of islanditoxin. J. Heterocycl. Chem. 1980, 17, 1809–1812. [Google Scholar] [CrossRef]
- Gulder, T.; Hong, H.; Correa, J.; Egereva, E.; Wiese, J.; Imhoff, J.; Gross, H. Isolation, structure elucidation and total synthesis of lajollamide A from the marine fungus Asteromyces cruciatus. Mar. Drugs 2012, 10, 2912–2935. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Itoh, M.; Izumikawa, M.; Sakata, N.; Tsuchida, T.; Shin-ya, K. Novel arginine-containing peptides MBJ-0173 and MBJ-0174 from Mortierella alpine f28740. J. Antibiot. 2017, 70, 226–229. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Teng, X.; Wang, Y.; Liu, P.; Wang, H.; Li, J.; Li, G.; Zhu, W. Cyclopeptides and polyketides from coral-associated fungus, Aspergillus versicolor LCJ-5-4. Tetrahedron 2011, 67, 7085–7089. [Google Scholar] [CrossRef]
- Zhou, L.-N.; Gao, H.-Q.; Cai, S.-X.; Zhu, T.-J.; Gu, Q.-Q.; Li, D.-H. Two new cyclic pentapeptides from the marine-derived fungus Aspergillus versicolor. Helv. Chim. Acta 2011, 94, 1065–1070. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Hu, Z.-Y.; Shen, Y.-M. Two new cyclopeptides and one new nonenolide from Xylaria sp. 101. Nat. Prod. Commun. 2011, 6, 1843–1846. [Google Scholar] [PubMed]
- Mizuno, K.; Yagi, A.; Satoi, S.; Takada, M.; Hayashi, M.; Asano, K.; Matsuda, T. Studies on aculeacin. I. Isolation and characeterazation of aculeacin A. J. Antibiot. 1977, 30, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hiratani, T.; Iwata, K.; Yamamoto, Y. Studies on the mechanism of antifungal action of aculeacin A. J. Antibiot. 1982, 35, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Song, J.C.; Stevens, D.A. Caspofungin: Pharmacodynamics, pharmacokinetics, clinical uses and treatment outcomes. Crit. Rev. Microbiol. 2016, 42, 813–846. [Google Scholar] [CrossRef] [PubMed]
- Somer, A.; Torun, S.H.; Salman, N. Caspofungin therapy in immunocompromised children and neonates. Expert Rev. Anti-Infect. Ther. 2011, 9, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, S. Micafungin: A sulfated echinocandin. J. Antibiot. 2009, 62, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, T.; Ganguli, B.N.; Fehlhaber, H.W.; Kogler, H.; Vertesy, L. Mulundocandin, a new lipopeptide antibiotic. II. Structure elucidation. J. Antibiot. 1987, 40, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Bills, G.; Yue, Q.; Chen, L.; Li, Y.; An, Z.; Frisvad, J. Aspergillus mulundensis sp. nov., a new species for the fungus producing the antifungal echinocandin lipopeptides, mulundocandins. J. Antibiot. 2016, 69, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Kai, K.; Yoshikawa, H.; Kuo, Y.-H.; Akiyama, K.; Hayashi, H. Determination of absolute structures of cyclic peptides, PF1171A and PF1171C, from unidentified ascomycete OK-128. Biosci. Biotechnol. Biochem. 2010, 74, 1309–1311. [Google Scholar] [CrossRef] [PubMed]
- Prompanya, C.; Fernandes, C.; Cravo, S.; Pinoto, M.M.M.; Dethoup, T.; Silva, A.M.S.; Kijjoa, A. A new cyclic hexapeptide and a new isocoumarin derivative from the marine sponge-associated fungus Aspergillus similanenesis KUFA 0013. Mar. Drugs 2015, 13, 1432–1450. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Tanaka, R.; Ganesan, A.; Doi, T. Structure revision of similanamide to PF1171C by total synthesis. J. Nat. Prod. 2015, 78, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- Hensens, O.D.; Liesch, J.M.; Zink, D.L.; Smith, J.L.; Wichmann, C.F.; Schwartz, R.E. Pneumocandins from Zalearion arboricola. III. Structure elucidation. J. Anibiot. 1992, 45, 1875–1885. [Google Scholar] [CrossRef]
- Schmatz, D.M.; Abruzzo, G.; Powles, M.A.; Mcfadden, D.C.; Balkovec, J.M.; Black, R.M.; Nollstadt, K.; Bartizal, K. Pneumocandins from Zalearion arboricola. IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumoystis carinii and Candida species. J. Anibiot. 1992, 45, 1886–1891. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, H.; Hong, K.; Wang, Y.; Liu, P.; Wang, X.; Peng, X.; Zhu, W. Novel cyclic hexapeptides from marine-derived fungus, Aspergillus sclerotiorum PT06-1. Org. Lett. 2009, 11, 5262–5265. [Google Scholar] [CrossRef] [PubMed]
- Liermann, J.C.; Thines, E.; Anke, H.; Opatz, T. Anthranicine, an unusual cyclic hexapeptide from Acremonium sp. A29-2004. Z. Naturforsch. B 2009, 64, 727–730. [Google Scholar]
- Nakamura, I.; Yoshimura, S.; Masaki, T.; Takase, S.; Ohsumi, K.; Hashimoto, M.; Furukawa, S.; Fujie, A. ASP2397: A novel antifungal agent produced by Acremonium persicinum MF-347833. J. Antibiot. 2017, 70, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A.; Miller, R.V.; Martinez-Miller, C.; Condron, M.M.; Teplow, D.B.; Hess, W.M. Cryptocandin, a potent antimycotic from the endophytic fungus Cryptosporiopsis c.f. quercina. Microbiology 1999, 145, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, T.; Roy, K.; Bhat, R.G.; Sawant, S.N.; Blumbach, J.; Ganguli, B.N.; Fehlhaver, H.W.; Kogler, H. Deoxymulundocandin-a new echinocandin type antifungal antibiotic. J. Antibiot. 1992, 45, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Toth, V.; Nagy, C.T.; Pocsi, I.; Emri, T. The echinocandin B producer fungus Aspergillus nidulans var. roseus ATCC 58397 does not possess innate resistance against its lipopeptide antimycotic. Appl. Microbiol. Biotechnol. 2012, 95, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Traber, R.; Keller-Juslen, C.; Loosli, H.R.; Kuhn, M.; Von Wartburg, A. Cyclopeptide antibiotics from Aspergillus species. Structure of echinocandins C and D. Helv. Chim. Acta 1979, 62, 1252–1267. [Google Scholar] [CrossRef]
- Bolker, M.; Basse, C.W.; Schirawski, J. Ustilago maydis secondary metabolism-from genomics to chemistry. Fungal Genet. Biol. 2008, 45, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Ohra, J.; Morita, K.; Tsujino, Y.; Tazaki, H.; Fujimori, T.; Goering, M.; Evans, S.; Zorner, P. Production of the phytotoxic metabolite, ferricrocin, by the fungus Colletotrichum gloeosporioides. Biosci. Biotechnol. Biochem. 1995, 59, 113–114. [Google Scholar] [CrossRef] [PubMed]
- Konetschny-Rapp, S.; Jung, G.; Huschka, H.G.; Winkelmann, G. Isolation and identification of the principal siderophore of the plant pathogenic fungus Botrytis cinerea. Biol. Met. 1988, 1, 90–98. [Google Scholar] [CrossRef]
- Kanasaki, R.; Kobyashi, M.; Fujine, K.; Sato, I.; Hashimoto, M.; Takase, S.; Tsurumi, Y.; Fujie, A.; Hino, M.; Hashimoto, S.; et al. FR227673 and FR190293, novel antifungal lipopeptides from Chalara sp. No. 22210 and Tolypocladium parasiticum No. 16616. J. Antibiot. 2006, 59, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, R.; Sakamoto, K.; Hashimoto, M.; Takase, S.; Tsurumi, Y.; Fujie, A.; Hino, M.; Hashimoto, S.; Hori, Y. FR209602 and related compounds, novel antifungal lipopeptides from Coleophoma craterifomis No. 738. J. Antibiot. 2006, 59, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, R.; Abe, F.; Kobayashi, M.; Katsuoka, M.; Hashimoto, M.; Takase, S.; Tsurumi, Y.; Fujie, A.; Hino, M.; Hashimoto, S.; et al. FR220897 and FR220899, novel antifungal lipopeptides from Coleophoma empetri No. 14573. J. Antibiot. 2006, 59, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Fujie, A. Discovery of micafungin (FK463): A novel antifungal drug derived from a natural product lead. Pure Appl. Chem. 2007, 79, 603–614. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, H.; Kamdem, R.S.T.; Orfali, R.S.; Dai, H.; Makhloufi, G.; Janiak, C.; Liu, Z.; Proksch, P. A new cyclohexapeptide, penitropeptide and a new polyketide, penitropone from the endophytic fungus Penicillium tropicum. Tetrahedron Lett. 2016, 57, 2998–3001. [Google Scholar] [CrossRef]
