Lipase-Catalyzed Transesterification of Egg-Yolk Phophatidylcholine with Concentrate of n-3 Polyunsaturated Fatty Acids from Cod Liver Oil
Abstract
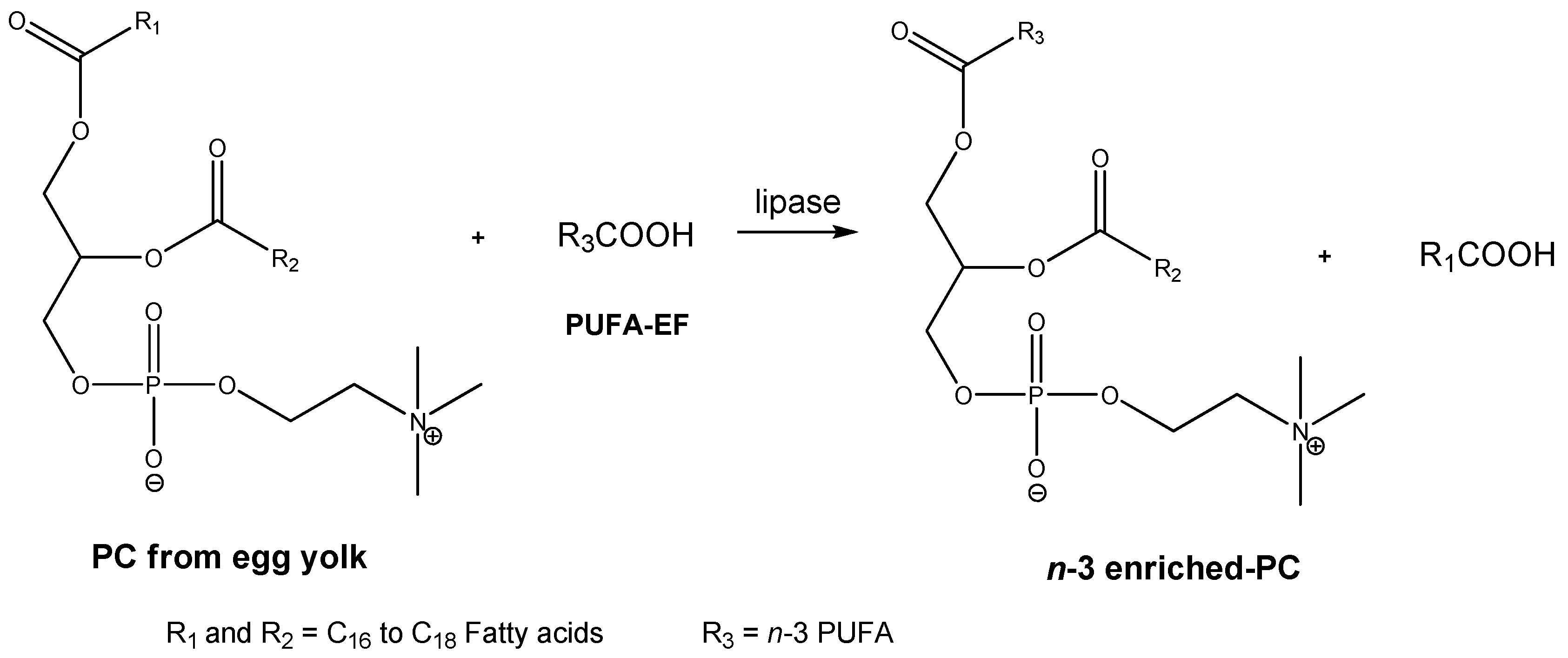
:1. Introduction
2. Results and Discussion
2.1. Screening of Enzymes
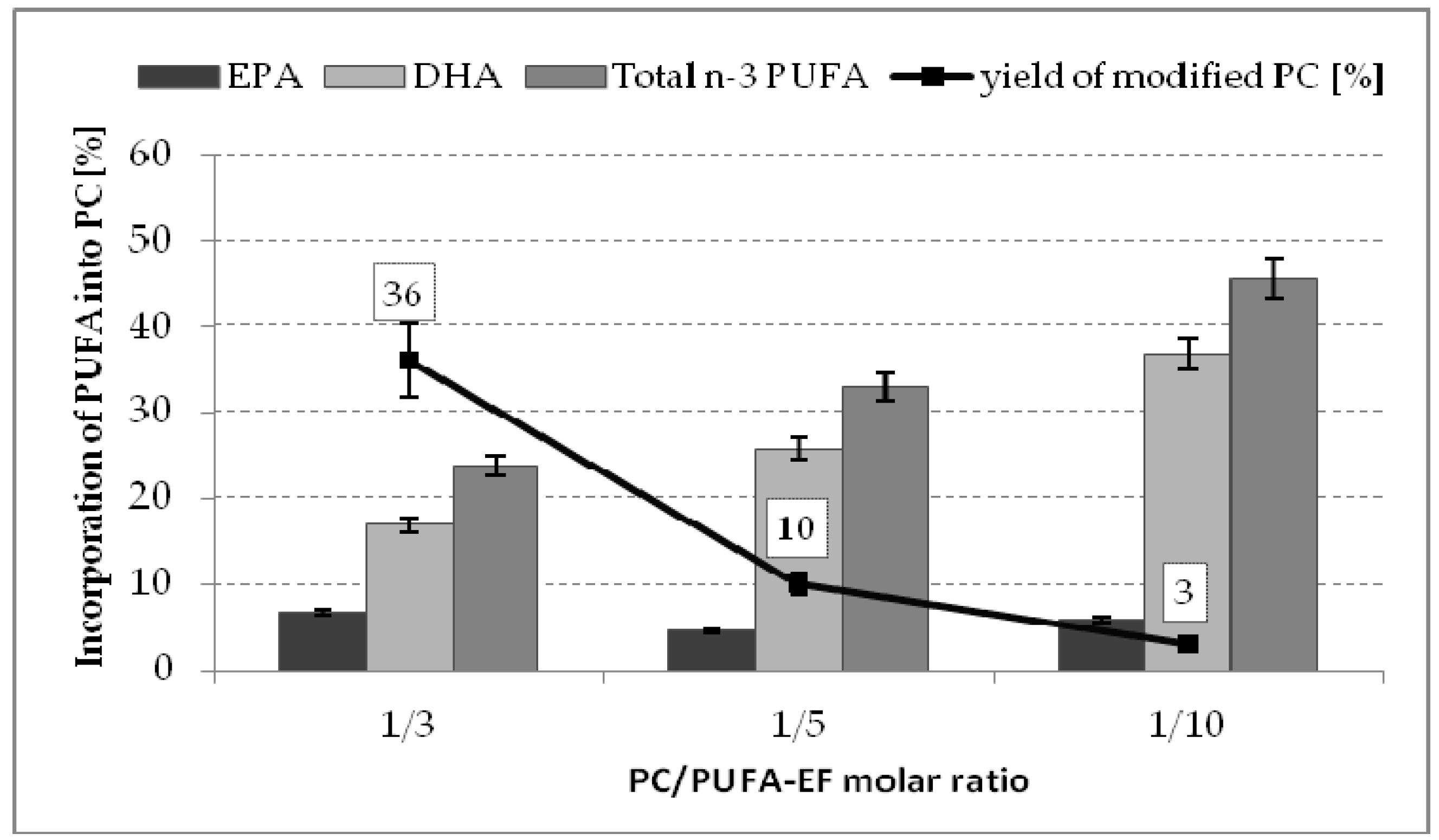
2.2. Effect of Substrate Molar Ratio
2.3. The Effect of Organic Solvent
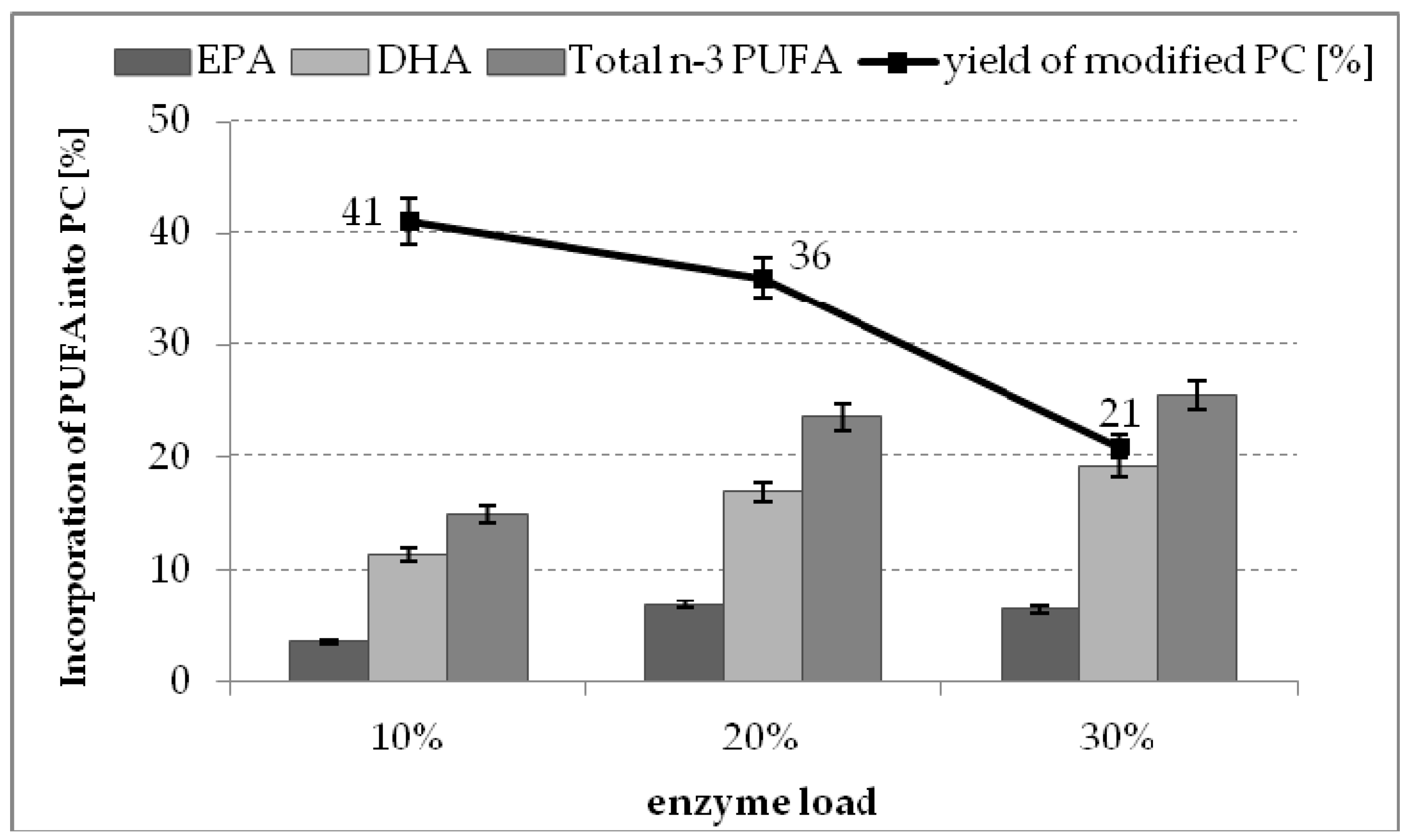
2.4. The Effect of Enzyme Dosage
2.5. Positional Analysis of Modified PC
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Isolation of PC from Egg Yolk
3.3. Production of n-3 PUFA Concentrate
3.4. The Lipase-Catalyzed Transesterifiction of PC with PUFA Concentrates
3.5. Analysis of Substrates and Products
3.6. Positional Analysis of Fatty Acids in Native and Modified PC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Flachs, P.; Mohammed-Ali, V.; Horakove, O.; Rossmeisl, M.; Hosseinzadeh-Attar, M.J.; Hensler, M.; Ruzickova, J.; Kopecky, J. Polyunsaturated fatty acids of marine origin induce adiponectin in mice fed a high-fat diet. Diabetologia 2006, 49, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Tandy, S.; Chung, R.W.; Wat, E.; Kamili, A.; Berge, K.; Griinari, M.; Cohn, J.S. Dietary krill oil supplementation reduces hepatic steatosis, glycemia, and hypercholesterolemia in high-fat-fed mice. J. Agric. Food Chem. 2009, 57, 9339–9345. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.M.; Myung, S.K.; Lee, Y.J.; Seo, H.G. Efficacy of omega-3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease: A meta-analysis of randomized, double-blind, placebo-controlled trials. Arch. Intern. Med. 2012, 172, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. Omega-3 fatty acids in inflammation and autoimmune diseases. J. Am. Coll. Nutr. 2002, 21, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Narayanan, N.K.; Reddy, B.S. Docosahexaenoic acid regulated genes and transcription factors inducing apoptosis in human colon cancer cells. Int. J. Oncol. 2001, 19, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.T.; Zelnik, D.L.; Dobs, A.S. Fish oil supplementation in the treatment of cachexia in pancreatic cancer patients. Int. J. Gastrointest. Cancer 2003, 34, 143–150. [Google Scholar] [CrossRef]
