GC-MS Profiling of Volatile Components in Different Fermentation Products of Cordyceps Sinensis Mycelia
Abstract
:1. Introduction
2. Results and Discussion
2.1. SDE Extraction of Essential Oils
2.2. GC-MS Volatile Profiling and Method Validation
2.3. Identification of Volatile Components in Fermentation Products
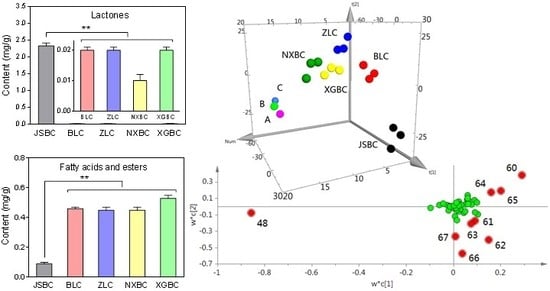
2.4. Multivariate PLS-DA Analysis
3. Materials and Methods
3.1. Reagents and Materials
3.2. Sample Preparation
3.3. GC-MS Analysis
3.4. Analytical Method Validation
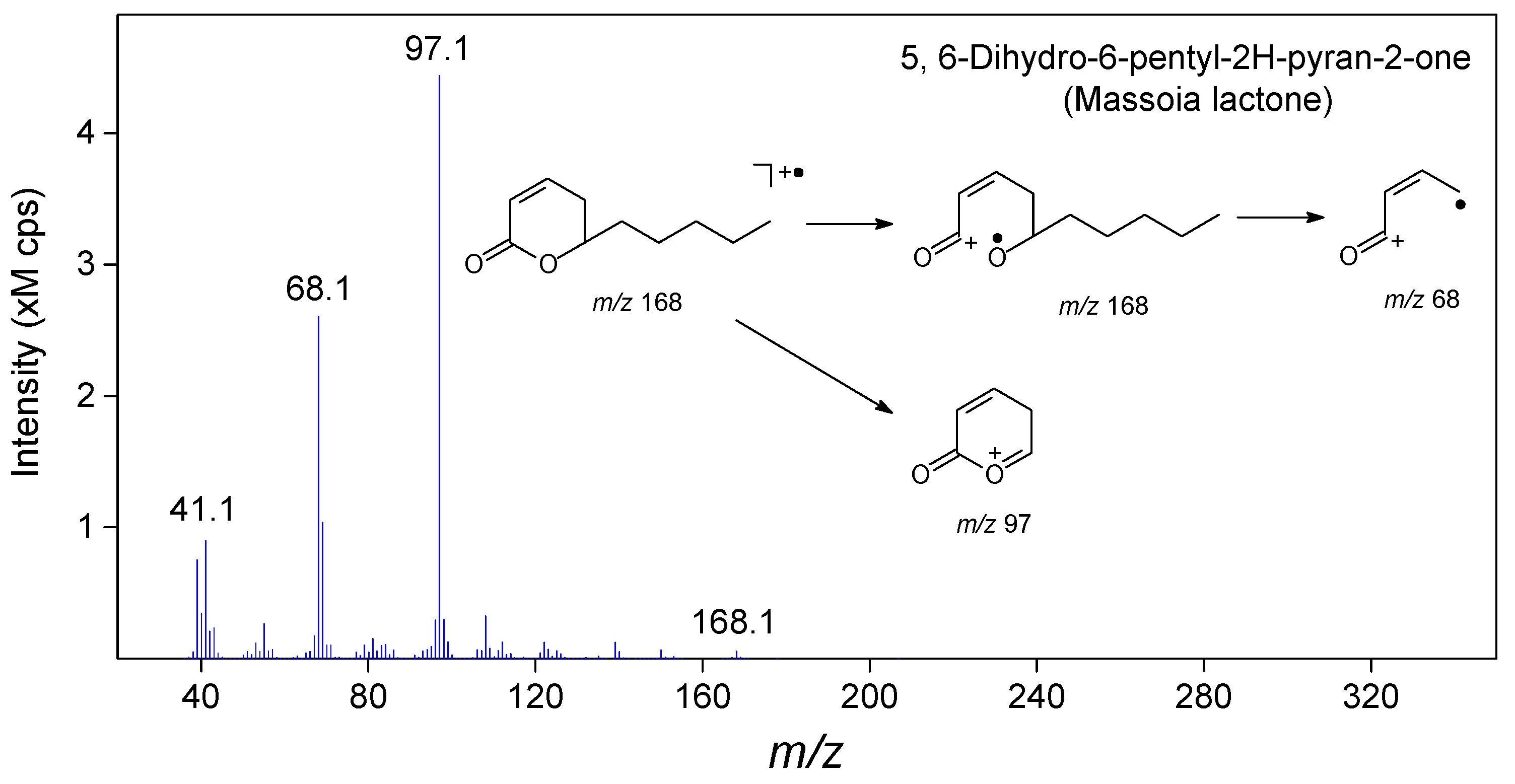
3.5. Isolation and Characterization of Massoia Lactone
3.6. Chemometric Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, S.P.; Yang, F.Q.; Tsim, K.W. Quality control of Cordyceps sinensis, a valued traditional Chinese medicine. J. Pharm. Biomed. Anal. 2006, 41, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Yue, K.; Ye, M.; Zhou, Z.J.; Sun, W.; Lin, X. The genus Cordyceps: A chemical and pharmacological review. J. Pharm. Pharmacol. 2013, 65, 474–493. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.W.; Gong, Z.H.; Su, Y.; Lin, J.; Tang, K.X. Cordyceps fungi: Natural products, pharmacological functions and developmental products. J. Pharm. Pharmacol. 2009, 61, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Shashidhar, M.G.; Giridhar, P.; Sankar, K.U.; Manohar, B. Bioactive principles from Cordyceps sinensis: A potent food supplement–A review. J. Funct. Food 2013, 5, 1013–1030. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Liu, X.Z.; Wang, M. Cloning, expression, and characterization of two novel cuticle-degrading serine proteases from the entomopathogenic fungus Cordyceps sinensis. Res. Microbiol. 2008, 159, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.K.; Wang, W.Q.; Wu, J.Y. Recent advances in Cordyceps sinensis polysaccharides: Mycelial fermentation, isolation, structure, and bioactivities: A review. J. Funct. Food 2014, 6, 33–47. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Cordyceps—A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 69, 1469–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, Q.W.; Xiao, X.Y.; Lin, R.C. The research situation of related preparation of submerged fermentation of Cordyceps sinensis. Chin. J. Pharm. Anal. 2009, 29, 680–687. [Google Scholar] [CrossRef]