- Kuo, Y.-H.; Kai, K.; Akiyama, K.; Hayashi, H. Novel bioactive peptides, PF1171F and PF1171G, from unidentified ascomycete OK-128. Tetrahedron Lett. 2012, 53, 429–431. [Google Scholar] [CrossRef]
- Wichmann, C.F.; Liesch, J.M.; Schwartz, R.E. L-671,329, a new antifungal agent. II. Structure determination. J. Antibiot. 1989, 42, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Nong, X.-H.; Huang, Z.-H.; Qi, S.-H. Antifungal and antiviral cyclic peptides from the deep-sea-derived fungus Simplicillium obclavatum EIODSF 020. J. Agric. Food Chem. 2017, 65, 5114–5121. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Chantaruk, S.; Tansakul, C.; Saithong, S.; Chaicharemwimonkoon, L.; Pakawatchai, C.; Isaka, M.; Intereya, K. A cyclopeptide from the insect pathogenic fungus Cordyceps sp. BCC 1788. J. Nat. Prod. 2006, 69, 305–307. [Google Scholar] [CrossRef] [PubMed]
- Isaka, M.; Srisanoh, U.; Lartpornmatulee, N.; Boonruangprapa, T. ES-242 derivatives and cycloheptapeptides from Cordyceps sp. strains BCC 16173 and BCC 16176. J. Nat. Prod. 2007, 70, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, Y.; Chen, Y.; Huang, H.; Zhang, W.; Ju, J. Cyclic heptapeptides, cordyheptapeptides C–E, from the marine-derived fungus Acremonium persicinum SCSIO 115 and their cytotoxic activities. J. Nat. Prod. 2012, 75, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Vetter, J. Toxins of Amanita phalloides. Toxicon 1998, 36, 13–24. [Google Scholar] [CrossRef]
- Walton, J.D.; Hallen-Adams, H.E.; Luo, H. Ribosomal biosynthesis of the cyclic peptide toxins of Amanita mushrooms. Biopolymers 2010, 94, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Boot, C.M.; Amagata, T.; Tenney, K.; Compton, J.E.; Pietraszkiewicz, H.; Valeriote, F.A.; Crews, P. Four classes of structurally unusual peptides from two marine-derived fungi: Structures and bioactivities. Tetrahedron 2007, 63, 9903–9914. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.; Cheng, X.C.; Jensen, P.R.; Fenical, W. Scytalidamides A and B, new cytotoxic cyclic heptapeptides from a marine fungus of the genus Scytalidium. J. Org. Chem. 2003, 68, 8767–8773. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, J. Uguisins A and B. New cyclic peptides from the marine-derived fungus Emericella unguis. J. Nat. Prod. 1999, 62, 787–789. [Google Scholar] [CrossRef] [PubMed]
- Malmstrom, J.; Ryager, A.; Anthoni, U.; Nielsen, P.H. Unguisin C, a GABA-containing cyclic peptide from the fungus Emericella unguis. Phytochemistry 2002, 60, 869–872. [Google Scholar] [CrossRef]
- Liu, S.; Shen, Y. A new cyclic peptide from the marine fungal strain Aspergillus sp. AF119. Chem. Nat. Compd. 2011, 47, 786–788. [Google Scholar] [CrossRef]
- Akone, S.H.; Daletos, G.; Lin, W.; Proksch, P. Unguisin F, a new cyclic peptide from the endophytic fungus Mucor irregularis. Z. Natufors. C 2016, 71c, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Ariawan, A.D.; Webb, J.E.A.; Howe, E.N.W.; Gale, P.A.; Thordarson, P.; Hunter, L. Cyclic peptide unguisin A is an anion receptor with high affinity for phosphate and pyrophosphate. Org. Biomol. Chem. 2017, 15, 2962–2967. [Google Scholar] [CrossRef] [PubMed]
- Loranger, A.; Tuchweber, B.; Gicquaud, C.; St-Pierre, S.; Cote, M.G. Toxicity of peptides of Amanita virosa mushrooms in mice. Fundam. Appl. Toxicol. 1985, 5, 1144–1152. [Google Scholar] [CrossRef]
- Koulman, A.; Lee, T.V.; Fraser, K.; Johnson, L.; Arcus, V.; Lott, J.S.; Rasmussen, S.; Lane, G. Identification of extracellular siderophores and a related peptide from the endophytic fungus Epichloe festucae in culture and endophyte-infected Lolium perenne. Phytochemistry 2012, 75, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, A.L.; Berrue, F.; Robertson, A.W.; Overy, D.P.; Kerr, R.G. Mortiamides A–D, cyclic heptapeptides from a novel Mortierella sp. obtained from Frobisher Bay. J. Nat. Prod. 2017, 80, 2677–2683. [Google Scholar] [CrossRef] [PubMed]
- Krasnoff, S.B.; Keresztes, I.; Gillilan, R.E.; Szebenyi, D.M.E.; Donzelli, B.G.G.; Churchill, A.C.L.; Gibson, D.M. Serinocyclins A and B, cyclic heptapeptides from Metarhizium anisopliae. J. Nat. Prod. 2007, 70, 1919–1924. [Google Scholar] [CrossRef] [PubMed]
- Dewapriya, P.; Prasad, P.; Damodar, R.; Salim, A.A.; Capon, R.J. Talarolide A, a cyclic heptapeptide hydroxamate from an Australian marine tunicate-associated fungus, Talaromyces sp. (CMB-TU011). Org. Lett. 2017, 19, 2046–2049. [Google Scholar] [CrossRef] [PubMed]
- Bara, R.; Aly, A.H.; Wray, V.; Lin, W.H.; Proksch, P.; Debbab, A. Talaromins A and B, new cyclic peptides from the endophytic fungus Talaromyces wortmannii. Tetrahedron Lett. 2013, 54, 1686–1689. [Google Scholar] [CrossRef]
- Feng, Y.; Blunt, J.W.; Cole, A.L.J.; Cannon, J.F.; Robinson, W.T.; Munro, M.H.G. Two novel cytotoxic cyclodepsipeptides from a mycoparasitic Cladobotryum sp. J. Org. Chem. 2003, 68, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Seto, Y.; Takahashi, K.; Matsuura, H.; Kogami, Y.; Yada, H.; Yoshihara, T.; Nabeta, K. Novel cyclic peptide, epichlicin, from the endophytic fungus Epichloe typhina. Biosci. Biotechnol. Biochem. 2007, 71, 1470–1475. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Belofsky, G.N.; Gloer, J.B.; Wicklow, D.T.; Dowd, P.F. Shearamide A: A new cyclic peptide from the ascostromata of Eupenicillium shearii. Tetrahedron Lett. 1998, 39, 5497–5500. [Google Scholar] [CrossRef]
- Xue, J.-H.; Wu, P.; Chi, Y.-L.; Xu, L.-X.; Wei, X.-Y. Cyclopeptides from Amanita exitialis. Nat. Prod. Bioprospect. 2011, 1, 52–56. [Google Scholar] [CrossRef]
- Kim, K.H.; Choi, S.U.; Park, K.M.; Seok, S.J.; Lee, K.R. Cytotoxic constituents of Amanita subjunquillea. Arch. Pharm. Res. 2008, 31, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Adachi, K.; Kanoh, K.; Wisespongp, P.; Nishijima, M.; Shizuri, Y. Clonostachysins A and B, new anti-dinoflagellate cyclic peptides from a marine-derived fungus. J. Antibiot. 2005, 58, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.; Erosa, G.; Sterner, O.; Anke, T. Cylindrocyclin A, a new cytotoxic cyclopeptide from Cylindrocarpon sp. J. Antibiot. 2006, 59, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Azzolin, L.; Antolini, N.; Calderan, A.; Ruzza, P.; Sciacovelli, M.; Marin, O.; Stefano, M.; Paolo, B.; Rasola, A. Antamanide, a derivative of Amantia phalloides, is a novel inhibitor of the mitochondrial permeability transition pore. PLoS ONE 2011, 6, e16280. [Google Scholar] [CrossRef] [PubMed]
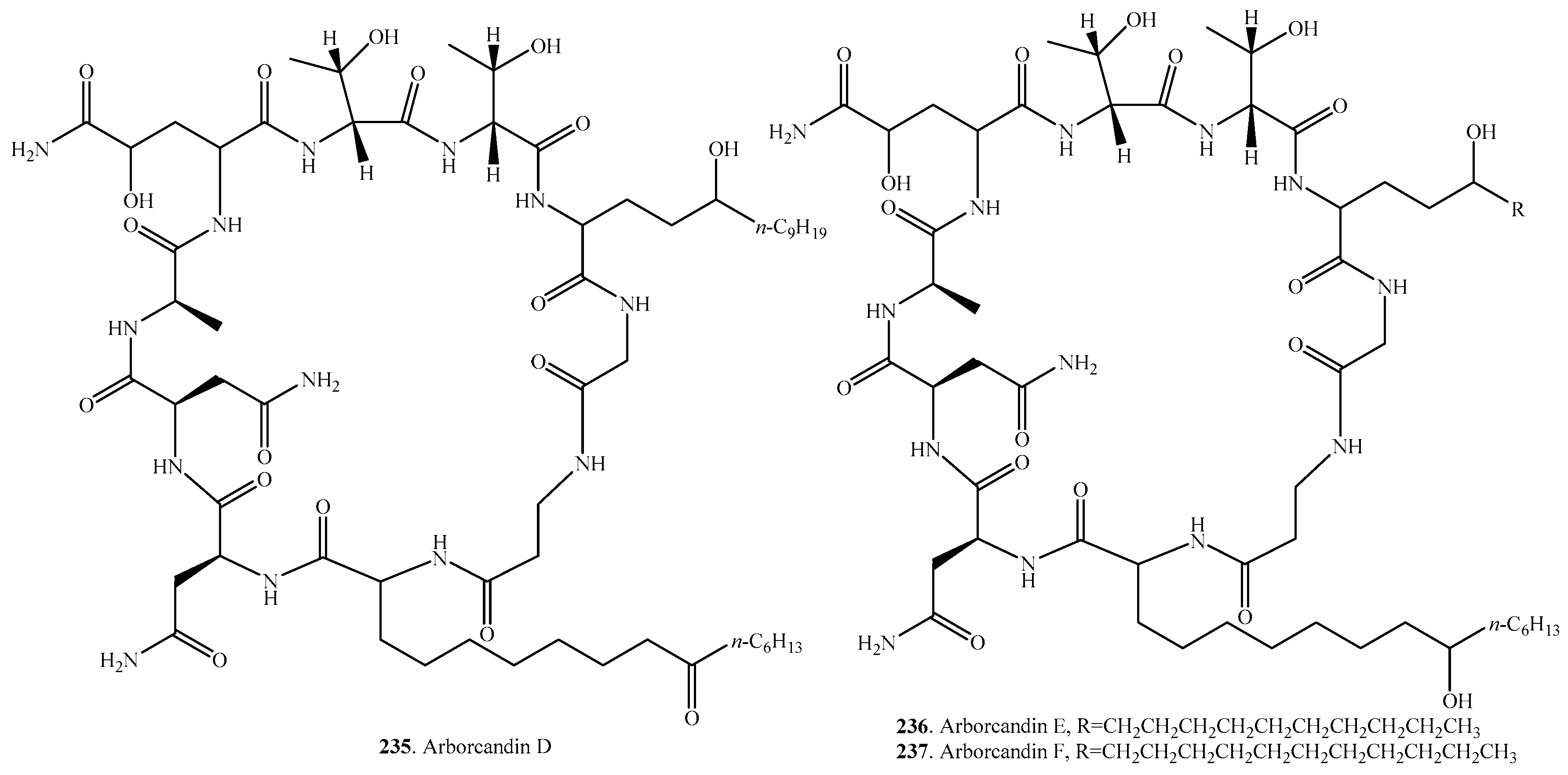
- Ohyama, T.; Kurihara, Y.; Ono, Y.; Ishikawa, T.; Miyakoshi, S.; Hamano, K.; Arai, M.; Suzuki, T.; Igari, H.; Suzuki, Y. Arborcandins A, B, C, D, E and F, novel 1,3-β-glucan synthase inhibitors: Production and biological activity. J. Antibiot. 2000, 53, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Iwadate-kurihara, Y.; Ishikawa, T.; Miyakoshi, S.; Hamano, K.; Inukai, M. Arborcandins A, B, C, D, E and F, novel 1,3-β-glucan synthase inhibitors: Physico-chemical properties and structure elucidation. J. Antibiot. 2003, 56, 1024–1032. [Google Scholar] [CrossRef]