- Alessandri, J.M.; Guesnet, P.; Vancassel, V.; Astorg, P.; Denis, I.; Langelier, B.; Aïd, S.; Poumès-Ballihaut, C.; Champeil-Potokar, G.; Lavialle, M. Polyunsaturated fatty acids in the nervous system: Evolution of concepts and nutritional implications throughout life. Reprod. Nutr. Dev. 2004, 44, 509–538. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid and the aging brain. J. Nutr. 2008, 138, 2510–2514. [Google Scholar] [CrossRef] [PubMed]
- Birch, E.E.; Hoffman, D.R.; Uauy, R.; Birch, D.G.; Prestidge, C. Visual acuity and the essentiality of docosahexaenoic acid and arachidonic acid in the diet of term infants. Pediatr. Res. 1998, 44, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.T.; Tisdale, M.J. Effect of eicosapentaenoic acid (EPA) on expression of a lipid mobilizing factor in adipose tissue in cancer cachexia. Prostaglandins Leukot. Essent. Fatty Acids 2005, 72, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Burri, L.; Hoem, N.; Banni, S.; Berge, K. Marine Omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci. 2012, 13, 15401–15419. [Google Scholar] [CrossRef] [PubMed]
- Schuchardt, J.P.; Neubronner, J.; Kressel, G.; Merkel, M.; von Schacky, C.; Hahn, A. Moderate doses of EPA and DHA from re-esterified triacylglycerols but not from ethyl-esters lower fasting serum triacylglycerols in statin-treated dyslipidemic subjects: Results from a six month randomized controlled trial. Prostaglandins Leukot. Essent. Fatty Acids 2011, 85, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterization of lipids from Antarctic krill (Euphausia superb). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Haraldsson, G.G. The application of lipase for preparing various lipids enriched with Omega-3 fatty acids. Rit Fiskideildar 1999, 16, 97–105. [Google Scholar]