- Zhang, P.; Zheng, T.J.; Zhang, W.J.; Jiao, K.; Wei, F.; Liu, B. HPLC characteristic fingerprint and chemical pattern recognition of fermentation mycelium preparations. Chin. Pharm. J. 2015, 50, 293–298. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, J.; Wang, L.Y.; Li, S.P. Advanced development in chemical analysis of Cordyceps. J. Pharm. Biomed. Anal. 2014, 87, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.H.; Wang, W.; Zhang, H.Y.; Zhang, X.L.; Han, C.C. The chemical constituents and pharmacological actions of Cordyceps sinensis. Evid.-Based Complement. Altern. Med. 2015, 2015, 575063. [Google Scholar] [CrossRef]
- Zong, S.Y.; Han, H.; Wang, B.; Li, N.; Dong, T.T.X.; Zhang, T.; Tsim, K.W.K. Fast simultaneous determination of 13 nucleosides and nucleobases in Cordyceps sinensis by UHPLC-ESI-MS/MS. Molecules 2015, 20, 21816–21825. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Ye, M.Q.; Yu, H.D.; Fan, Y.; Li, H.P.; Zou, G.L. Chemical composition of essential oil in cultured Cordyceps sinensis. Chin. Tradit. Herb. Drugs 2004, 35, 975–977. [Google Scholar] [CrossRef]
- Yang, F.Q.; Feng, K.; Zhao, J.; Li, S.P. Analysis of sterols and fatty acids in natural and cultured Cordyceps by one-step derivatization followed with gas chromatography–mass spectrometry. J. Pharm. Biomed. Anal. 2009, 49, 1172–1178. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.Z.; Zhuang, M.Z.; Lei, F.F.; Yang, B.; Zhao, M.M. GC/MS analysis of volatiles obtained by headspace solid-phase microextraction and simultaneous-distillation extraction from Rabdosia serra (MAXIM.) HARA leaf and stem. Food Chem. 2013, 136, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; China Medical Science Press: Beijing, China, 2015; Volume 4, pp. 203–504. ISBN 978-7-5067-7539-7. [Google Scholar]