- Wieland, T. The discovery, isolation, elucidation of structure, and sysnthesis of antamanide. Angew. Chem. 1968, 7, 204–208. [Google Scholar] [CrossRef] [PubMed]
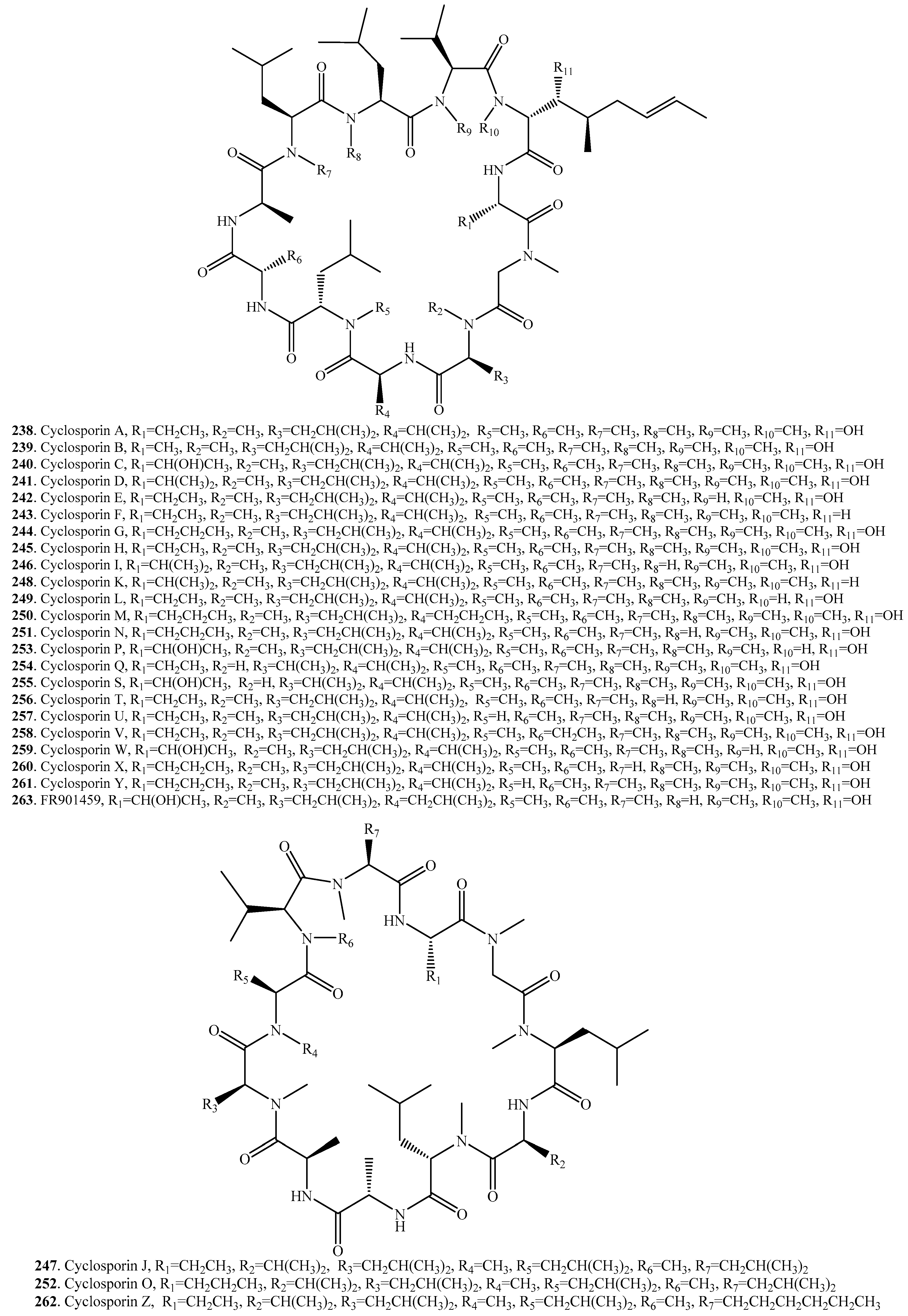
- Survase, S.A.; Kagliwal, L.D.; Annapure, U.S.; Singhal, P.S. Cyclosporin A—A review on fermentative production, donstream processing and pharmacological applications. Biotechnol. Adv. 2011, 29, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Ismaiel, A.A. Production of the immunosuppressant cyclosporine A by a new soil isolate, Aspergillus fumigatus, in submerged culture. Appl. Microbiol. Biotechnol. 2017, 101, 3305–3317. [Google Scholar] [CrossRef] [PubMed]
- Sallam, L.A.R.; El-Refai, A.H.; Hamdi, A.A.; El-Minofi, A.H.; Abd-Elsalam, S.I. Role of some fermentation parameters on cyclosporine A production by a new isolate of A. terreus. J. Gen. Appl. Microbiol. 2003, 49, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Margaritis, A.; Chahal, P.S. Development of a fructose based medium for biosynthesis of cyclosporine-A by Bearuveria nivea. Biotechnol. Lett. 1989, 11, 765–768. [Google Scholar] [CrossRef]
- Sharmila, K.; Thillaimaharani, K.A.; Logesh, A.R.; Sathishkumar, A.; Kalaiselvam, M. Production of cyclosporin A by saprophytic filamentous fungus Fusarium oxysporum. Int. J. Pharm. Pharm. Sci. 2012, 4, 149–153. [Google Scholar]
- Traber, R.; Kuhn, M.; Loosli, H.R.; Pache, W.; Von Wartburg, A. Neue cycolpeptide aus Trichoderma polysporum (LINK EX PERS.) RIFAI: Die cyclosporine B, D und E. Helv. Chim. Acta 1977, 60, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Kobel, H.; Traber, R. Directed biosynthesis of cyclosporins. Eur. J. Appl. Microbiol. Biotechnol. 1982, 14, 237–240. [Google Scholar] [CrossRef]
- Sekar, C.; Balaraman, K. Immobilization of the fungus, Tolypocladium sp. for the production of cyclosporin A. Bioprocess Eng. 1998, 19, 281–283. [Google Scholar] [CrossRef]
- Sakamoto, K.; Tsujii, E.; Miyauchi, M.; Nakanishi, T.; Yamashita, M.; Shigematsu, N.; Tada, T.; Izumi, S.; Okuhara, M. FR901459, a novel immunosuppressant isolated from Stachybotrys chartarum No. 19392. Taxonomy of the producing organism, fermentation, isolation, phytico-chemical properties and biological activities. J. Antibiot. 1993, 46, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, Y.; Furuichi, Y.; Tojo, N.; Moriguchi, A.; Maemoto, T.; Nakada, H.; Hino, M.; Matsuoka, N. Neuroprotective efficacy of FR901459, a novel derivative of cyclosporine A, in vitro mitochondrial damage and in vivo transient cerebral ischemia models. Brain Res. 2007, 1149, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Traber, R.; Hofmann, H.; Loosli, H.R.; Ponelle, M.; von Warburg, A. Neue cyclosprorine aus Tolypocladium inflatum. Die cyclosporine K–Z. Helv. Chim. Acta 1987, 70, 13–36. [Google Scholar] [CrossRef]
- Moussaif, M.; Jacques, P.; Schaarwachter, P.; Budzikiewicz, H.; Thonart, P. Cyclosporin C is the main antifungal compound produced by Acremonium luzulae. Appl. Environ. Microbiol. 1997, 63, 1739–1743. [Google Scholar] [PubMed]
- Traber, R.; Loosli, H.R.; Hofmann, H.; Kuhn, M.; Von Wartburg, A. Isolation and structure dertermination of the new cyclosporins E, F, G, H and I. Helv. Chim. Acta 1982, 65, 1655–1677. [Google Scholar] [CrossRef]
- Jegorov, A.; Matha, V.; Sedmera, P.; Havlicek, V.; Josef, S.; Seidel, P.; Simek, P. Cyclosporins from Tolypocladium terricola. Phytochemistry 1995, 38, 403–407. [Google Scholar] [CrossRef]
- Song, Q.-Y.; Nan, Z.-B.; Gao, K.; Song, H.; Tian, P.; Zhang, X.-X.; Li, C.-J.; Xu, W.-B.; Li, X.-Z. Antifungal, phytotoxic, and cytotoxic activities of metabolites from Epichloe bromicola, a fungus obtained from Elymus tangutorum grass. J. Agric. Food Chem. 2015, 63, 8787–8792. [Google Scholar] [CrossRef] [PubMed]
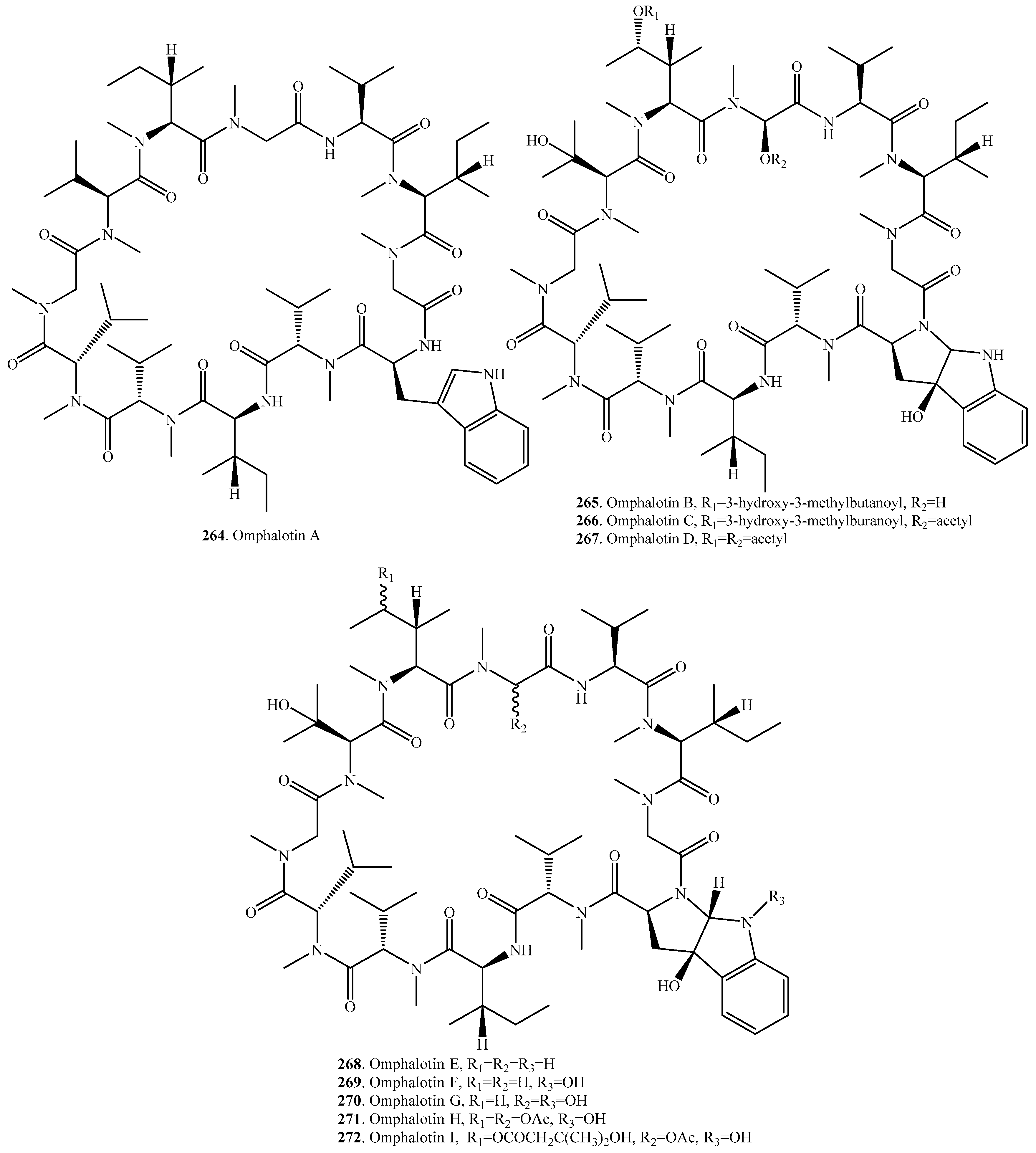
- Buchel, E.; Martini, U.; Mayer, A.; Anke, H.; Sterner, O. Omphalotins B, C and D, nematicidal cyclopeptides from Omphalotus olearius. Absolute configuration of omphalotin A. Tetradedron 1998, 54, 5345–5352. [Google Scholar] [CrossRef]
- Liermann, J.C.; Opatz, T.; Kolshorn, H.; Antelo, L.; Hof, C.; Anke, H. Omphalotins E–I, five oxidatively modified nematicidal cyclopeptides from Omphalotus olearius. Eur. J. Org. Chem. 2009, 1256–1262. [Google Scholar] [CrossRef]
- Anke, H.; Sterner, O. Nematicidal metabolites from higher fungi. Curr. Org. Chem. 1997, 1, 361–374. [Google Scholar]
- Ramm, S.; Krawczyk, B.; Muhlenweg, A.; Poch, A.; Mosker, E.; Sussmuth, R.D. A self-sacrificing N-methyltransferase is the precursor of the fungal natural product omphalotin. Angew. Chem. Int. Ed. 2017, 56, 9994–9997. [Google Scholar] [CrossRef] [PubMed]
- Aldemir, H.; Gulder, T.A.M. Expanding the structural space of ribosomal peptides: Autocatalytic N-methylation in omphalotin biosynthesis. Angew. Chem. Int. Ed. 2017, 56, 13570–13572. [Google Scholar] [CrossRef] [PubMed]
- Van der Velden, N.S.; Kalin, N.; Helf, M.J.; Piel, J.; Freeman, M.F.; Kunzler, M. Autocatalytic backbone N-methylation in a family of ribosomal peptide natural products. Nat. Chem. Biol. 2017, 13, 833–835. [Google Scholar] [CrossRef] [PubMed]
- Mayer, A.; Anke, H.; Sterner, O. Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius I. Fermentation and biological activity. Nat. Prod. Lett. 1997, 10, 25–32. [Google Scholar] [CrossRef]
- Sterner, O.; Etzel, W.; Mayer, A.; Anke, H. Omphalotin, a new cyclic peptide with potent nematicidal activity from Omphalotus olearius II. Isolation and structure determination. Nat. Prod. Lett. 1997, 10, 33–38. [Google Scholar] [CrossRef]
- Mayer, A.; Kilian, M.; Hoster, B.; Sterner, O.; Anke, H. In-vitro and in-vivo nematicidal activities of the cyclic dodecapeptide omphalotin A. Pestic. Sci. 1999, 55, 27–30. [Google Scholar] [CrossRef]
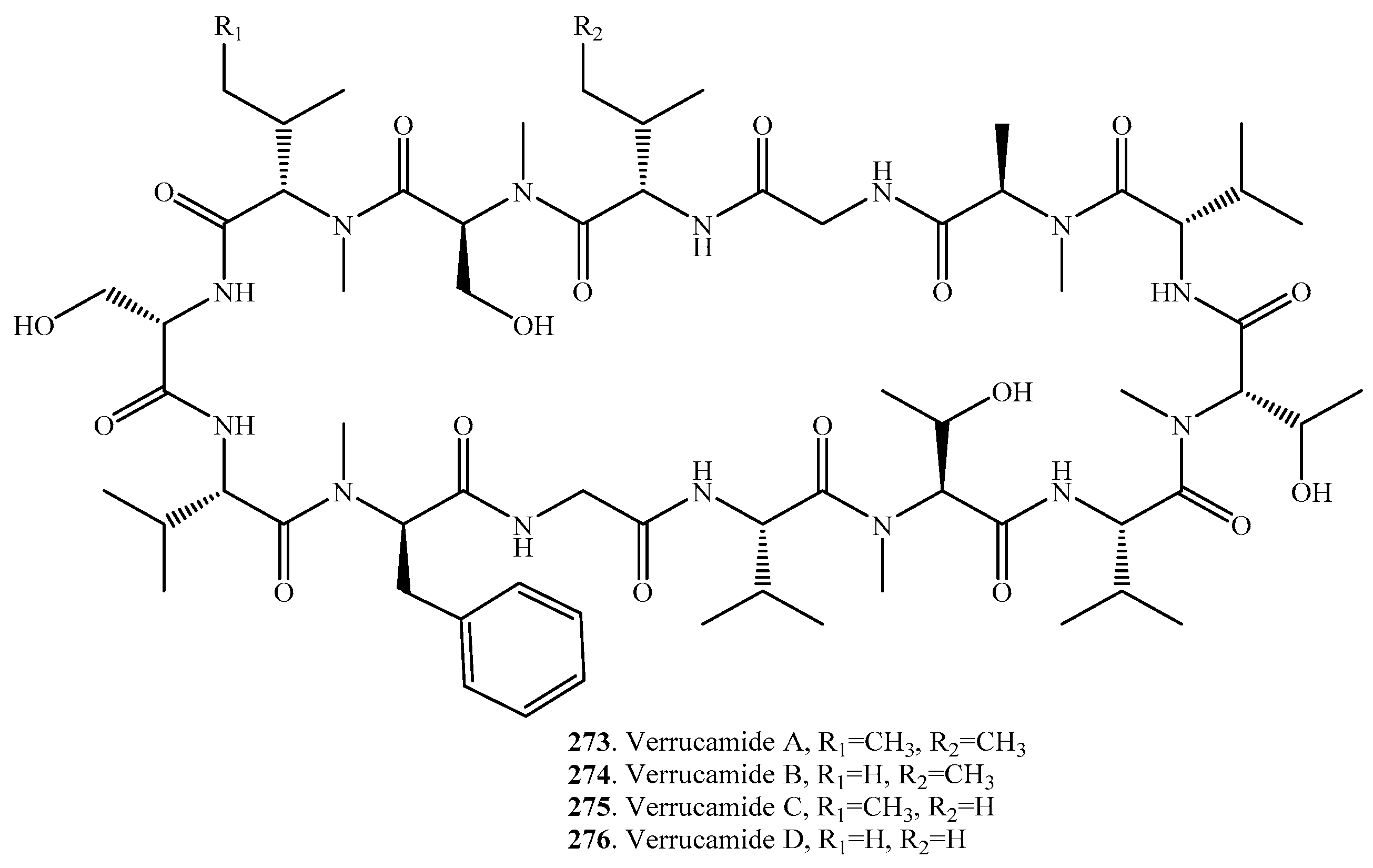
- Zou, X.; Niu, S.; Ren, J.; Li, E.; Liu, X.; Che, Y. Verrucamides A–D, antibacterial cyclopeptides from Myrothecium verrucaria. J. Nat. Prod. 2011, 74, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
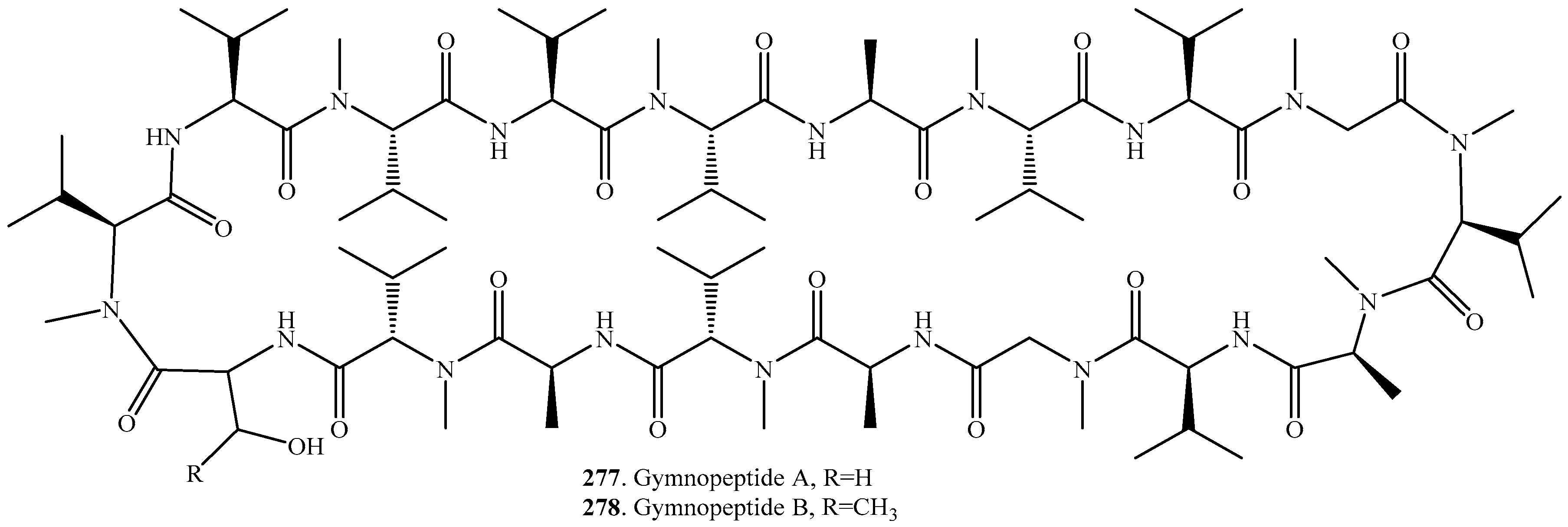
- Vanyolos, A.; Dekany, M.; Kovacs, B.; Kramos, B.; Berdi, P.; Zupko, I.; Hohmann, J.; Beni, Z. Gymnopeptides A and B, cyclic octadecapeptides from the mushroom Gymnopus fusipes. Org. Lett. 2016, 18, 2688–2691. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Wu, C.; Wang, W.; Cheng, Z.; Yao, G.; Liu, K.; Li, H.; Fang, L.; Su, W. Total synthesis and stereochemical assignment of gymnopeptides A and B. Org. Lett. 2017, 19, 4420–4423. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Umemura, M.; Izumikawa, M.; Kawano, J.; Ishii, T.; Kikuchi, M.; Tomii, K.; Kumagai, T.; Yoshimi, A.; Machida, M.; et al. Class of cyclic ribosomal peptide synthetic genes in filamentous fungi. Fungal Genet. Biol. 2016, 86, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Kono, Y.; Takeuchi, S.; Daly, J.M. Structure of HV-toxin M, a host-specific toxin-related compound produced by Helminthosporium victoriae. Agric. Biol. Chem. 1989, 53, 1283–1290. [Google Scholar] [CrossRef]
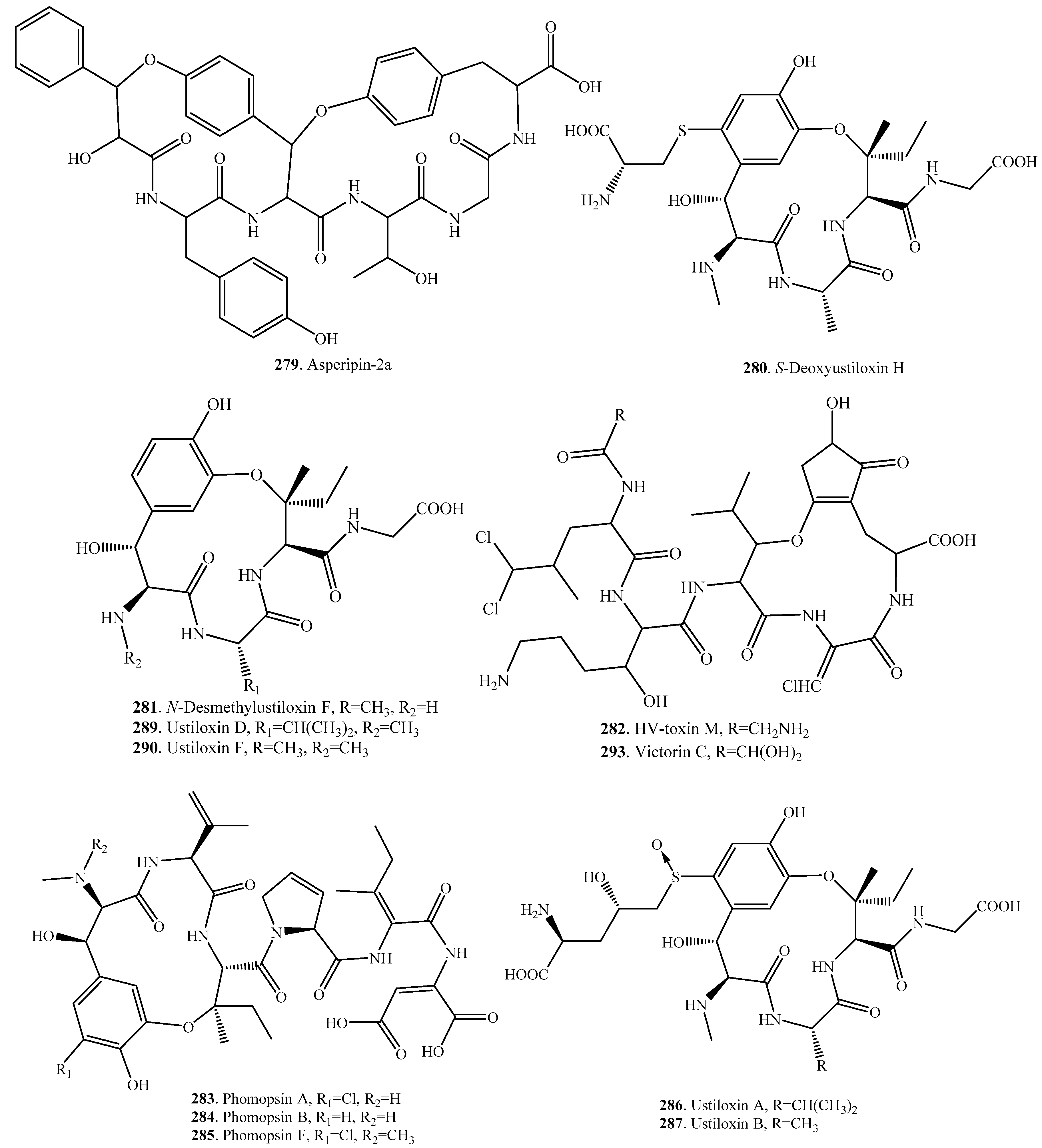