- Subbaiah, P.V.; Dammanahalli, K.J.; Yang, P.; Bi, J.; O’Donnell, J.M. Enhanced incorporation of dietary DHA into lymph phospholipids by altering its molecular carrier. Biochim. Biophys. Acta 2016, 1861, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, M.; Bernoud, N.; Brossard, N.; Lemaitre-Delaunay, D.; Thies, F.; Croset, M.; Lecerf, J. Lysophosphatidylcholine as a preferred carrier form of docosahexaenoic acid to the brain. J. Mol. Neurosci. 2001, 16, 201–204. [Google Scholar] [CrossRef]
- Bartelt, A.; Weigelt, C.; Cherradi, M.L.; Niemeier, A.; Todter, K.; Heeren, J.; Scheja, L. Effects of adipocyte lipoprotein lipase on de novo lipogenesis and white adipose tissue browning. Biochim. Biophys. Acta 2013, 1831, 934–942. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre-Delauney, D.; Pachiaudi, C.; Laville, M.; Pousin, J.; Armstrong, M.; Lagarde, M. Blood compartmental metabolism of docosahexaenoic acid (DHA) in humans after ingestion of a single dose of [13C]DHA in phosphatidylcholine. J. Lipid Res. 1999, 40, 1867–1874. [Google Scholar]
- Coste, T.C.; Gerbi, A.; Vague, P.; Pieroni, G.; Raccah, D. Neuroprotective effect of docosahexaenoic acid-enriched phospholipids in experimental diabetic neuropathy. Diabetes 2003, 52, 2578–2585. [Google Scholar] [CrossRef] [PubMed]
- Rossmeisl, M.; Jilkova, Z.M.; Kuda, O.; Jelenik, T.; Medrikova, D.; Stankova, B.; Kristinsson, B.; Haraldsson, G.G.; Svensen, H.; Stoknes, I.; et al. Metabolic effects of n-3 PUFA as phospholipids are superior to triglycerides in mice fed a high-fat diet: Possible role of endocannabinoids. PLoS ONE 2012, 7, e38834. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Banni, S.; Carta, G. Nutritional properties of dietary omega-3-enriched phospholipids. Biomed. Res. Int. 2013, 965417. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Perez, S.; Luna, P.; Senorans, F.J.; Guisan, J.M.; Fernandez-Lorente, G. Enzymatic synthesis of triacylglycerols of docosahexaenoic acid: Transesterification of its ethyl esters with glycerol. Food Chem. 2015, 187, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Haraldsson, G.G.; Thorarensen, A. The generation of glyceryl ether lipids highly enriched with eicosapentaenoic acid and docosahexaenoic acid by lipase. Tetrahedron Lett. 1994, 35, 7681–7684. [Google Scholar] [CrossRef]