- Pino, J.A.; Mesa, J.; Munoz, Y.; Marti, M.P.; Marbot, R. Volatile components from mano (Mangifera indica L.) cultivars. J. Agric. Food Chem. 2005, 23, 2213–2223. [Google Scholar] [CrossRef] [PubMed]
- Kaseleht, K.; Leitner, E.; Paalme, T. Determining aroma-active compounds in Kama flour using SPME-GC/MS and GC–olfactometry. Flavour Frag. J. 2011, 26, 122–128. [Google Scholar] [CrossRef]
- Lv, S.D.; Wu, Y.S.; Wei, J.F.; Lian, M.; Wang, C.; Gao, X.M.; Meng, Q.X. Application of gas chromatography-mass spectrometry and chemometrics methods for assessing volatile profiles of Pu-erh tea with different processing methods and ageing years. RSC Adv. 2015, 5, 87806–87817. [Google Scholar] [CrossRef]
- Wu, Y.B.; Lin, X.H.; Wu, J.G.; Yi, J.; Zheng, Y.Z.; Wu, J.Z. Volatile components from fruits of Ligustrum lucidum Ait. stimulate proliferation and differentiation of rat calvarial osteoblasts. Afr. J. Biotechnol. 2011, 10, 8662–8668. [Google Scholar] [CrossRef]
- Radulovic, N.; Dekic, M.; Radic, Z.S.; Palic, R. Chemical composition and antimicrobial activity of the essential oils of Geranium columbinum L. and G. lucidum L. (Geraniaceae). Turk. J. Chem. 2011, 35, 499–512. [Google Scholar] [CrossRef]
- Pellati, F.; Prencipe, F.P.; Benvenuti, S. Headspace solid-phase microextraction-gas chromatography–mass spectrometry characterization of propolis volatile compounds. J. Pharm. Biomed. Anal. 2013, 84, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Takeoka, G.R.; Wong, R.Y.; Dao, L.; Felker, P. Identification of 5,6-dihydro-6-propyl-2H-pyran-2-one as the major volatile constituent in mesquite (Prosopis) flour. Food Chem. 2009, 115, 1025–1027. [Google Scholar] [CrossRef]
- Simonsen, H.T.; Riedel, C.; Gade, L.B.; Jebjerg, C.P.; Guzman, A.; Molgaard, P. Chemical composition and antibacterial activity of the leaf essential oil of Baccharis magellanica (Lam.) Pers. and Baccharis elaeoides Remy from Chile. J. Essent. Oil Res. 2009, 21, 377–380. [Google Scholar] [CrossRef]