- Culvenor, C.C.J.; Edgar, J.A.; Mackay, M.F.; Gorst-Allman, C.P.; Marasas, W.F.O.; Steyn, P.S.; Vleggaar, R.; Wessels, P.L. Structure elucidation and absolute configuration of phomopsin A, a hexapeptide mycotoxin produced by Phomopsis leptostromiformis. Tetrahedron 1989, 45, 2351–2372. [Google Scholar] [CrossRef]
- Allen, J.G.; Hancock, G.R. Evidence that phomopsins A and B are not the only toxic metabolites produced by Phomopsis leptostromiformis. J. Appl. Toxicol. 1989, 9, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Schlob, S.; Hackl, T.; Herz, C.; Lamy, E.; Koch, M.; Rohn, S.; Maul, R. Dectection of a toxic methylated derative of phomopsin A produced by the legume-infesting fungus Diaporthe toxica. J. Nat. Prod. 2017, 80, 1930–1934. [Google Scholar]
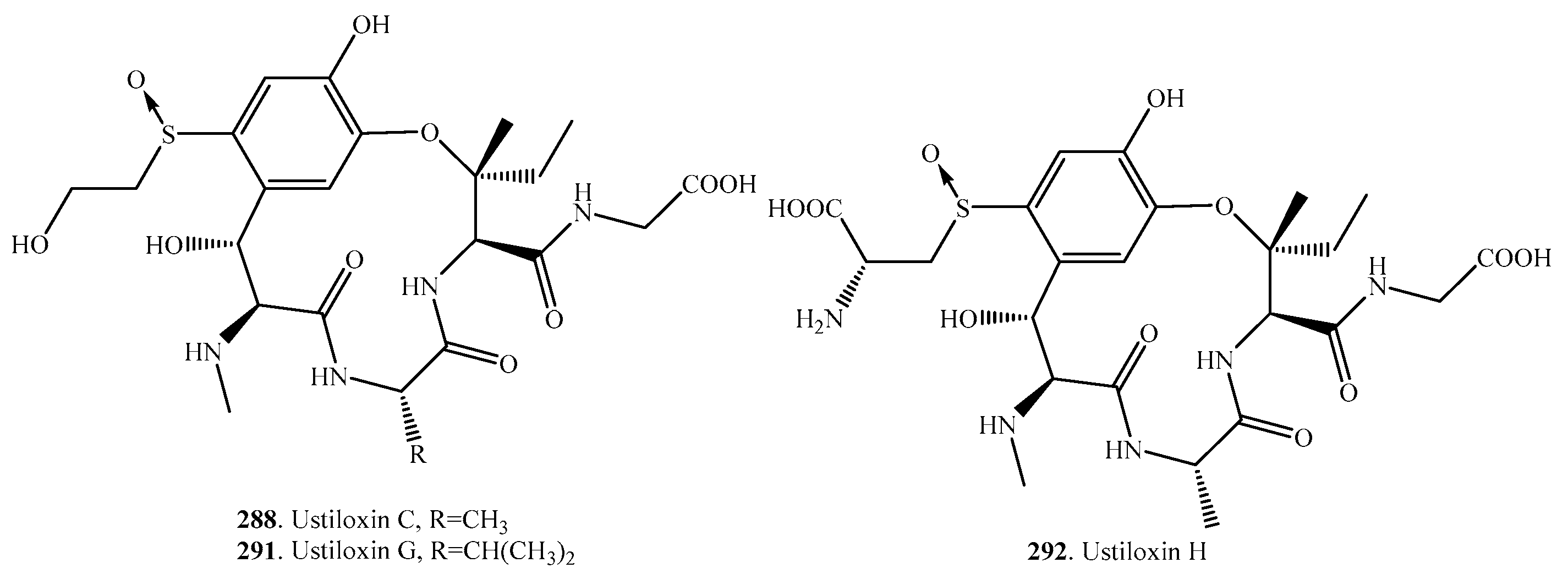
- Koiso, Y.; Li, Y.; Iwasaki, S.; Hanaoka, K.; Kobayashi, T.; Sonoda, R.; Fujita, Y.; Yaegashi, H.; Sato, Z. Ustiloxins, antimitotic cyclic peptides from false smut balls on rice panicles caused by Ustilaginoidea virens. J. Antibiot. 1994, 47, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Koiso, Y.; Morisaki, N.; Yamashita, Y.; Mitsui, Y.; Shirai, R.; Hashimoto, Y.; Iwasaki, S. Isolation and structure of an antimitotic cyclic peptide, ustiloxin F: Chemical interrelation with a homologous peptide, ustiloxin B. J. Antibiot. 1998, 51, 418–422. [Google Scholar] [CrossRef] [PubMed]
- Shan, T.; Sun, W.; Wang, X.; Fu, X.; Sun, W.; Zhou, L. Purification of ustiloxins A and B from rice false smut balls by macroporous resins. Molecules 2013, 18, 8181–8199. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Fu, X.; Lin, F.; Sun, W.; Meng, J.; Wang, A.; Lai, D.; Zhou, L.; Liu, Y. The contents of ustiloxins A and B along with their distribution in rice false smut balls. Toxins 2016, 8, 262. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Lai, D.; Wang, W.; Dai, J.; Zhou, L.; Liu, Y. Ustiloxin G, a new cyclopeptide mycotoxin from rice false smut balls. Toxins 2017, 9, 54. [Google Scholar] [CrossRef] [PubMed]
- Umemura, M.; Nagano, N.; Koike, H.; Kawano, J.; Ishi, T.; Miyamura, Y.; Kikuchi, M.; Tamano, K.; Yu, J.; Shin-ya, K.; et al. Characterization of the biosynthetic gene cluster for the ribosomally synthesized cyclic peptide ustiloxin B in Aspergillus flavus. Fungal Genet. Biol. 2014, 68, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Minami, A.; Igarashi, Y.; Izumikawa, M.; Umemura, M.; Nagano, N.; Mashida, M.; Kawahara, T.; Shin-ya, K.; Gomi, K.; et al. Unveiling the biosynthetic pathway of the ribosomally synthesized and post-trnslationally modified peptide ustiloxin B in filamentous fungi. Angew. Chem. Int. Ed. 2016, 55, 8072–8075. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, T.J.; Macko, V.; Acklin, W.; Jaun, B.; Seibl, J.; Meili, J.; Arigoni, D. Structure of victorin C, the major host-selective toxin from Cochliobolus victoriae. Experientia 1985, 41, 1524–1529. [Google Scholar] [CrossRef]
- Hallen, H.E.; Luo, H.; Scott-Craig, J.S.; Walton, J.D. Gene family encoding the major toxins of lethal Amanita mushrooms. Proc. Natl. Acad. Sci. USA 2007, 104, 19097–19101. [Google Scholar] [CrossRef] [PubMed]
- Hershenhorn, J.; Casella, F.; Vurro, M. Weed biocontrol with fungi: Past, present and future. Biocontrol Sci. Technol. 2016, 26, 1313–1328. [Google Scholar] [CrossRef]
- Brindisi, M.; Maramai, S.; Brogi, S.; Fanigliulo, E.; Butini, S.; Guarino, E.; Casagni, A.; Lamponi, S.; Bonechi, C.; Nathwani, S.M.; et al. Development of novel cyclic peptides as pro-apoptotic agens. Eur. J. Med. Chem. 2016, 117, 301–320. [Google Scholar] [CrossRef] [PubMed]
- Bills, G.; Li, Y.; Chen, L.; Yue, Q.; Niu, X.-M.; An, Z. New insights into the echinocandins and other fungal non-ribosomal peptides and peptaibiotics. Nat. Prod. Rep. 2014, 31, 1348–1375. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Echinocandin antifngal drugs. Lancet 2003, 362, 1142–1151. [Google Scholar] [CrossRef]
- Enoch, D.A.; Idris, S.F.; Aliyu, S.H.; Micallef, C.; Sule, O.; Karas, J.A. Micafungin for the treatment of invasive apsergillosis. J. Infect. 2014, 68, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Matasyoh, J.C. Endophytes as producers of peptides: An overview about the recently discovered peptides from endophytic microbes. Nat. Prod. Bioprospect. 2014, 4, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Drugs and drug candidates from marine source: An assessment of the current “State of Play”. Planta Med. 2016, 82, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Phat, C.; Hong, S.-C. Structural diversity of marine cyclic peptides and their molecular mechanisms for anticancer, antibacterial, antifungal, and other clinical applications. Peptides 2017, 95, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Klimova, E.; Rodriguez-Pena, K.; Sanchez, S. Endophytes as sources of antibiotics. Biochem. Pharmacol. 2017, 134, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef] [PubMed]
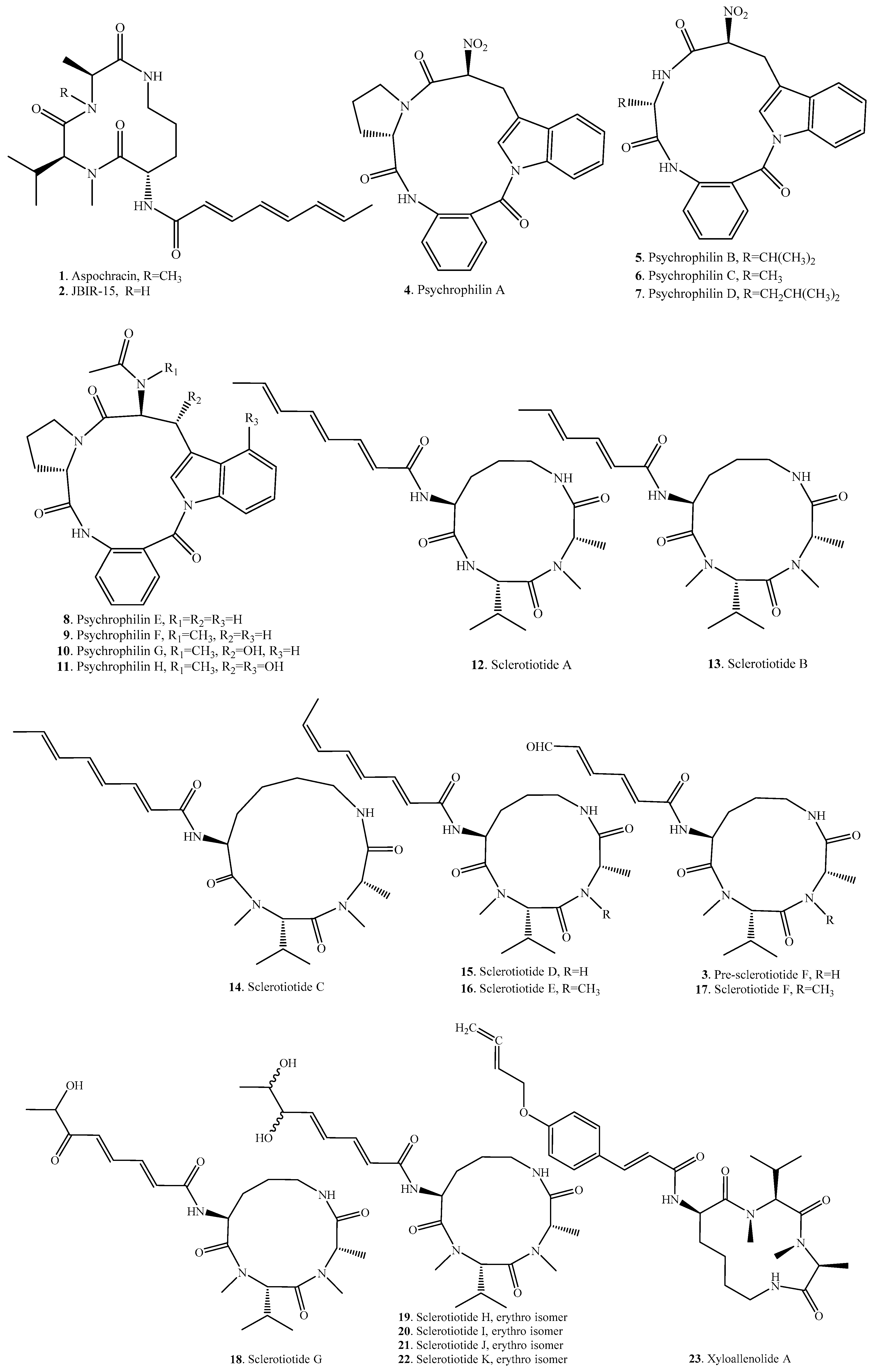
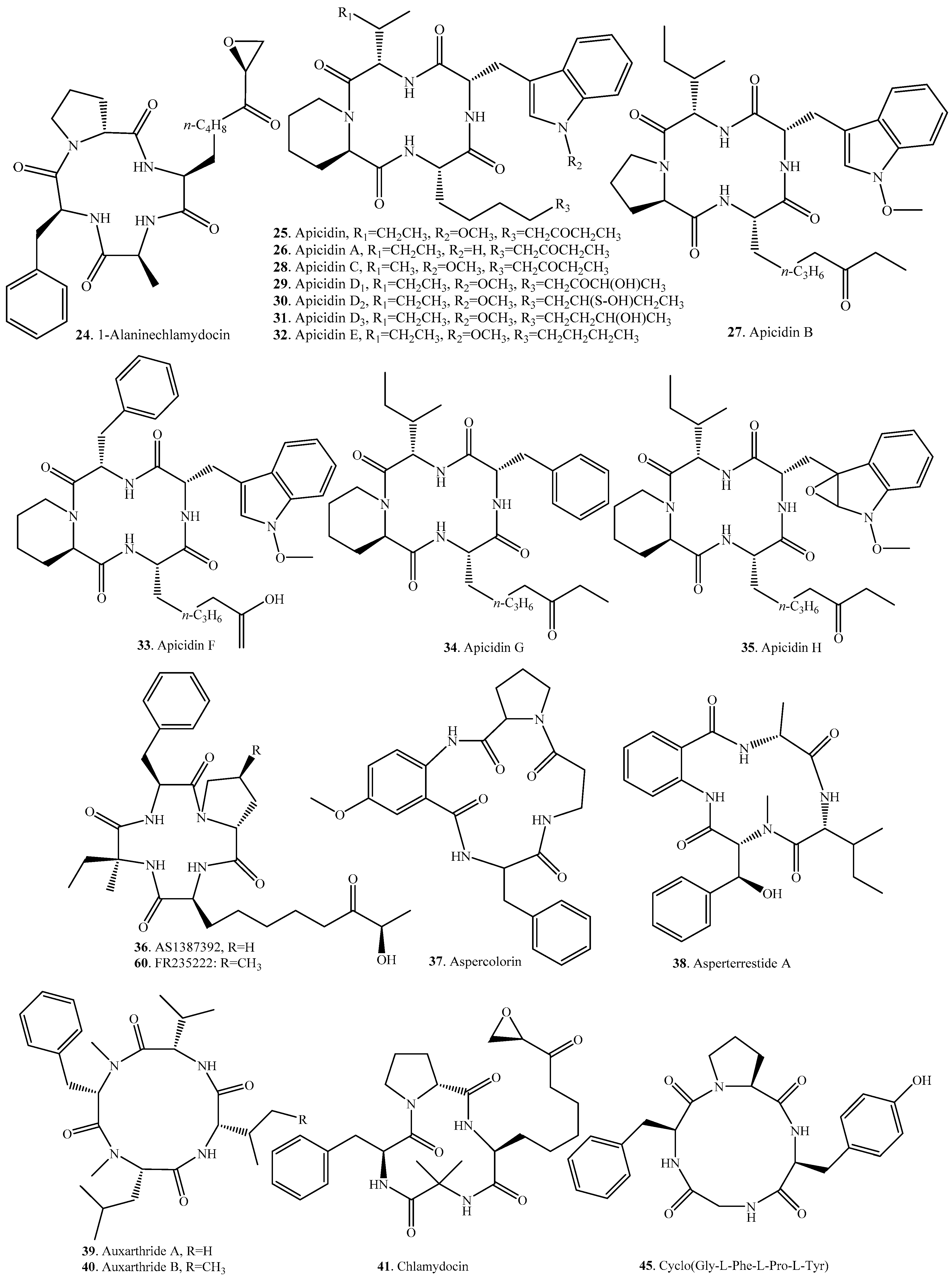
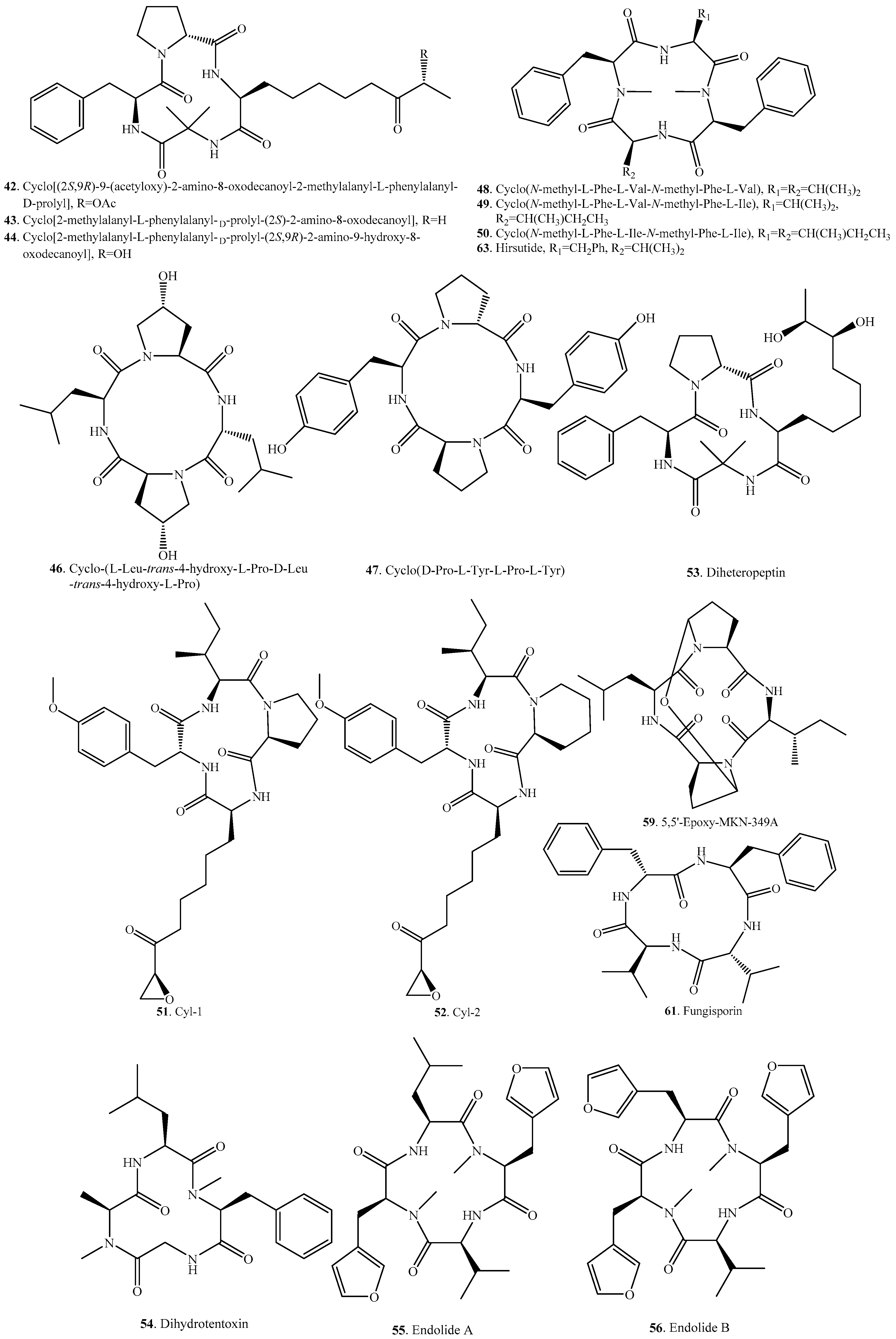
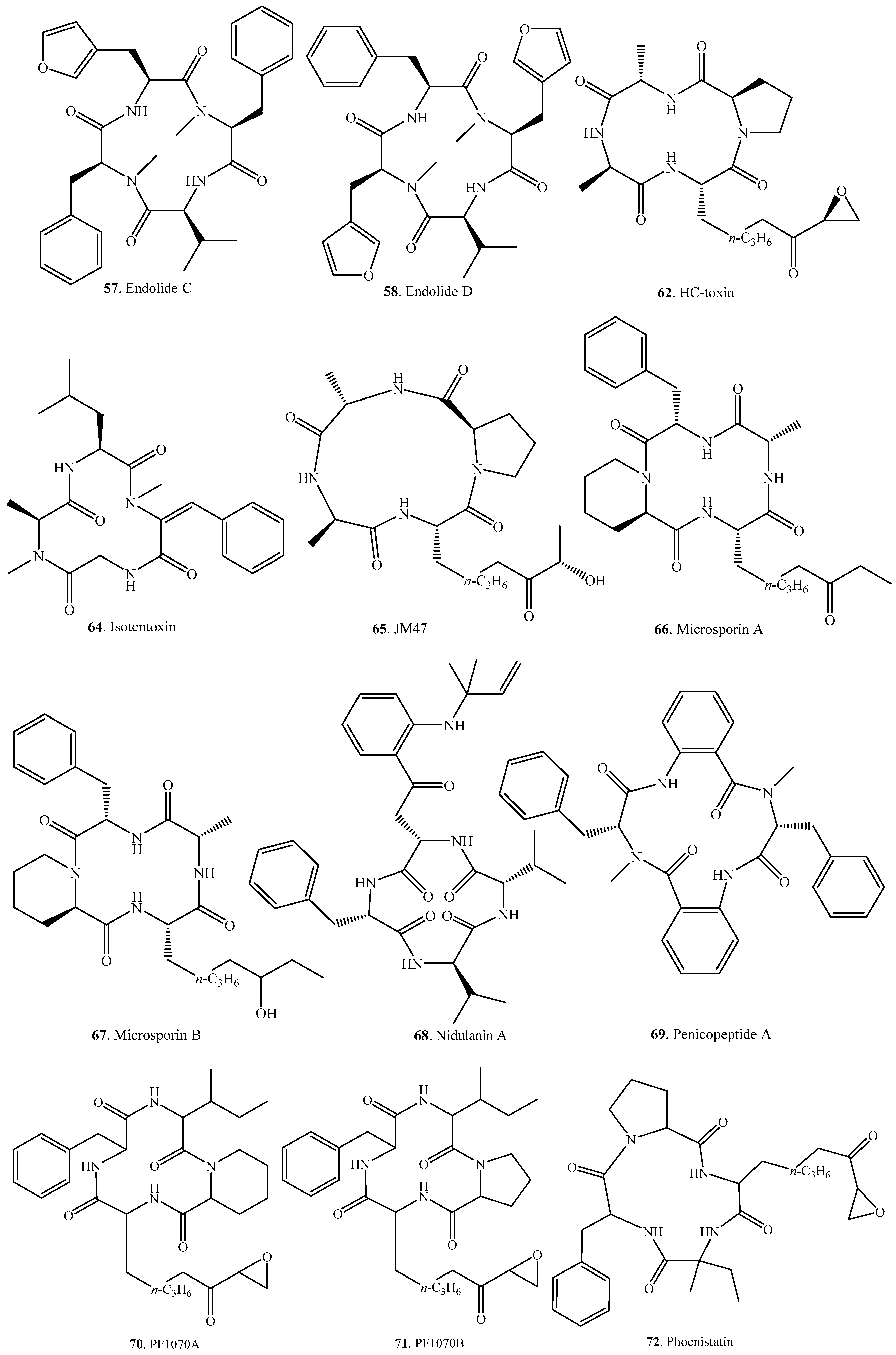


| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Aspochracin (1) | Aspergillus kumbius | - | [24] |
| Aspergillus ochraceus | Insecticidal activity | [17] | |
| Aspergillus sclerotiorum PT06-1 | - | [19] | |
| JBIR-15 (2) | Aspergillus sclerotiorum | Antifungal activity | [19,25] |
| Pre-sclerotiotide F (3) | Aspergillus insulicola | - | [26] |
| Psychrophilin A (4) | Penicillium ribeum from a soil under the tree Ribes sp. | - | [20] |
| Psychrophilin B (5) | Penicillium ribeum from a soil under the tree Ribes sp. | - | [21] |
| Psychrophilin C (6) | Penicillium ribeum from a soil under the tree Ribes sp. | - | [21] |
| Psychrophilin D (7) | Penicillium algidum | Cytotoxic activity | [18] |
| Psychrophilin E (8) | Aspergillus versicolor ZLN-60 | Antiproliferative activity | [22,23] |
| Psychrophilin F (9) | Aspergillus versicolor ZLN-60 | - | [23] |
| Psychrophilin G (10) | Aspergillus versicolor ZLN-60 | Lipid-lowering effect | [23] |
| Psychrophilin H (11) | Aspergillus versicolor ZLN-60 | - | [23] |
| Sclerotiotide A (12) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Sclerotiotide B (13) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Sclerotiotide C (14) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide D (15) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide E (16) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide F (17) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Aspergillus insulicola | - | [26] | |
| Sclerotiotide G (18) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide H (19) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide I (20) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [19] |
| Sclerotiotide J (21) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Sclerotiotide K (22) | Aspergillus sclerotiorum PT06-1 | - | [19] |
| Xyloallenolide A (23) | Xylaria sp. No. 2508 | - | [27] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| 1-Alaninechlamydocin (24) | Tolypocladium sp. | Inhibitory activity on histone deacetyl (HDAC) activity, antiproliferation, cytotoxicity, cell cycle arrest, and apoptosis induction | [30] |