- Mustranta, A.; Forssell, P.; Aura, A.M.; Suortti, T.; Poutanen, K. Modification of phospholipids with lipases and phospholipases. Biocatalysis 1994, 9, 181–194. [Google Scholar] [CrossRef]
- Peng, L.; Xu, X.; Mu, H.; Høy, C.-E.; Adler-Nissen, J. Production of structured phospholipids by lipase-catalyzed acidolysis: Optimization using response surface methodology. Enzyme Microb. Technol. 2002, 31, 523–532. [Google Scholar] [CrossRef]
- Chojnacka, A.; Gładkowski, W.; Kiełbowicz, G.; Wawrzeńczyk, C. Enzymatic enrichment of egg-yolk phosphatidylcholine with α-linolenic acid. Biotechnol. Lett. 2009, 31, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, M.; Bhattacharyya, D.K. Soy lecithin—Monoester interchange reaction by microbial lipase. J. Am. Oil Chem. Soc. 1997, 74, 761–763. [Google Scholar] [CrossRef]
- Park, C.W.; Kwon, S.J.; Han, J.J.; Rhee, J.S. Transesterification of phosphatidylcholine with eicosapentaenoic acid ethyl ester using phospholipase A2 in organic solvent. Biotechnol. Lett. 2000, 22, 147–150. [Google Scholar] [CrossRef]
- Kaki, S.S.; Ravinder, T.; Ashwini, B.; Rao, B.V.S.K.; Prasad, R.B.N. Enzymatic modification of phosphatidylcholine with n-3 PUFA from silkworm oil fatty acids. Grasas Aceites 2014, 65, e021. [Google Scholar] [CrossRef]
- Asomaning, J.; Curtis, J.M. Enzymatic modification of egg lecithin to improve properties. Food Chem. 2017, 220, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Chojnacka, A.; Gladkowski, W.; Kielbowicz, G.; Gliszczynska, A.; Niezgoda, N.; Wawrzenczyk, C. Lipase-catalyzed interesterification of egg-yolk phosphatidylcholine and plant oils. Grasas Aceites 2014, 65, e053. [Google Scholar] [CrossRef]
- Chojnacka, A.; Gładkowski, W.; Gliszczyńska, A.; Niezgoda, N.; Kiełbowicz, G.; Wawrzeńczyk, C. Synthesis of structured phosphatidylcholine containing punicic acid by the lipase-catalyzed transesterification with pomegranate seed oil. Catal. Commun. 2016, 75, 60–64. [Google Scholar] [CrossRef]