- Barros, M.E.S.B.; Freitas, J.C.R.; Oliveira, J.M.; da Cruz, C.H.B.; da Silva, P.B.N.; de Araujo, L.C.C.; Militao, G.C.G.; da Silva, T.G.; Oliveira, R.A.; Menezes, P.H. Synthesis and evaluation of (−)-Massoialactone and analogues as potential anticancer and anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Urbain, A.; Corbeiller, P.; Aligiannis, N.; Halabalaki, M.; Skaltsounis, A.L. Hydrostatic countercurrent chromatography and ultra high pressure LC: Two fast complementary separation methods for the preparative isolation and the analysis of the fragrant massoia lactones. J. Sep. Sci. 2010, 33, 1198–1203. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, N.; Sugihara, S.; Mochida, K.; Fujita, T. In vitro antifungal and antiviral activities of γ and δ-lactone analogs utilized as food flavoring. Biocontrol Sci. 2005, 10, 31–36. [Google Scholar] [CrossRef]
- Richard, D.; Kefi, K.; Barbe, U.; Bausero, P.; Visioli, F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008, 57, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Endo, J.; Arita, M. Cardioprotective mechanism of omega-3 polyunsaturated fatty acids. J. Cardiol. 2016, 67, 22–27. [Google Scholar] [CrossRef] [PubMed]
- De Assis, A.M.; Rech, A.; Longoni, A.; Morrone, M.D.; Pasquali, M.A.D.; Perry, M.L.S.; Souza, D.O.; Moreira, J.C.F. Dietary n-3 polyunsaturated fatty acids revert renal responses induced by a combination of 2 protocols that increase the amounts of advanced glycation end product in rats. Nutr. Res. 2015, 35, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Leung, K.S.Y.; Jiang, Z.H.; Dong, X.P.; Zhao, Z.Z. Establishment of GC-MS fingerprint of fresh Houttuynia cordata. Chem. Pharm. Bull. 2005, 53, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the fermentation products used in this study are available from the authors. |




| Samples | Batch No. | Yields 2 | Average Yield | RSD% |
|---|---|---|---|---|
| JSBC-1 | 131004 | 2.9 | ||
| JSBC-2 | 130913 | 2.8 | 3.0 | 7.0 |
| JSBC-3 | 140208 | 3.2 | ||
| BLC-1 | 121243 | 0.7 | ||
| BLC-2 | 130749 | 0.8 | 0.7 | 7.8 |