| Apicidin (25) | Fusarium pallidoroseum | Antimalarial activity | [32] |
| Fusarium semitectum | Phytotoxic activity | [53] | |
| - | Antiprotozoal activity by inhibiting parasite HDAC | [35] | |
| Apicidin A (26) | Fusarium pallidoroseum | Antimalarial activity | [32] |
| Fusarium semitectum | - | [53] | |
| Apicidin B (27) | Fusarium pallidoroseum | Antiprotozoal activity | [33] |
| Apicidin C (28) | Fusarium pallidoroseum | Antiprotozoal activity | [33] |
| Apicidin D1 (29) | Fusarium pallidoroseum | Antiprotozoal activity | [34] |
| Apicidin D2 (30) | Fusarium pallidoroseum | Antiprotozoal activity | [34] |
| Fusarium semitectum | Phytotoxic activity | [53] | |
| Apicidin D3 (31) | Fusarium pallidoroseum | Antiprotozoal activity | [34] |
| Apicidin E (32) | Fusarium semitectum mutant | - | [54] |
| Apicidin F (33) | Fusarium fujikuroi | Antimalarial activity | [55] |
| Apicidin G (34) | Fusarium semitectum | - | [56] |
| Apicidin H (35) | Fusarium semitectum | - | [56] |
| AS1387392 (36) | Acremonium sp. No. 27082 | Immunosuppressive activity | [37] |
| Aspercolorin (37) | Aspergillus versicolor | - | [57] |
| Asperterrestide A (38) | Aspergillus terreus SCSGAF0162 | Cytotoxic and antiviral activity | [38] |
| Auxarthride A (39) | Coprophilous fungus Auxarthron pseudauxarthron | - | [58] |
| Auxarthride B (40) | Coprophilous fungus Auxarthron pseudauxarthron | - | [58] |
| Chlamydocin (41) | Diheterospora chlantydosporia | Cytostatic activity | [39] |
| Penicillium sp. BCC18034 | Antimalarial and cytotoxic activity | [40] | |
| Cyclo[(2S,9R)-9-(acetyloxy)-2-amino-8-oxodecanoyl-2-methylalanyl-l-phenylalanyl-d-prolyl] (42) | Peniophora sp. | Plant growth-retardant activity | [41] |
| Cyclo[2-methylalanyl-l-phenylalanyl-d-prolyl-(2S)-2-amino-8-oxodecanoyl] (43) | Peniophora sp. | Plant growth-retardant activity | [41] |
| Cyclo[2-methylalanyl-l-phenylalanyl-d-prolyl-(2S,9R)-2-amino-9-hydroxy-8-oxodecanoyl] (44) | Peniophora sp. | Plant growth-retardant activity | [41] |
| Verticillium coccosporum | Phytotoxic activity | [59] | |
| Cyclo(Gly-l-Phe-l-Pro-l-Tyr) (45) | Co-culture broth of two mangrove fungi Phomopsis sp. K38 and Alternaria sp. E33 | Antifungal activity | [42] |
| Cyclo(l-Leu-trans-4-hydroxy-l-Pro-d-Leu-trans-4-hydroxy-l-Pro) (46) | Co-culture broth of two mangrove fungi Phomopsis sp. K38 and Alternaria sp. E33 | Antifungal activity | [43] |
| Cyclo(d-Pro-l-Tyr-l-Pro-l-Tyr) (47) | Co-culture broth of two mangrove fungi Phomopsis sp. K38 and Alternaria sp. E33 | Antifungal activity | [42] |
| Cyclo(N-methyl-l-Phe-l-Val-N-methyl-Phe-l-Val) (48) | Onychocola sclerotica | Cardiac calcium channel blocker | [44] |
| Cyclo(N-methyl-l-Phe-l-Val-N-methyl-l-Phe-l-Ile) (49) | Onychocola sclerotica | Cardiac calcium channel blocker | [44] |
| Cyclo(N-methyl-l-Phe-l-Ile-N-methyl-l-Phe-l-Ile) (50) | Onychocola sclerotica | Cardiac calcium channel blocker | [44] |
| Cyl-1 (51) | Cylindrocladium scoparium | Phytotoxic activity | [45,46] |
| Cyl-2 (52) | Cylindrocladium scoparium | Phytotoxic activity | [46,60] |
| Diheteropeptin (53) | Diheterospora sp. | TGF-β-like activity | [61] |
| Diheterospora chlamydosporia | TGF-β-like activity | [62] | |
| Dihydrotentoxin (54) | Alternaria porri | - | [63] |
| Phoma sp. | - | [51] | |
| Endolide A (55) | Stachylideium sp. from the sponge Callyspongia sp. | Affinity to the vaspopressin receptor 1A with a Ki of 7.04 μM | [64] |
| Endolide B (56) | Stachylideium sp. from the sponge Callyspongia sp. | Be selevtive toward the serotonin receptor 5HT2b with a Ki of 0.77 μM | [64] |
| Endolide C (57) | Marine-sponge-derived Stachylidium sp. 293 K04 | - | [65] |
| Endolide D (58) | Marine-sponge-derived Stachylidium sp. 293 K04 | - | [65] |
| 5,5’-Epoxy-MKN-349A (59) | Endophytic fungus Penicillium sp. GD6 from the mangrove Bruguiera gymnorrhiza | - | [66] |
| FR235222 (60) | Acremonium sp. No. 27082 | Immunosuppressant that inhibits mammalian histone deacetylase | [67,68,69] |
| Fungisporin (61) | Penicillium chrysogenum | - | [70] |
| HC-toxin (62) | Helminthosporium carbonum = Cochliobolus carbonum | Phytotoxic activity | [71,72] |
| Hirsutide (63) | Spider-derived fungus Hirsutella sp. | - | [73] |
| Isotentoxin (64) | Alternaria porri | - | [63] |
| JM47 (65) | Marine-derived Fusarium sp. from the alga Codium fragile | - | [29] |
| Microsporin A (66) | Microsporum cf. gypseum CNL-692 | Inhibitory activity on histone deacetylase; cytotoxic activity | [47] |
| Microsporin B (67) | Microsporum cf. gypseum CNL-692 | Inhibitory activity on histone deacetylase; cytotoxic activity | [47] |
| Nidulanin A (68) | Aspergillus nidulans | - | [74] |
| Penicopeptide A (69) | Endophytic Penicillium commune from Vitis vinifera | Inhibitrory activity on 11β-hydroxysteroid dehydrogense | [48] |
| PF1070A (70) | Calonectria ilicicola | Phytotoxic activity | [75] |
| Humicola sp. | Antitumour activity | [76] | |
| PF1070B (71) | Humicola sp. | Antitumour activity | [76] |
| Phoenistatin (72) | Acremonium fusigerum | Enhancing activity on gene expression | [77] |
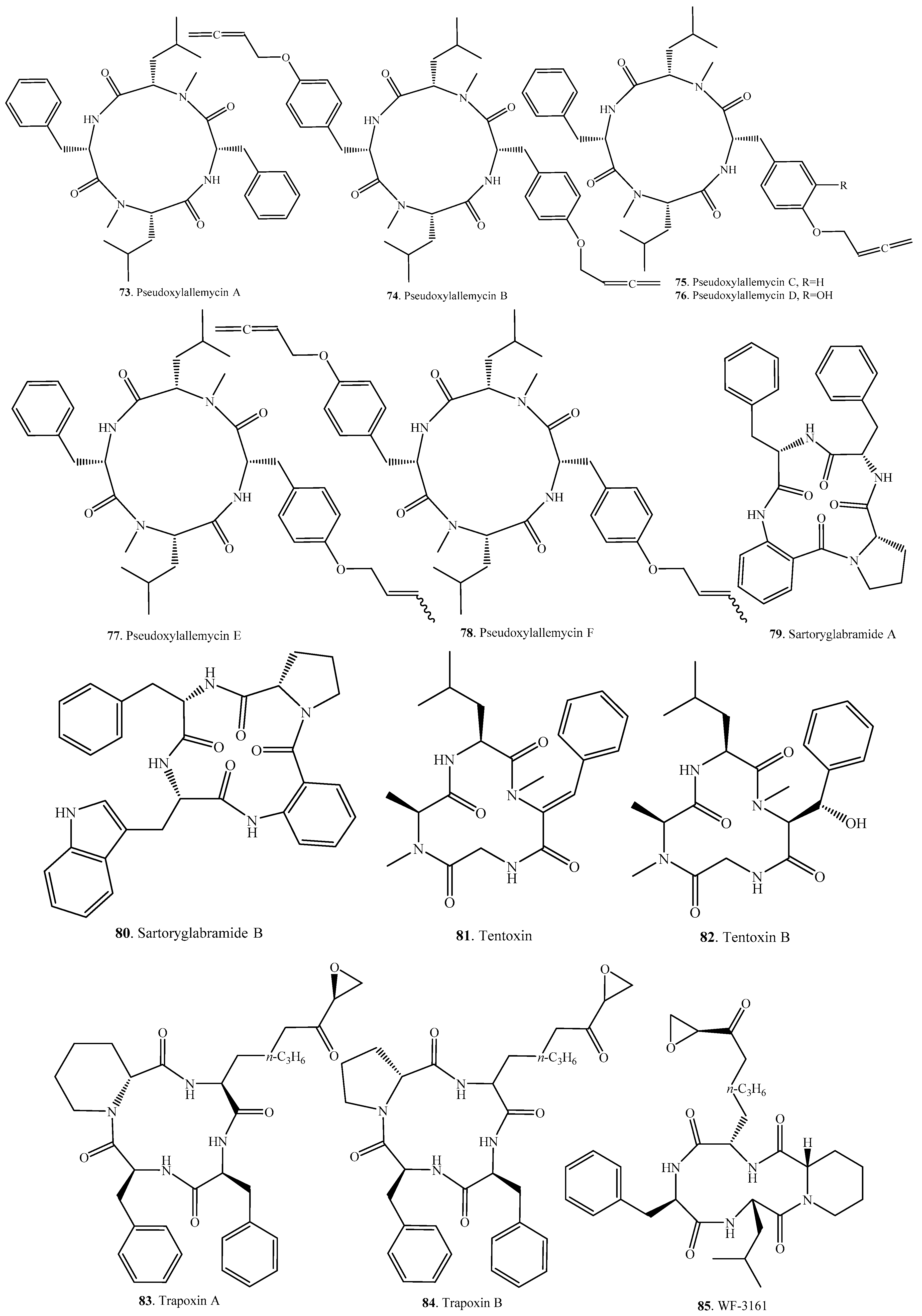
| Pseudoxylallemycin A (73) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin B (74) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin C (75) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin D (76) | Termite-associated fungus Pseudoxylaria sp. X802 | Antibacterial and antiproliferative activities | [49] |
| Pseudoxylallemycin E (77) | Termite-associated fungus Pseudoxylaria sp. X802 | - | [49] |
| Pseudoxylallemycin F (78) | Termite-associated fungus Pseudoxylaria sp. X802 | - | [49] |
| Sartoryglabramide A (79) | Marine-derived Neosartorya glabra KUFA 0702 from the sponge Mycale sp. | - | [78] |
| Sartoryglabramide B (80) | Marine-derived Neosartorya glabra KUFA 0702 from the sponge Mycale sp. | - | [78] |
| Tentoxin (81) | Alternaria linicola | Phytotoxic activity | [79] |
| Alternaria porri | - | [63] | |
| Phoma sp. | - | [51] | |
| Tentoxin B (82) | Marine-derived fungus Phoma sp. from the giant jellyfish Nemopilema nomurai | Weak suppressive effect on the production of nitric oxide in murine macrophage cells without notable cytotoxicity | [51] |
| Trapoxin A (83) | Helicoma ambiens RF-1023 | Detransformation activity against v-sis oncogene-transformed NIH3T3 cells | [52] |
| - | HDAC inhibitory activity | [31] | |
| Trapoxin B (84) | Helicoma ambiens RF-1023 | Detransformation activity against v-sis oncogene-transformed NIH3T3 cells | [52] |
| WF-3161 (85) | Petriella guttlata | Antitumor activity | [80] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
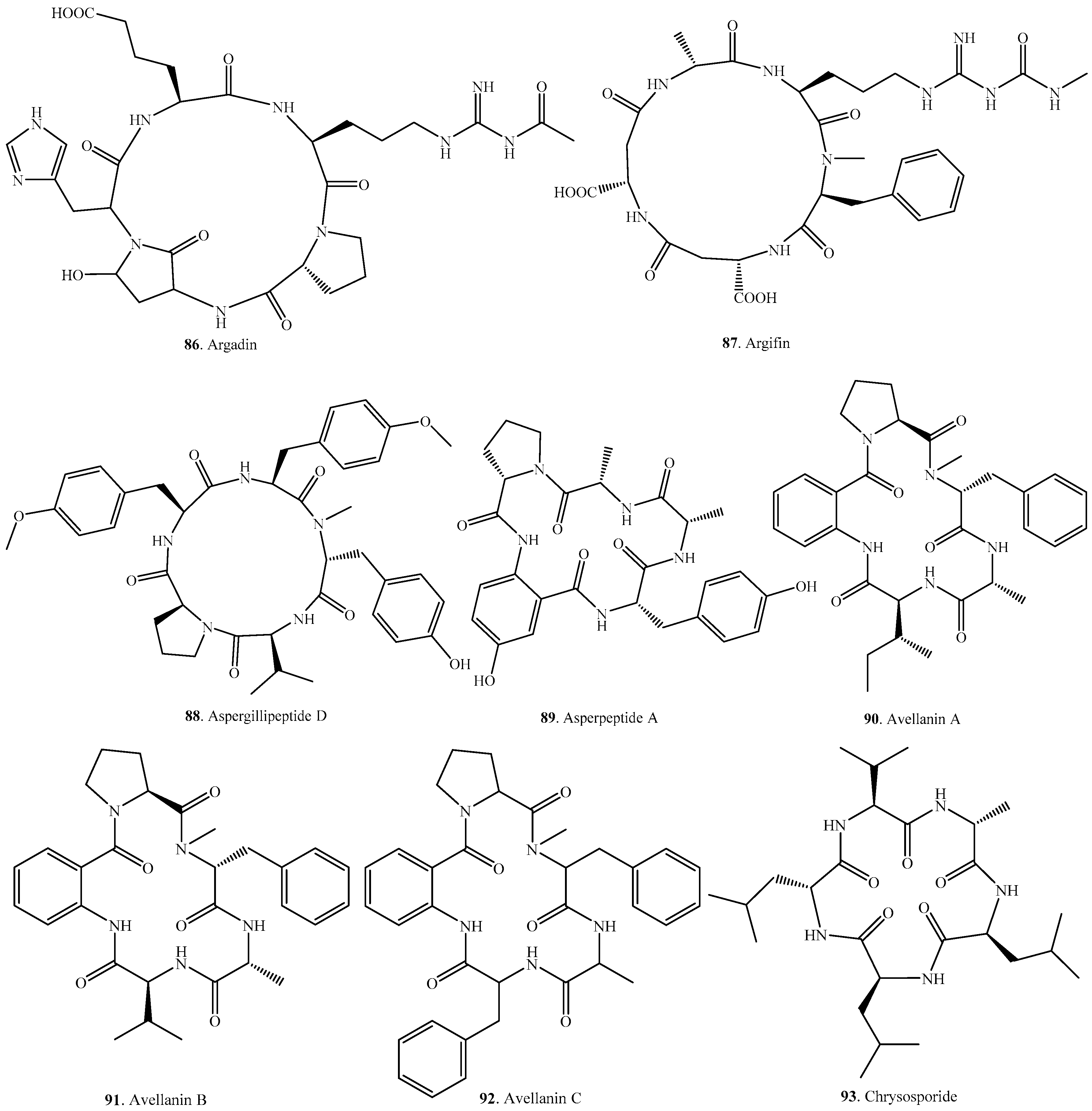
| Argadin (86) | Clonostachys sp. FO-7314 | Chitinase inhibitor | [81] |
| Argifin (87) | Gliocladium sp. FTD-0668 | Chitinase inhibitor | [82,97] |
| Aspergillipeptide D (88) | Marine gorgonian-derived fungus Aspergillus sp. SCSIO 41501 | Antiviral activity | [84] |
| Asperpeptide A (89) | Marine gorgonian-derived Aspergillus sp. XS-20090B15 | Antibacterial activity | [98] |
| Avellanin A (90) | Hamigera sp. | Vasoconstriction and pressor effect | [99] |
| Avellanin B (91) | Hamigera sp. | Vasoconstriction and pressor effect | [99] |
| Avellanin C (92) | Hamigera paravellanea | Vasoconstriction and pressor effect | [99] |