- Niezgoda, N.; Gliszczyńska, A.; Gładkowski, W.; Chojnacka, A.; Kiełbowicz, G.; Wawrzeńczyk, C. Production of concentrates of CLA obtained from sunflower and safflower and their application to the lipase-catalyzed acidolysis of egg yolk phosphatidylcholine. Eur. J. Lipid Sci. Technol. 2016, 118, 1566–1578. [Google Scholar] [CrossRef]
- Hayes, D.G.; Bengtsson, Y.C.; Alstine, J.M.; Setterwall, F. Urea complexation for the rapid, ecologically responsible fractionation of fatty acids from seed oil. J. Am. Oil Chem. Soc. 1998, 75, 1403–1409. [Google Scholar] [CrossRef]
- Gładkowski, W.; Kiełbowicz, G.; Chojnacka, A.; Gil, M.; Trziszka, T.; Dobrzański, Z.; Wawrzeńczyk, C. Fatty acid composition of egg yolk phospholipid fractions following feed supplementation of Lohmann Brown hens with humic-fat preparations. Food Chem. 2011, 126, 1013–1018. [Google Scholar] [CrossRef]
- Milinsk, M.C.; Murakami, A.E.; Gomes, S.T.M.; Matsushita, M.; de Souza, N.E. Fatty acid profile of egg yolk lipids from hens fed diets rich in n-3 acids. Food Chem. 2003, 83, 287–292. [Google Scholar] [CrossRef]
- Gładkowski, W.; Kiełbowicz, G.; Chojnacka, A.; Bobak, Ł.; Spychaj, R.; Dobrzański, Z.; Trziszka, T.; Wawrzeńczyk, C. The effect of feed supplementation with dietary sources of n-3 polyunsaturated fatty acids, flaxseed and algae Schizochytrium sp., on their incorporation into lipid fractions of Japanese quail eggs. Int. J. Food Sci. Technol. 2014, 49, 1876–1885. [Google Scholar] [CrossRef]
- Lyberg, A.-M.; Adlercreutz, D.; Adlercreutz, P. Enzymatic and chemical synthesis of phosphatidylcholine regioisomers containing eicosapentaenoic acid or docosahexaenoic acid. Eur. J. Lipid Sci. Technol. 2005, 107, 279–290. [Google Scholar] [CrossRef]