| BLC-3 | 131128 | 0.7 | ||
| ZLC-1 | 130406 | 0.7 | ||
| ZLC-2 | 130703 | 0.7 | 0.7 | 7.8 |
| ZLC-3 | 130902 | 0.8 | ||
| NXBC-1 | 1401001 | 0.6 | ||
| NXBC-2 | 1401003 | 0.7 | 0.7 | 8.6 |
| NXBC-3 | 1306002 | 0.7 | ||
| XGBC-1 | 130407 | 1.0 | ||
| XGBC-2 | 18130101 | 1.1 | 1.0 | 5.6 |
| XGBC-3 | 18140104 | 1.0 |
| No. | tR (min) | Compound 1 | Class | Formula | Average Peak Area Percentage (%, n = 3) | RI 2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| JSBC | BLC | ZLC | NXBC | XGBC | ||||||
| 1 | 5.28 | Hexanal | Aldehyde | C6H12O | 0.06 | - | - | 0.16 | - | 801 3 |
| 2 | 6.13 | Furfural | Aldehyde | C5H4O2 | 0.01 | - | - | - | - | 829 3 |
| 3 | 6.36 | 3-Methylbutanoic acid | Fatty acid | C5H10O2 | 0.02 | - | 0.21 | 0.35 | 0.59 | 831 3 |
| 4 | 6.60 | 2-Methylbutanoic acid | Fatty acid | C5H10O2 | 0.01 | - | 0.20 | 0.45 | 0.43 | 841 3 |
| 5 | 6.73 | 2-Furanmethanol | Alcohol | C5H6O2 | 0.22 | - | 0.19 | 0.16 | 0.50 | 853 3 |
| 6 | 7.77 | 2-Heptanone | Ketone | C7H14O | 0.01 | - | - | - | - | 895 3 |
| 7 | 8.09 | Heptanal | Aldehyde | C7H14O | 0.01 | - | - | - | - | 901 3 |
| 8 | 10.14 | Benzaldehyde | Aldehyde | C7H6O | 0.05 | - | - | - | 0.30 | 964 3 |
| 9 | 10.29 | 5-Methylfurfural | Aldehyde | C6H6O2 | 0.01 | - | - | - | - | 978 3 |
| 10 | 10.95 | Hexanoic acid | Fatty acid | C6H12O2 | 0.07 | - | 0.27 | 0.33 | 0.50 | 982 3 |
| 11 | 11.37 | 2-Ethyl-5-methylpyrazine | Pyrazine | C7H10N2 | - | - | 0.08 | 0.07 | - | 993 3 |
| 12 | 11.44 | 2-Ethyl-6-methylpyrazine | Pyrazine | C7H10N2 | 0.01 | - | 0.08 | - | - | 1003 3 |
| 13 | 11.47 | 2,3,5-Trimethylpyrazine | Pyrazine | C7H10N2 | 0.01 | - | - | - | - | 1005 3 |
| 14 | 12.26 | Benzyl alcohol | Alcohol | C7H8O | 0.01 | - | 0.08 | 0.05 | 0.18 | 1035 3 |
| 15 | 12.70 | Phenylacetaldehyde | Aldehyde | C8H8O | 0.20 | 0.65 | 0.17 | 0.17 | 2.92 | 1049 3 |
| 16 | 12.80 | 2-Acetylpyrrole | Pyrrole | C6H7NO | 0.05 | 0.20 | 0.16 | 0.06 | 0.34 | 1055 3 |
| 17 | 12.93 | Acetophenone | Ketone | C8H8O | 0.01 | - | 0.12 | 0.10 | - | 1064 3 |