| Chrysosporide (93) | Sepedonium chrysospermum | Weak cytotoxic to murine leukemia cell line | [100] |
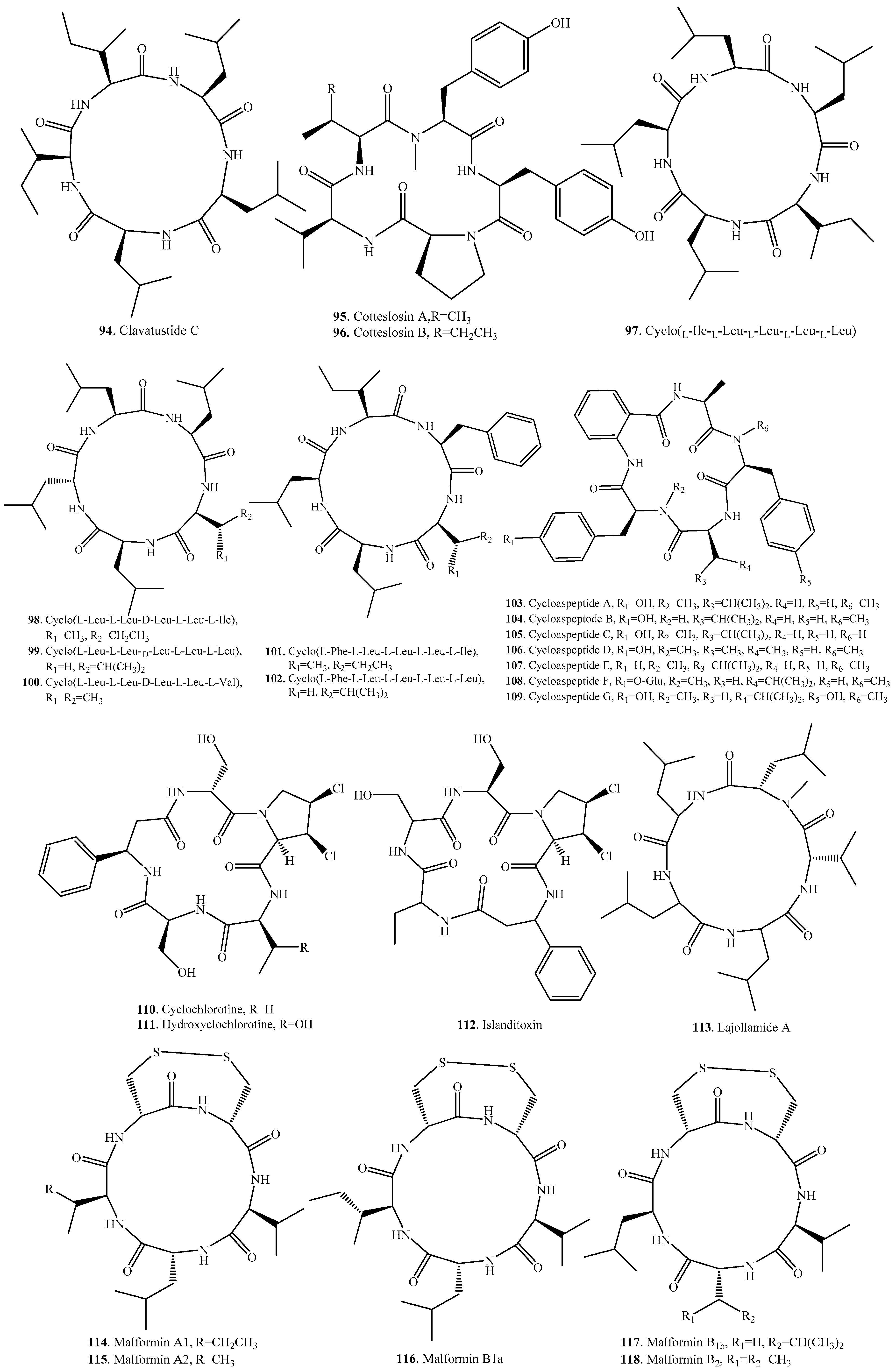
| Clavatustide C (94) | Aspergillus clavatus C2WU | - | [101] |
| Cotteslosin A (95) | Aspergillus versicolor | Weak cytotoxic to human cancer cell lines | [102] |
| Cotteslosin B (96) | Aspergillus versicolor | Weak cytotoxic to human cancer cell lines | [102] |
| Cyclo(l-Ile-l-Leu-l-Leu-l-Leu-l-Leu) (97) | Endophytic fungus Cryptosporiopsis sp. of Zanthoxylum leprieurii | Motility inhibitory and lytic activities against zoospores; Antifungal activity; weak cytotoxic activity on brine shrimp larvae | [103] |
| Cyclo(l-Leu-l-Leu-d-Leu-l-Leu-l-Ile) (98) | Endophytic fungus Fusarium decemcellulare LG53 from Chinese medicinal plant Mahonia fortunei | Antagonistic effect on fungus | [104] |
| Cyclo(l-Leu-l-Leu-d-Leu-l-Leu-l-Leu) (99) | Endophytic fungus Fusarium decemcellulare LG53 from Chinese medicinal plant Mahonia fortunei | Antagonistic effect on fungus | [104] |
| Cyclo(l-Leu-l-Leu-d-Leu-l-Leu-l-Val) (100) | Endophytic fungus Fusarium decemcellulare LG53 from Chinese medicinal plant Mahonia fortunei | Antagonistic effect on fungus | [104] |
| Cyclo(l-Phe-l-Leu-l-Leu-l-Leu-l-Ile) (101) | Unidentified endophytic fungus No. 2524 from the mangrove Avicennia marina | - | [105] |
| Cyclo(l-Phe-l-Leu-l-Leu-l-Leu-l-Leu) (102) | Endophytic fungus Cryptosporiopsis sp. of Zanthoxylum leprieurii | Motility inhibitory and lytic activities against zoospores; Antifungal activity; Weak cytotoxic activity on brine shrimp larvae | [103] |
| Cycloaspeptide A (103) | Aspergillus sp. NE-45 | - | [85] |
| Penicillium algidum | Antiplasmodial activity | [18] | |
| Penicillium jamesonlandense | - | [106] | |
| Penicillium janczewskii | - | [107] | |
| Penicillium lanosum | - | [106] | |
| Penicillium ribium | - | [106] | |
| Penicillium ribeum from a soil under the tree Ribes sp. | - | [20] | |
| Penicillium soppii | - | [106] | |
| Cycloaspeptide B (104) | Aspergillus sp. NE-45 | - | [85] |
| Cycloaspeptide C (105) | Aspergillus sp. NE-45 | - | [85] |
| Cycloaspeptide D (106) | Penicillium algidum | Antiplasmodial activity | [18] |
| Penicillium ribeum from a soil under the tree Ribes sp. | - | [20] | |
| Cycloaspeptide E (107) | Penicillia sp. | Insecticidal activity | [86] |
| Tricothecium sp. | - | [86] | |
| Cycloaspeptide F (108) | Isaria farinosa | Cytotoxic activity | [87] |
| Cycloaspeptide G (109) | Isaria farinosa | Cytotoxic activity | [87] |
| Cyclochlorotine (110) | Penicillium islandicum | Liver fibrosis, liver injuries | [108,109,110] |
| Hydroxycyclochlorotine (111) | Penicillium islandicum | - | [108] |
| Islanditoxin (112) | Penicillium islandicum | - | [111] |
| Lajollamide A (113) | Marine-derived fungus Asteromyces cruciatusis | Weak antibacterial on Gram-positive bacteria | [112] |
| Malformin A1 (114) | Aspergillus niger | Malformation activity in the corn root; Cytotoxic activity | [92,93] |
| Aspergillus tubingensis | Cytotoxic activity; Anti-TMV activity | [89,90] | |
| Malformin A2 (115) | Aspergillus niger | - | [92] |
| Malformin B1a (116) | Aspergillus niger | Curvature activity in the corn root test | [88] |
| Malformin B1b (117) | Aspergillus niger | - | [88] |
| Malformin B2 (118) | Aspergillus niger | - | [88] |
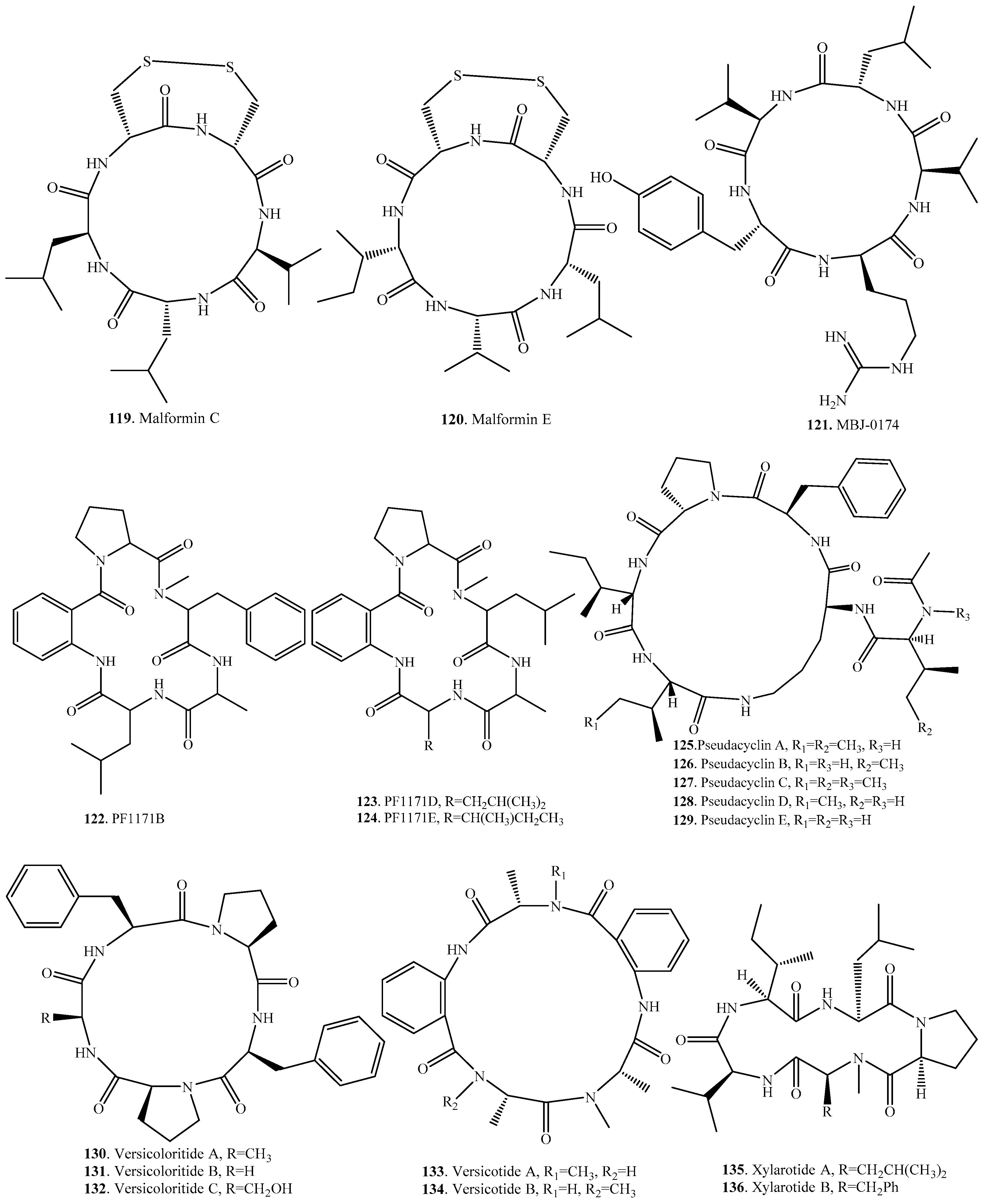
| Malformin C (119) | Aspergillus niger | Anticancer activity | [94,95] |
| Malformin E (120) | Endophytic fungus Aspergillus tamari from Ficus carica | Cytotoxic and antibiotic activity | [91] |
| MBJ-0174 (121) | Mortierella alpine f28740 from a soil smaple collected in Ise, Japan | Stimulators of cellular fibrinolytic activity | [113] |
| PF1171B (122) | Hamigera sp. | - | [99] |
| PF1171D (123) | Hamigera sp. | - | [99] |
| PF1171E (124) | Hamigera sp. | - | [99] |
| Pseudacyclin A (125) | Animal fungal pathogen Pseudallescheria boydii | Immunosuppressive activity | [96] |
| Pseudacyclin B (126) | Animal fungal pathogen Pseudallescheria boydii | - | [96] |
| Pseudacyclin C (127) | Animal fungal pathogen Pseudallescheria boydii | - | [96] |
| Pseudacyclin D (128) | Animal fungal pathogen Pseudallescheria boydii | - | [96] |
| Pseudacyclin E (129) | Anmal fungal pathogen Pseudallescheria boydii | - | [96] |
| Versicoloritide A (130) | Aspergillus versicolor LCJ-5-4 | - | [114] |
| Versicoloritide B (131) | Aspergillus versicolor LCJ-5-4 | - | [114] |
| Versicoloritide C (132) | Aspergillus versicolor LCJ-5-4 | - | [114] |
| Versicotide A (133) | Aspergillus versicolor ZLN-60 from the sediment of the Yellow Sea | - | [115] |
| Versicotide B (134) | Aspergillus versicolor ZLN-60 from the sediment of the Yellow Sea | - | [115] |
| Xylarotide A (135) | Xylaria sp. 101 | - | [116] |
| Xylarotide B (136) | Xylaria sp. 101 | - | [116] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Aculeacin A (137) | Aspergillus aculeatus M-4214 | Antifungal activity by inhibiting synthesis of β-1,3-glucan | [117,118] |
| Anthranicine (138) | Acremonium sp. A29-2004 | - | [130] |
| ASP2397 (139) | Acremonium persicinum MF-347833 from Malaysian leaf litter | Antifungal activity | [131] |
| AS2524371 (140) | Acremonium persicinum MF-347833 from Malaysian leaf litter | - | [131] |
| AS2488059 (141) | Acremonium persicinum MF-347833 from Malaysian leaf litter | - | [131] |
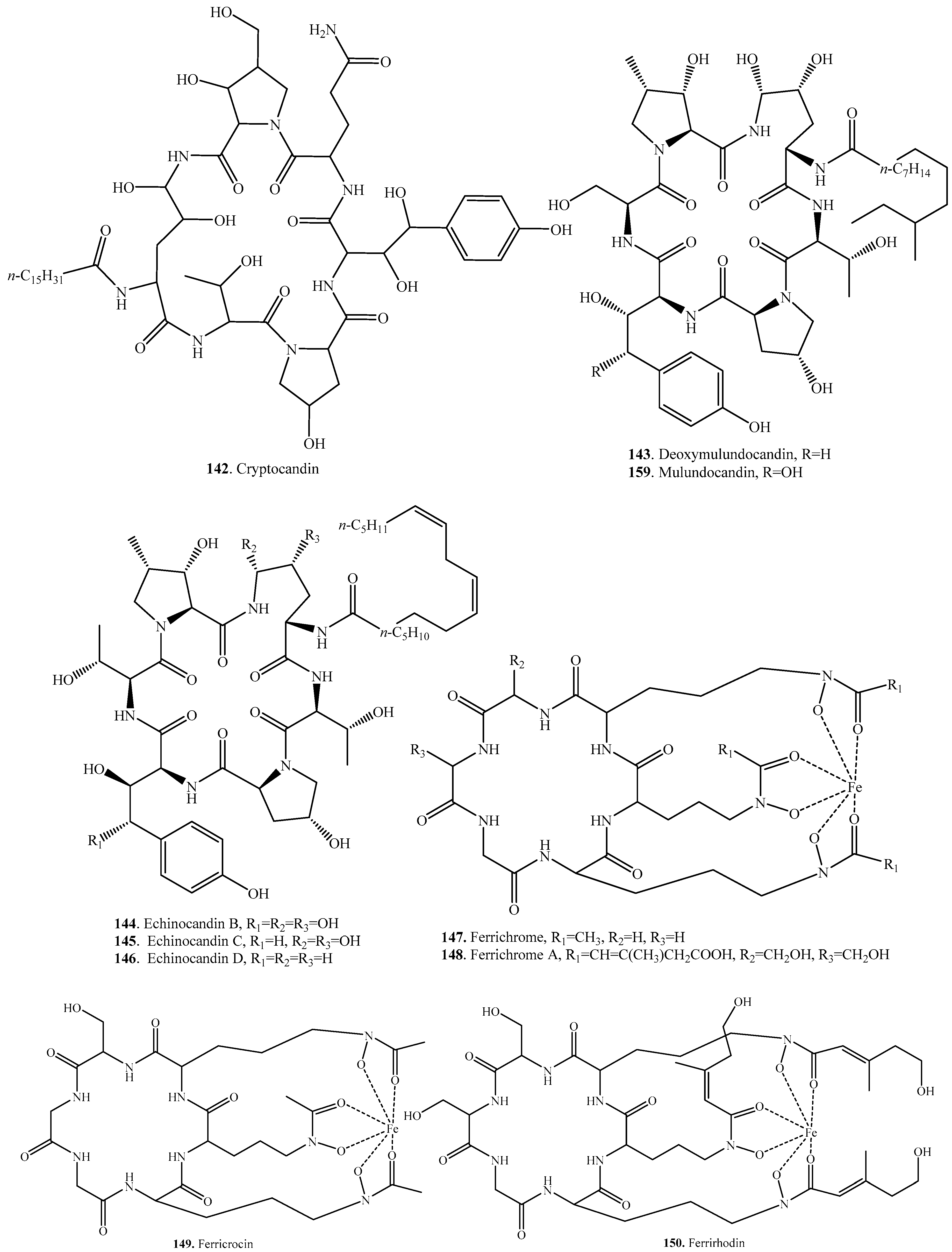
| Cryptocandin (142) | Cryptosporiopsis cf. quercina | Antifungal activity | [132] |
| Deoxymulundocandin (143) | Aspergillus sydowii | Antifungal activity | [133] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Echinocandin B (144) | Aspergillus nidulans var. roseus ATCC 58397 | Antifungal activity | [134] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Echinocandin C (145) | Aspergillus sp. | Antifungal activity | [135] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Echinocandin D (146) | Aspergillus sp. | Antifungal activity | [135] |
| Ferrichrome (147) | Ustilago maydis | - | [136] |
| Ferrichrome A (148) | Ustilago maydis | - | [136] |
| Ferricrocin (149) | Colletotrichum gloeosporioides | Phytotoxic activity | [137] |
| Ferrirhodin (150) | Botrytis cinerea | - | [138] |
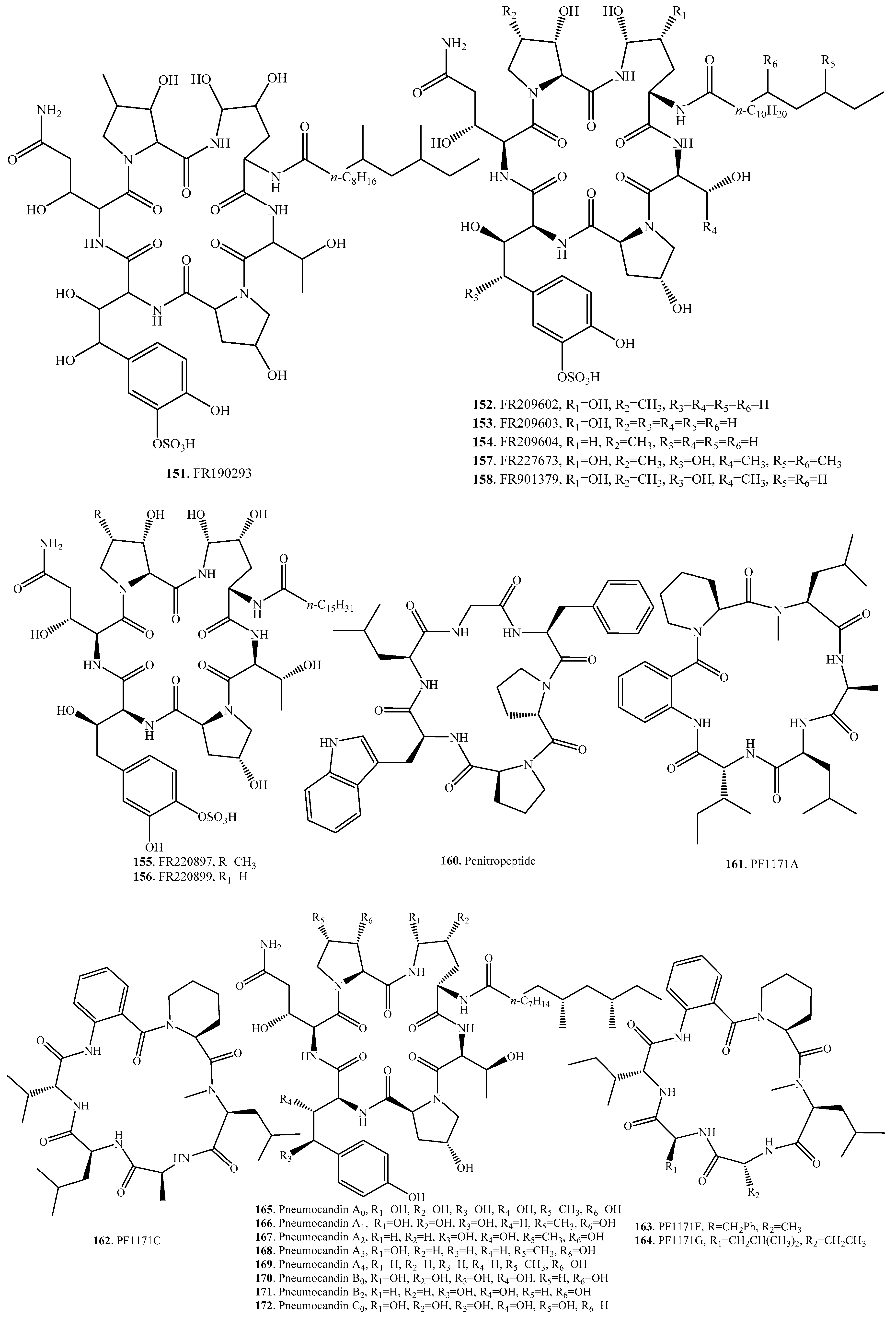
| FR190293 (151) | Tolypocladium parasiticum No. 16616 | Antifungal activity | [139] |
| FR209602 (152) | Coleophoma crateriformis No. 738 | Antifungal activity | [140] |
| FR209603 (153) | Coleophoma crateriformis No. 738 | Antifungal activity | [140] |
| FR209604 (154) | Coleophoma crateriformis No. 738 | Antifungal activity | [140] |
| FR220897 (155) | Coleophoma empetri No. 14573 | Antifungal activity | [141] |
| FR220899 (156) | Coleophoma empetri No. 14573 | Antifungal activity | [141] |
| FR227673 (157) | Chalara sp. No. 22210 | Antifungal activity | [139] |
| FR901379 (158) | Coleophoma empetri F-11899 | Antifungal activity | [142] |
| Mulundocandin (159) | Aspergillus sydowii | Antifungal activity | [122] |
| Aspergillus mulundensis | Antifungal activity | [123] | |
| Penitropeptide (160) | Endophytic fungus Penicillium tropicum isolated from leaves of Sapium ellipticum | - | [143] |
| PF1171A (161) | Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [124] |