- Adlercreutz, D.; Budde, H.; Wehtje, E. Synthesis of phosphatidylcholine with denied fatty acid in the sn-1 position by lipase-catalyzed esterification and transesterification reaction. Biotechnol. Bioeng. 2002, 78, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Vikbjerg, A.F.; Mu, H.; Xu, X. Lipase-catalyzed acyl exchange of soybean phosphatidylcholine in n-hexane: A critical evaluation of both acyl incorporation and product recovery. Biotechnol. Prog. 2005, 21, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Mutua, L.N.; Akoh, C.C. Lipase-catalyzed modification of phospholipids: Incorporation of n-3 fatty acids into biosurfactants. J. Am. Oil Chem. Soc. 1993, 70, 125–128. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Mizuta, E.; Ito, M.; Harata, M.; Hiramoto, S.; Hara, S. Lipase-catalyzed preparation of phospholipids containing n-3 polyunsaturated fatty acids from soy phospholipids. J. Oleo Sci. 2014, 63, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Totani, Y.; Hara, S. Preparation of polyunsaturated phospholipids by lipase-catalyzed transesterification. J. Am. Oil Chem. Soc. 1991, 68, 848–851. [Google Scholar] [CrossRef]
- Kim, I.H.; Garcia, H.S.; Hill, C.G. Phospholipase A1-catalyzed synthesis of phospholipids enriched in n-3 polyunsaturated fatty acid residues. Enzyme Microb. Technol. 2007, 40, 1130–1135. [Google Scholar] [CrossRef]
- Zhao, T.T.; Kim, B.H.; Garcia, H.S.; Kim, Y.; Kim, I.H. Immobilized phospholipase A1-catalyzed modification of phosphatidylcholine with n-3 polyunsaturated fatty acid. Food Chem. 2014, 157, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Garcia, H.S.; Kim, I.; Lopez-Hernandez, A.; Hill, C.G., Jr. Enrichment of lecithin with n-3 fatty acids by acidolysis using immobilized phospholipase A1. Grasas Aceites 2008, 59, 368–374. [Google Scholar] [CrossRef]
- Härröd, M.; Elfman, I. Enzymatic synthesis of phosphatidylcholine with fatty acids, isooctane, carbon dioxide, and propane as solvents. J. Am. Oil Chem. Soc. 1995, 72, 641–646. [Google Scholar] [CrossRef]
- Na, A.; Eriksson, C.; Eriksson, S.-G.; Österberg, E.; Holmberg, K. Synthesis of phosphatidylcholine with (n-3) fatty acids by phospholipase A2 in microemulsion. J. Am. Oil Chem. Soc. 1990, 67, 766–770. [Google Scholar] [CrossRef]