| 18 | 13.08 | p-Cresol | Phenol | C7H8O | 0.01 | 0.18 | 0.29 | 0.10 | - | 1072 3 |
| 19 | 13.16 | 2,5-Dimethyl-3-ethylpyrazine | Pyrazine | C8H12N2 | 0.02 | - | 0.15 | 0.58 | 0.56 | 1082 3 |
| 20 | 13.18 | 1-Ethenyl-3-ethylbenzene | Hydrocarbon | C10H12 | 0.01 | 0.16 | 0.28 | 0.11 | - | 1084 |
| 21 | 13.26 | 1-Ethenyl-4-ethylbenzene | Hydrocarbon | C10H12 | 0.02 | 0.36 | 0.25 | 0.09 | 0.54 | 1089 |
| 22 | 13.35 | 2-Methoxyphenol | Phenol | C7H8O2 | 0.01 | - | 0.06 | - | - | 1092 3 |
| 23 | 13.51 | Undecane | Hydrocarbon | C11H24 | 0.01 | - | 0.06 | - | - | 1100 3 |
| 24 | 13.76 | 1,3-Diethenylbenzene | Hydrocarbon | C10H10 | 0.02 | 0.62 | 0.36 | 1.99 | 1.27 | 1114 |
| 25 | 13.85 | 1,3-Dichloro-2-methylbenzene | Aromatic hydrocarbon | C7H6Cl2 | 0.01 | 0.33 | - | 0.10 | 0.29 | 1117 |
| 26 | 13.86 | Phenylethyl alcohol | Alcohol | C8H10O | 0.01 | - | - | 0.13 | - | 1119 3 |
| 27 | 13.88 | 1,4-Dichloro-2-methylbenzene | Aromatic hydrocarbon | C7H6Cl2 | 0.02 | 0.52 | 0.55 | 0.14 | 0.49 | 1121 |
| 28 | 13.94 | 4-Nonen-2-one | Ketone | C9H16O | 0.04 | - | 0.05 | - | - | 1123 |
| 29 | 13.96 | 1,4-Diethenylbenzene | Hydrocarbon | C10H10 | 0.01 | 0.21 | 0.13 | 0.05 | 0.14 | 1125 |
| 30 | 14.15 | 3-Nonen-2-one | Ketone | C9H16O | 0.06 | - | - | - | - | 1132 3 |
| 31 | 14.31 | 1,2-Dichloro-4-methylbenzene | Aromatic hydrocarbon | C7H6Cl2 | 0.01 | 0.33 | 0.24 | 0.10 | 0.28 | 1146 |
| 32 | 14.39 | 2,3-Diethyl-5-methylpyrazine | Pyrazine | C9H14N2 | - | - | 0.06 | 0.07 | - | 1157 3 |
| 33 | 14.42 | 3,5-Diethyl-2-methylpyrazine | Pyrazine | C9H14N2 | 0.03 | - | - | 0.09 | 0.37 | 1159 3 |
| 34 | 14.46 | Benzoic acid | Fatty acid | C7H6O2 | 0.03 | - | 0.42 | 0.29 | 0.54 | 1162 3 |
| 35 | 14.52 | 4-Ethylphenol | Phenol | C8H10O | 0.04 | - | 0.51 | 2.27 | 0.37 | 1169 3 |
| 36 | 14.55 | Octanoic acid | Fatty acid | C8H16O2 | 0.03 | 0.44 | 0.79 | 0.06 | 0.48 | 1171 3 |
| 37 | 14.88 | 2-Decanone | Ketone | C10H20O | 0.01 | - | 0.12 | 0.07 | - | 1209 3 |
| 38 | 15.02 | 2,5-Dimethyl-3-(2-methylpropyl)pyrazine | Pyrazine | C10H16N2 | 0.02 | - | 0.16 | 0.13 | 0.11 | 1217 |