| PF1171C = Similanamide (162) | Marine sponge-associated fungus Aspergillus similanensis KUFA 0013 | Weak cytotoxic activity against the cancer cell lines | [125,126] |
| Hamigera sp. | - | [99] | |
| Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [124] | |
| PF1171F (163) | Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [144] |
| PF1171G (164) | Unidentified ascomycete OK-128 | Paralytic activity against silkworms | [144] |
| Pneumocandin A0 = L-671,329 (165) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Zalerion arboricola | Antifungal activity | [145] | |
| Pneumocandin A1 (166) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin A2 (167) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin A3 (168) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin A4 (169) | Zalerion arboricola | Anticandida activity and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin B0 (170) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3-β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin B2 (171) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3- β-d-glucan synthesis inhibition activity | [127,128] |
| Pneumocandin C0 (172) | Zalerion arboricola | Antipneumocystis, anticandida and Candida albicans 1,3- β-d-glucan synthesis inhibition activity | [127,128] |
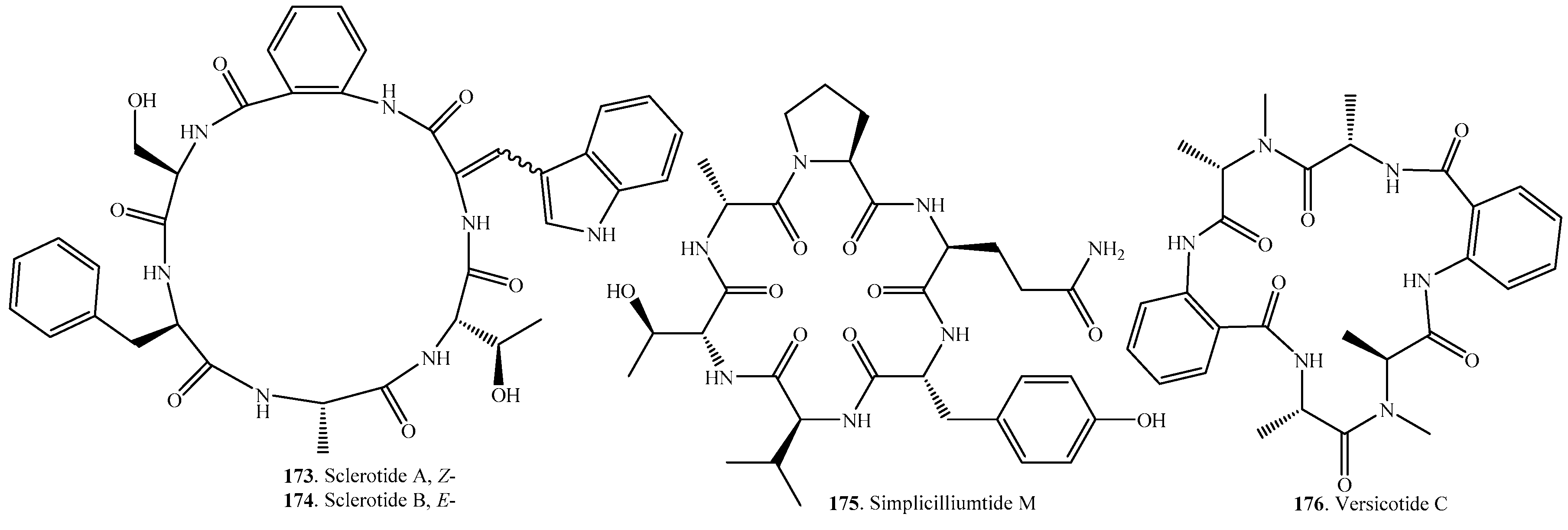
| Sclerotide A (173) | Aspergillus sclerotiorum PT06-1 | Antifungal activity | [129] |
| Sclerotide B (174) | Aspergillus sclerotiorum PT06-1 | Antifungal, antibacterial and cytotoxic activity | [129] |
| Simplicilliumtide M (175) | Deep-sea derived Simplicillium obclavatum EIODSF 020 | - | [146] |
| Versicotide C (176) | Aspergillus versicolor ZLN-60 | - | [23] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
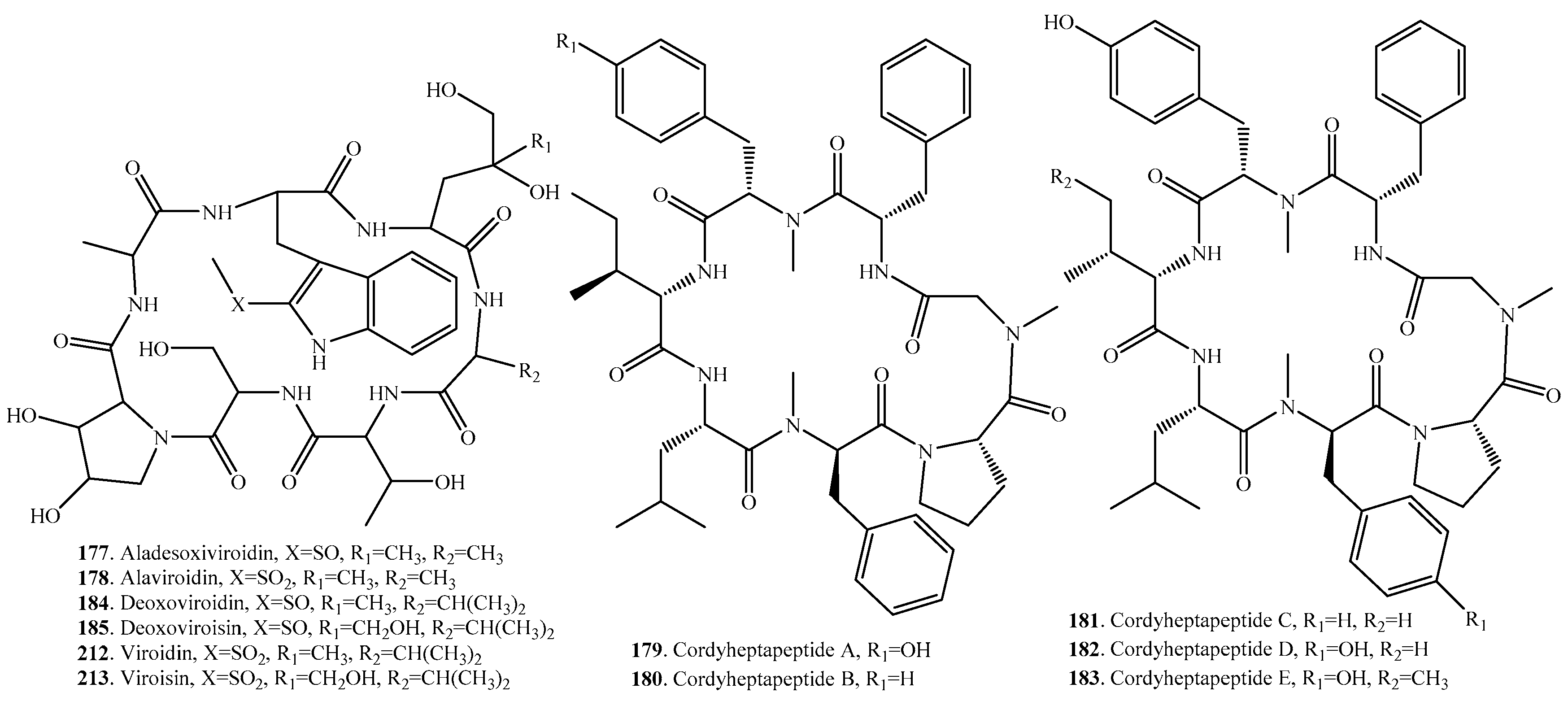
| Aladesoxiviroidin (177) | Amanita phalloides | Toxic to some animal species | [150] |
| Alaviroidin (178) | Amanita phalloides | Toxic to some animal species | [150] |
| Cordyheptapeptide A (179) | Cordyceps sp. BCC1788 | Antimalarial activity against Plasmodium falciparum K1 | [147] |
| Cordyceps sp. BCC 16176 | Cytotoxic activity | [148] | |
| Cordyheptapeptide B (180) | Cordyceps sp. BCC 16176 | Cytotoxic activity | [148] |
| Cordyheptapeptide C (181) | Acremonium persicinum SCSIO 115 | Cytotoxic activity | [149] |
| Cordyheptapeptide D (182) | Acremonium persicinum SCSIO 115 | - | [149] |
| Cordyheptapeptide E (183) | Acremonium persicinum SCSIO 115 | Cytotoxic activity | [149] |
| Deoxoviroidin (184) | Mushroom Amanita virosa | Hepatotoxicity | [159] |
| Deoxoviroisin (185) | Mushroom Amanita virosa | Hepatotoxicity | [159] |
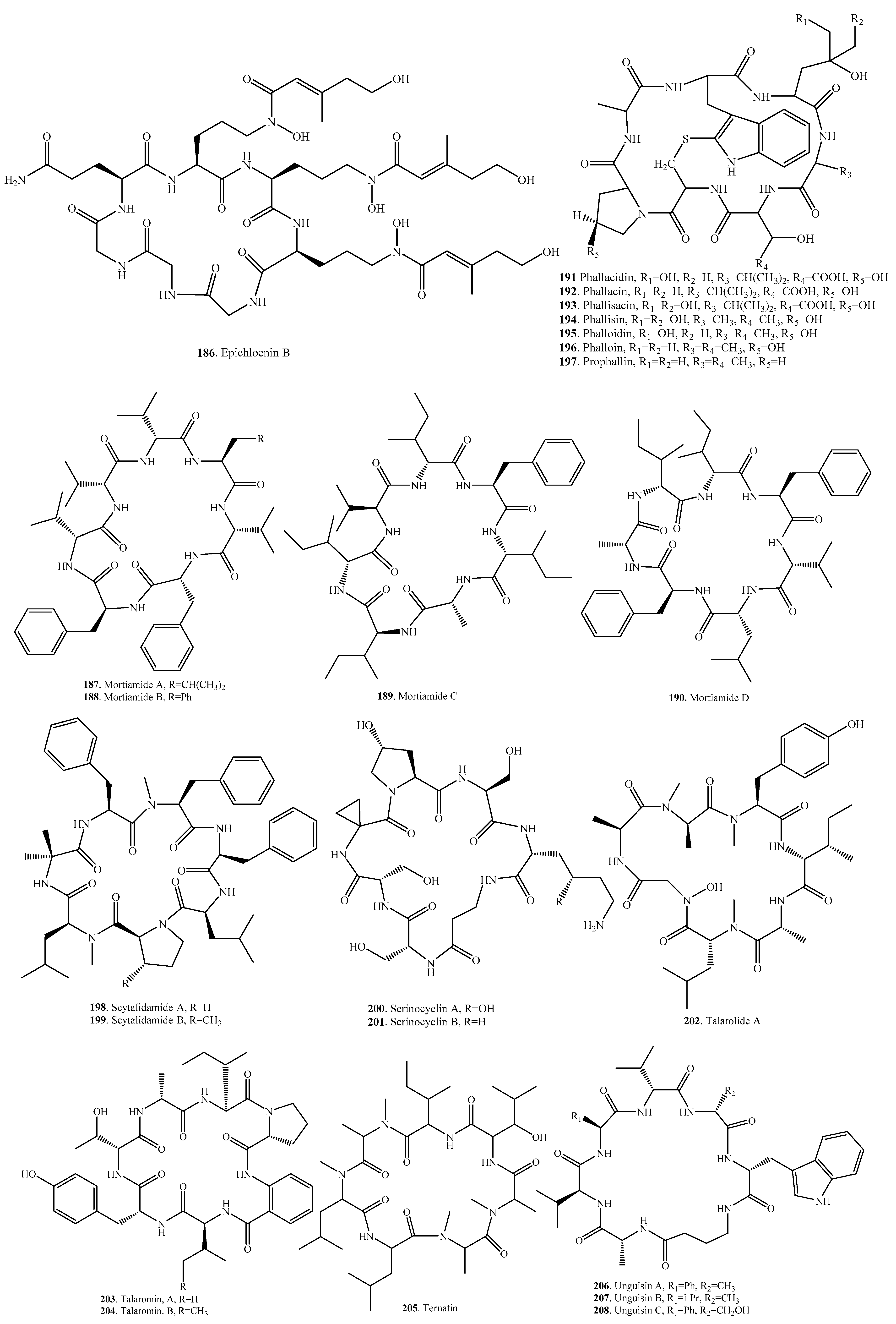
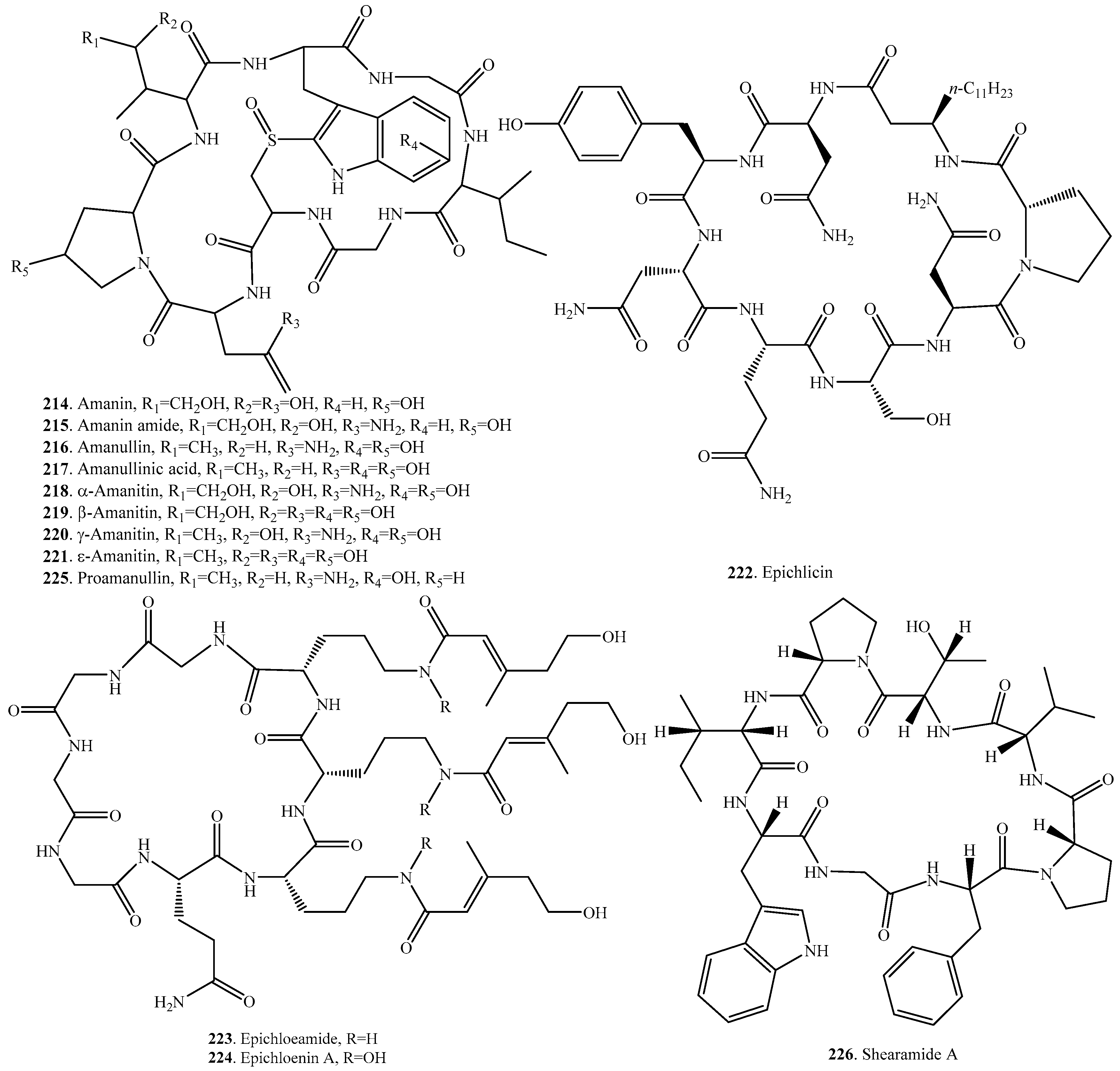
| Epichloenin B (186) | Epichloe festucae | - | [160] |
| Mortiamide A (187) | Marine-derived Mortierella sp. | - | [161] |
| Mortiamide B (188) | Marine-derived Mortierella sp. | - | [161] |
| Mortiamide C (189) | Marine-derived Mortierella sp. | - | [161] |
| Mortiamide D (190) | Marine-derived Mortierella sp. | - | [161] |
| Phallacidin (191) | Amanita phalloides | Toxic to some animal species | [150] |
| Phallacin (192) | Amanita phalloides | Toxic to some animal species | [150] |
| Phallisacin (193) | Amanita phalloides | Toxic to some animal species | [150] |
| Phallisin (194) | Amanita phalloides | Toxic to some animal species | [150] |
| Phalloidin (195) | Amanita phalloides | Toxic to some animal species | [150] |
| Phalloin (196) | Amanita phalloides | Toxic to some animal species | [150] |
| Prophallin (197) | Amanita phalloides | Toxic to some animal species | [150] |
| Scytalidamide A (198) | Acremonium sp. | - | [152] |
| Scytalidiu sp. | Cytotoxic activity on HCT-116 | [153] | |
| Scytalidamide B (199) | Acremonium sp. | - | [152] |
| Scytalidiu sp. | Cytotoxic activity on HCT-116 | [153] | |
| Serinocyclin A (200) | Metarhizium anisopliae | Insecticidal activity on mosquito larvae | [162] |
| Serinocyclin B (201) | Metarhizium anisopliae | - | [162] |
| Talarolide A (202) | Marine tunicte-associated fungus Talaromyces sp. CMB-TU011 | - | [163] |
| Talaromin A (203) | Talaromyces wortmannii | - | [164] |
| Talaromin B (204) | Talaromyces wortmannii | - | [164] |
| Ternatin (205) | Cladobotryum sp. | Cytotoxic activity; antifungal activity | [165] |
| Unguisin A (206) | Marine-derived Emericella unguis | Moderate antibacterial activity | [154,155] |
| Unguisin B (207) | Marine-derived Emericella unguis | Moderate antibacterial activity | [154,155] |
| Unguisin C (208) | Marine-derived Emericella unguis | - | [155] |
| Unguisin D (209) | Marine-derived Emericella unguis | - | [155] |
| Unguisin E (210) | Aspergillus sp. AF119 | - | [156] |
| Endophytic fungus Mucor irregularis from the medicinal plant Moringa stenopetala | - | [157] | |
| Unguisin F (211) | Endophytic fungus Mucor irregularis from the medicinal plant Moringa stenopetala | - | [157] |
| Viroidin (212) | Mushroom Amanita virosa | - | [159] |
| Amanita phalloides | Toxic to some animal species | [150] | |
| Viroisin (213) | Mushroom Amanita virosa | - | [159] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Amanin (214) | Amanita phalloides | Toxic to some animal species | [150] |
| Amanin amide (215) | Amanita phalloides | Toxic to some animal species | [150] |
| Amanullin (216) | Amanita phalloides | Toxic to some animal species | [150] |
| Amanullinic acid (217) | Amanita phalloides | Toxic to some animal species | [150] |
| α-Amanitin (218) | Amanita exitialis | - | [168] |
| Amanita subjunquillea | Cytotoxic activity | [169] | |
| Amanita phalloides | Toxic to some animal species | [150] | |
| β-Amanitin (219) | Amanita exitialis | - | [168] |
| Amanita subjunquillea | Cytotoxic activity | [169] | |
| Amanita phalloides | Toxic to some animal species | [150] | |
| γ-Amanitin (220) | Amanita phalloides | Toxic to some animal species | [150] |
| ε-Amanitin (221) | Amanita phalloides | Toxic to some animal species | [150] |
| Epichlicin (222) | Endophytc Epichloe typhina from Phleum pratense | Inhibitory activity on the spore germination of Cladosporium phlei | [166] |
| Epichloeamide (223) | Epichloe festucae | - | [160] |
| Epichloenin A (224) | Epichloe festucae | - | [160] |
| Proamanullin (225) | Amanita phalloides | Toxic to some animal species | [150] |
| Shearamide A (226) | Eupenicillium shearii | Insecticidal effect | [167] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Amanexitide (227) | Amanita exitialis | - | [168] |
| Clonostachysin A (228) | Marine-derived fungus Clonostachys rogersoniana HJK9 from sponge | Anti-dinoflagellate activity | [170] |
| Clonostachysin B (229) | Marine-derived fungus Clonostachys rogersoniana HJK9 from sponge | Anti-dinoflagellate activity | [170] |
| Cylindrocyclin A (230) | Cylindrocarpon sp. | Cytotoxic activity | [171] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Antamanide (231) | Amanita phalloides | Inhibition of the mitochondrial permeability transition pore, antidote activity against phallotoxins and amatoxins, inhibition of tumor cell growth in vitro | [172,175] |