- Xi, X.; Feng, X.M.; Shi, N.R.; Ma, X.X.; Lin, H.; Han, Y.Q. Immobilized phospholipase A1-catalyzed acidolysis of phosphatidylcholine from Antarctic krill (Euphausia superba) for docosahexaenoic acid enrichment under supercritical conditions. J. Mol. Catal. B 2016, 126, 46–55. [Google Scholar] [CrossRef]
- Haraldsson, G.G.; Thorarensen, A. Preparation of phospholipids highly enriched with n-3 polyunsaturated fatty acids by lipase. J Am. Oil. Chem. Soc. 1999, 76, 1143–1149. [Google Scholar] [CrossRef]
- Patkowska-Sokoła, B.; Usydus, Z.; Szlinder-Richert, J.; Bodkowski, R. Technology for recovering omega-3 fatty acids from fish oils and protecting them against oxidative changes. Przem. Chem. 2009, 88, 548–553. (In Polish) [Google Scholar]
- Kiełbowicz, G.; Gładkowski, W.; Chojnacka, A.; Wawrzeńczyk, C. A simple method for positional analysis of phosphatidylcholine. Food Chem. 2012, 135, 2542–2548. [Google Scholar] [CrossRef] [PubMed]
- Kitson, A.P.; Metherel, A.H.; Chen, C.T.; Domenichiello, A.F.; Trépanier, M-O.; Berger, A.; Bazinet, R.P. Effect of dietary docosahexaenoic acid (DHA) in phospholipids or triglycerides on brain DHA uptake and accretion. J. Nutr. Biochem. 2016, 33, 91–102. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all compounds are available from the authors. |


| Fatty Acid | CLO | PUFA-EF | Native PC |
|---|---|---|---|
| C16:0 | 11.0 ± 0.64 | 1.0 ± 0.16 | 34.2 ± 0.11 |
| C18:0 | 2.3 ± 0.26 | 0.1 ± 0.04 | 15.7 ± 0.05 |
| C18:1 | 19.5 ± 0.88 | 3.9 ± 0.11 | 29.0 ± 0.04 |
| C18:2 n-6 | 1.6 ± 0.04 | 1.2 ± 0.03 | 15.3 ± 0.02 |
| C18:3 n-3 | 0.8 ± 0.03 | 2.3 ± 0.07 | - |
| C18:4 SDA n-3 | 2.7 ± 0.11 | 8.5 ± 0.45 | - |
| C20:4 n-6 | 0.9 ± 0.01 | 2.2 ± 0.05 | 2.0 ± 0.01 |
| C20:5 EPA n-3 | 10.4 ± 0.04 | 26.7 ± 0.11 | 0.6 ± 0.02 |
| C21:5 n-3 | 0.5 ± 0.02 | 1.7 ± 0.02 | - |
| C22:5 n-3 | 1.5 ± 0.04 | 2.5 ± 0.01 | - |
| C22:6 (DHA) n-3 | 11.2 ± 0.13 | 45.2 ± 0.35 | 3.2 ± 0.05 |
| Others | 37.6 | 4.7 | - |
| Total n-3 PUFA | 27.1 | 86.9 | 3.8 |
| Fatty Acid | Native PC | Modified PC | Modified LPC | ||||
|---|---|---|---|---|---|---|---|