| 39 | 15.29 | 2,5-Dimethyl-3-(1-propenyl)pyrazine | Pyrazine | C9H12N2 | 0.02 | - | 0.18 | 0.15 | 0.24 | 1238 |
| 40 | 15.63 | 2-Isoamyl-6-methylpyrazine | Pyrazine | C10H16N2 | 0.02 | 0.35 | 0.38 | 0.45 | - | 1260 |
| 41 | 15.83 | 5,6-Dihydro-6-propyl-2H-pyran-2-one | Lactone | C8H12O2 | 0.04 | - | 0.15 | - | - | 1275 |
| 42 | 15.87 | 2-Methyl-3-phenyl-2-propenal | Aldehyde | C10H10O | 0.02 | 0.34 | 0.32 | 0.34 | 0.66 | 1293 3 |
| 43 | 16.07 | 2-Undecanone | Ketone | C11H22O | 0.01 | 0.26 | 0.14 | 0.19 | 0.23 | 1295 3 |
| 44 | 16.34 | 2,4-Decadienal | Aldehyde | C10H16O | 0.05 | 1.80 | 1.08 | 2.59 | 3.91 | 1316 3 |
| 45 | 16.45 | 2,5-Dimethyl-3-(3-methylbutyl)pyrazine | Pyrazine | C10H18N2 | 0.01 | 0.42 | 0.14 | - | 0.24 | 1329 3 |
| 46 | 16.84 | Decanoic acid | Fatty acid | C10H20O2 | 0.03 | 0.96 | 2.96 | 0.18 | 0.50 | 1360 3 |
| 47 | 16.93 | γ-Nonanolactone | Lactone | C9H16O2 | 0.02 | 0.49 | 0.46 | 0.17 | 0.13 | 1364 3 |
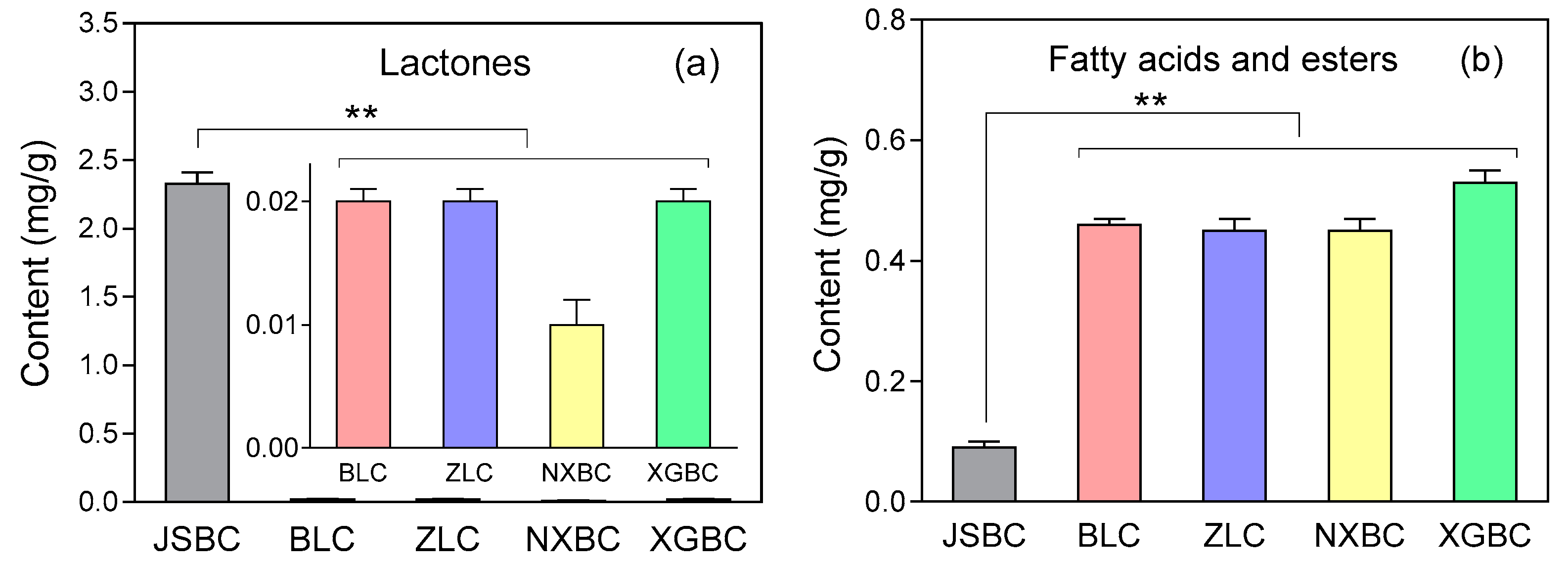
| 48 | 18.61 | 5,6-Dihydro-6-pentyl-2H-pyran-2-one | Lactone | C10H16O2 | 77.46 | 1.78 | 1.97 | 1.63 | 1.48 | 1476 3 |
| 49 | 18.80 | 5-Methyl-2-phenyl-2-hexenal | Aldehyde | C13H16O | 0.11 | 1.52 | 1.72 | 1.05 | 4.14 | 1483 |
| 50 | 19.05 | δ-Decalactone | Lactone | C10H18O2 | 0.18 | 0.44 | - | - | - | 1492 3 |
| 51 | 19.20 | Butylated hydroxytoluene | Alcohol | C15H24O | - | 1.93 | 0.35 | - | - | 1515 |
| 52 | 19.94 | Lauric acid | Fatty acid | C12H24O2 | 0.11 | 0.93 | 0.61 | 0.31 | 1.08 | 1557 3 |
| 53 | 23.73 | 2-Pentadecanone | Ketone | C15H30O | 0.01 | 0.64 | 0.42 | 0.24 | 0.65 | 1688 3 |
| 54 | 25.76 | Myristic acid | Fatty acid | C14H28O2 | 0.05 | 0.76 | 1.91 | 0.19 | 0.18 | 1768 3 |
| 55 | 27.05 | Ethyl myristate | Ester | C16H32O2 | - | 0.25 | 0.23 | - | - | 1809 3 |
| 56 | 29.46 | Pentadecanoic acid | Fatty acid | C15H30O2 | 0.03 | 0.47 | 0.36 | 0.38 | - | 1862 3 |
| 57 | 31.14 | 2-Heptadecanone | Ketone | C17H34O | 0.02 | 0.23 | 0.39 | 0.32 | 0.58 | 1902 3 |
| 58 | 32.14 | Methyl palmitate | Ester | C17H34O2 | 0.10 | 0.74 | 1.17 | 0.93 | 0.52 | 1928 3 |