| Arborcandin A (232) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin B (233) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin C (234) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin D (235) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin E (236) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Arborcandin F (237) | Unidentified fungus SANK 17397 | Inhibitory activity on 1,3-β-glucan synthase | [173,174] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Cyclosporin A (238) | Aspergillus fumigatus | - | [177] |
| Aspergillus terreus NRC FA 200 | - | [178] | |
| Beauveria nivea | - | [179] | |
| Fusarium oxysporum | Immunosuppressive, antifungal, anti-inflammatory, anti-parasitic and anticancer activities | [180] | |
| Tolypocladium inflatum | - | [182] | |
| Tolypocladium sp. | - | [183] | |
| Trichoderma polysporum | Antifungal activity | [181] | |
| Cyclosporin B (239) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186] |
| Trichoderma polysporum | Antifungal activity | [181] | |
| Cyclosporin C (240) | Acremonium luzulae | Antifungal activity | [187] |
| Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186] | |
| Cyclosporin D (241) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186] |
| Trichoderma polysporum | Antifungal activity | [181] | |
| Cyclosporin E (242) | Trichoderma polysporum | - | [181] |
| Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186,188] | |
| Cyclosporin F (243) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186,188] |
| Cyclosporin G (244) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [182,186,188] |
| Cyclosportin H (245) | Tolypocladium inflatum | - | [188] |
| Cyclosportin I (246) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186,188] |
| Cyclosportin J (247) | Tolypocladium terricola | Increased cell proliferation | [189] |
| Cyclosporin K (248) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin L (249) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin M (250) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin N (251) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin O (252) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin P (253) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin Q (254) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin S (255) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin T (256) | Epichloe bromicola | Antifungal activity | [190] |
| Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] | |
| Cyclosporin U (257) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin V (258) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin W (259) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin X (260) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin Y (261) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| Cyclosporin Z (262) | Tolypocladium inflatum | Immunosuppressive and antifungal activities | [186] |
| FR901459 (263) | Stachybotrys chartarum No. 19392 from a soil collected in Tokyo, Japan | Immunosuppressive activity | [184] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Omphalotin A = Omphalotin (264) | Mushroom Omphalotus olearius | Nematicidal activity | [191,197,198,199] |
| Omphalotin B (265) | Mushroom Omphalotus olearius | Nematicidal activity | [191] |
| Omphalotin C (266) | Mushroom Omphalotus olearius | Nematicidal activity | [191] |
| Omphalotin D (267) | Mushroom Omphalotus olearius | Nematicidal activity | [191] |
| Omphalotin E (268) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin F (269) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin G (270) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin H (271) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Omphalotin I (272) | Mushroom Omphalotus olearius | Nematicidal activity on Meloidogyne incognita | [192] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Verrucamide A (273) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
| Verrucamide B (274) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
| Verrucamide C (275) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
| Verrucamide D (276) | Plant pathogenic fungus Myrothecium verrucaria on crops and weeds | Antibacterial activity on Staphylococcus aureus | [200] |
| Name | Fungus and its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Gymnopeptide A (277) | Mushroom Gymnopus fusipes | Antiproliferative activity on several human cancer cell lines | [201] |
| Gymnopeptide B (278) | Mushroom Gymnopus fusipes | Antiproliferative activity on several human cancer cell lines | [201] |
| Name | Fungus and Its Origin | Biological Activity | Ref. |
|---|---|---|---|
| Asperipin-2a (279) | Aspergillus flavus | - | [203] |
| S-Deoxyustiloxin H (280) | Aspergillus flavus | - | [214] |
| N-Desmethylustiloxin F (281) | Aspergillus flavus | - | [214] |
| HV-toxin M (282) | Plant pathogenic fungus Cochliobus victoriae = Helminthosporium victoria from oat | Phytotoxic activity | [205] |
| Phomopsin A (283) | Plant pathogenic fungus Phomopsis leptostromiformis from lupinis (Lupinus spp.) | Phytotoxic activity, toxic to animals | [206] |
| Phomopsin B (284) | Plant pathogenic fungus Phomopsis leptostromiformis from lupinis (Lupinus spp.) | Toxic to animals | [207] |
| Phomopsin F (285) | Plant pathogenic fungus Diaporthe toxica | Cytotoxic activity against Hepg2 cells | [208] |
| Ustiloxin A (286) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [209,211] |
| Ustiloxin B (287) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [209,211] |
| Aspergillus flavus | - | [213] | |
| Ustiloxin C (288) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [208] |
| Ustiloxin D (289) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [208] |
| Ustiloxin F (290) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [209] |
| Ustiloxin G (291) | Plant pathogenic fungus Ustilaginoidea virens from rice false smut balls | Cytotoxic, phytotoxic activities | [212] |
| Ustiloxin H (292) | Aspergillus flavus | - | [214] |
| Victorin C (293) | Plant pathogenic fungus Cochliobus victoriae = Helminthosporium victoria | Phytotoxic activity | [215] |
| Product Name | Original Cyclic Peptide | Development Way | Application | Ref. |
|---|---|---|---|---|
| Anidulafungin | Echinocandin B (144) | Semisynthesis | Antifungal agent | [220] |
| CANCIDAS® | Pneumocandin B0 (170) | Semisynthesis | Antifungal agent | [14] |
| Caspofungin | Echinocandins | Direct application | Antifungal agent for many Candida infections | [120] |
| Cyclosporin A | Cyclosporin A (238) | Direct application | Immunosuppressive and antifungal drug | [176] |
| Micafungin | Echinocandins | Semisynthesis | Antifugal agent | [221] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides. Molecules 2017, 22, 2069. https://doi.org/10.3390/molecules22122069
Wang X, Lin M, Xu D, Lai D, Zhou L. Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides. Molecules. 2017; 22(12):2069. https://doi.org/10.3390/molecules22122069
Chicago/Turabian StyleWang, Xiaohan, Minyi Lin, Dan Xu, Daowan Lai, and Ligang Zhou. 2017. "Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides" Molecules 22, no. 12: 2069. https://doi.org/10.3390/molecules22122069
APA StyleWang, X., Lin, M., Xu, D., Lai, D., & Zhou, L. (2017). Structural Diversity and Biological Activities of Fungal Cyclic Peptides, Excluding Cyclodipeptides. Molecules, 22(12), 2069. https://doi.org/10.3390/molecules22122069