| Total | sn-1 | sn-2 | Total | sn-1 | sn-2 | ||
| C16:0 | 34.2 ± 0.11 | 64.1 ± 0.22 | 0.8 ± 0.04 | 15.7 ± 0.48 | 28.5 ± 0.65 | 0.5 ± 0.55 | 17.7 ± 0.22 |
| C18:0 | 15.7 ± 0.05 | 29.2 ± 0.55 | 1.1 ± 0.09 | 9.5 ± 0.27 | 19.5 ± 0.35 | 0.3 ± 0.13 | 10.8 ± 0.05 |
| C18:1 | 29.0 ± 0.04 | 5.5 ± 0.33 | 56.3 ± 0.32 | 30.1 ± 0.09 | 7.5 ± 0.12 | 52.1 ± 0.95 | 28.1 ± 0.56 |
| C18:2 | 15.3 ± 0.02 | 1.2 ± 0.05 | 28.8 ± 0.55 | 15.9 ± 0.34 | 1.5 ± 0.25 | 29.0 ± 0.84 | 20.5 ± 0.08 |
| C18:4 SDA n-3 | - | - | - | 2.1 ± 0.06 | 3.8 ± 0.22 | 0.2 ± 0.06 | 1.9 ± 0.02 |
| C20:4 n-6 | 2.0 ± 0.01 | - | 4.5 ± 0.06 | 1.8 ± 0.08 | 3.5 ± 0.12 | 0.5 ± 0.08 | 0.7 ± 0.03 |
| C20:5 EPA n-3 | 0.6 ± 0.02 | - | 1.4 ± 0.06 | 7.8 ± 0.13 | 14.5 ± 0.35 | 1.5 ± 0.12 | 5.9 ± 0.05 |
| C22:6 (DHA) n-3 | 3.2 ± 0.05 | - | 7.1 ± 0.22 | 17.6 ± 0.11 | 29.1 ± 0.84 | 3.2 ± 0.55 | 14.4 ± 0.07 |
| Total n-3 PUFA | 3.8 | 0 | 8.5 | 27.5 | 47.4 | 4.9 | 22.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacka, A.; Gładkowski, W.; Grudniewska, A. Lipase-Catalyzed Transesterification of Egg-Yolk Phophatidylcholine with Concentrate of n-3 Polyunsaturated Fatty Acids from Cod Liver Oil. Molecules 2017, 22, 1771. https://doi.org/10.3390/molecules22101771
Chojnacka A, Gładkowski W, Grudniewska A. Lipase-Catalyzed Transesterification of Egg-Yolk Phophatidylcholine with Concentrate of n-3 Polyunsaturated Fatty Acids from Cod Liver Oil. Molecules. 2017; 22(10):1771. https://doi.org/10.3390/molecules22101771
Chicago/Turabian StyleChojnacka, Anna, Witold Gładkowski, and Aleksandra Grudniewska. 2017. "Lipase-Catalyzed Transesterification of Egg-Yolk Phophatidylcholine with Concentrate of n-3 Polyunsaturated Fatty Acids from Cod Liver Oil" Molecules 22, no. 10: 1771. https://doi.org/10.3390/molecules22101771
APA StyleChojnacka, A., Gładkowski, W., & Grudniewska, A. (2017). Lipase-Catalyzed Transesterification of Egg-Yolk Phophatidylcholine with Concentrate of n-3 Polyunsaturated Fatty Acids from Cod Liver Oil. Molecules, 22(10), 1771. https://doi.org/10.3390/molecules22101771