| 59 | 32.76 | Palmitoleic acid | Fatty acid | C16H30O2 | 0.01 | 1.57 | 1.70 | 0.66 | 0.85 | 1941 3 |
| 60 | 33.82 | Palmitic acid | Fatty acid | C16H32O2 | 1.32 | 19.55 | 18.28 | 32.23 | 27.07 | 1969 3 |
| 61 | 34.20 | Ethyl palmitoleate | Ester | C18H34O2 | 0.06 | 4.72 | 5.21 | 0.05 | 0.31 | 1978 3 |
| 62 | 35.07 | Ethyl palmitate | Ester | C18H36O2 | 0.19 | 7.80 | 7.92 | 1.13 | 2.82 | 1996 3 |
| 63 | 38.28 | Methyl linoleate | Ester | C19H34O2 | 0.19 | 2.45 | 2.95 | 1.56 | 1.44 | 2095 3 |
| 64 | 38.46 | Methyl oleate | Ester | C19H36O2 | 0.14 | 1.81 | 1.81 | 2.24 | 1.47 | 2112 3 |
| 65 | 39.36 | Linoleic acid | Fatty acid | C18H32O2 | 0.22 | 4.12 | 5.37 | 17.74 | 6.07 | 2138 3 |
| 66 | 40.09 | Ethyl linoleate | Ester | C20H36O2 | 0.23 | 9.57 | 7.90 | 2.36 | 3.40 | 2163 3 |
| 67 | 40.25 | Ethyl oleate | Ester | C20H38O2 | 0.13 | 5.87 | 4.49 | 2.15 | 3.40 | 2166 3 |
| 68 | 40.92 | Ethyl stearate | Ester | C20H40O2 | 0.02 | 3.08 | 1.23 | 0.29 | 1.09 | 2189 3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, Y.; Mi, J.; Zhang, M.; Wang, Y.; Jiang, Z.; Hu, P. GC-MS Profiling of Volatile Components in Different Fermentation Products of Cordyceps Sinensis Mycelia. Molecules 2017, 22, 1800. https://doi.org/10.3390/molecules22101800
Zhang H, Li Y, Mi J, Zhang M, Wang Y, Jiang Z, Hu P. GC-MS Profiling of Volatile Components in Different Fermentation Products of Cordyceps Sinensis Mycelia. Molecules. 2017; 22(10):1800. https://doi.org/10.3390/molecules22101800
Chicago/Turabian StyleZhang, Hongyang, Yahui Li, Jianing Mi, Min Zhang, Yuerong Wang, Zhihong Jiang, and Ping Hu. 2017. "GC-MS Profiling of Volatile Components in Different Fermentation Products of Cordyceps Sinensis Mycelia" Molecules 22, no. 10: 1800. https://doi.org/10.3390/molecules22101800
APA StyleZhang, H., Li, Y., Mi, J., Zhang, M., Wang, Y., Jiang, Z., & Hu, P. (2017). GC-MS Profiling of Volatile Components in Different Fermentation Products of Cordyceps Sinensis Mycelia. Molecules, 22(10), 1800. https://doi.org/10.3390/molecules22101800