Pterodontic Acid Isolated from Laggera pterodonta Inhibits Viral Replication and Inflammation Induced by Influenza A Virus
Abstract
:1. Introduction
2. Results
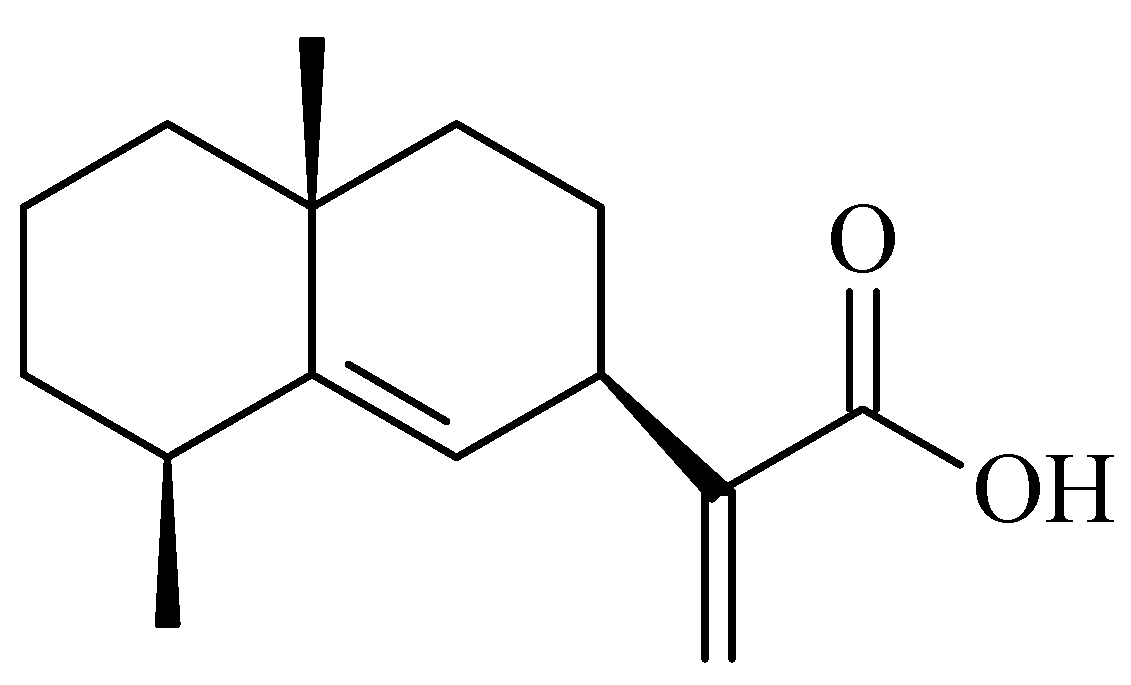
2.1. Identification of Pterodontic Acid by NMR
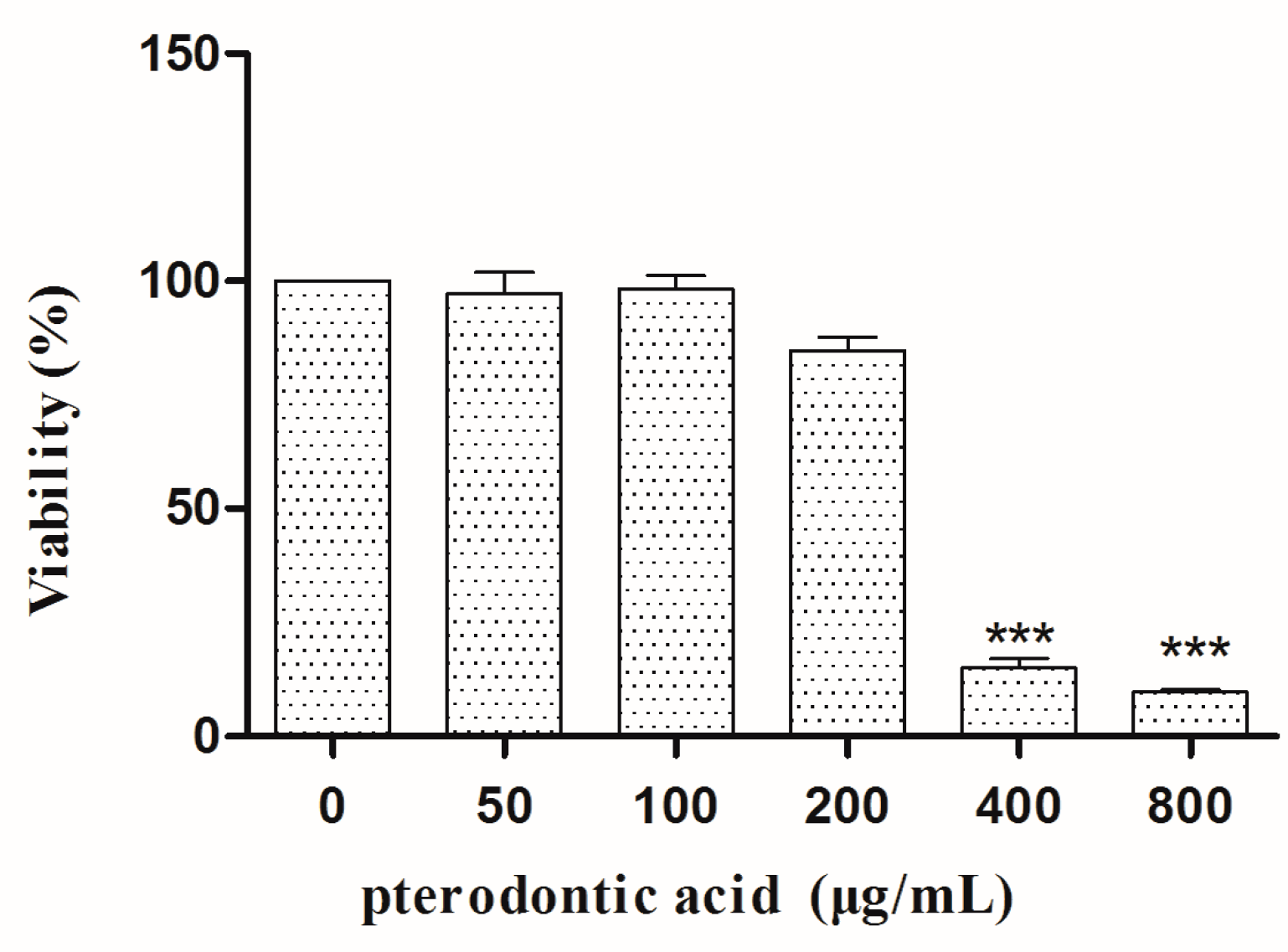
2.2. Cytotoxicity In Vitro
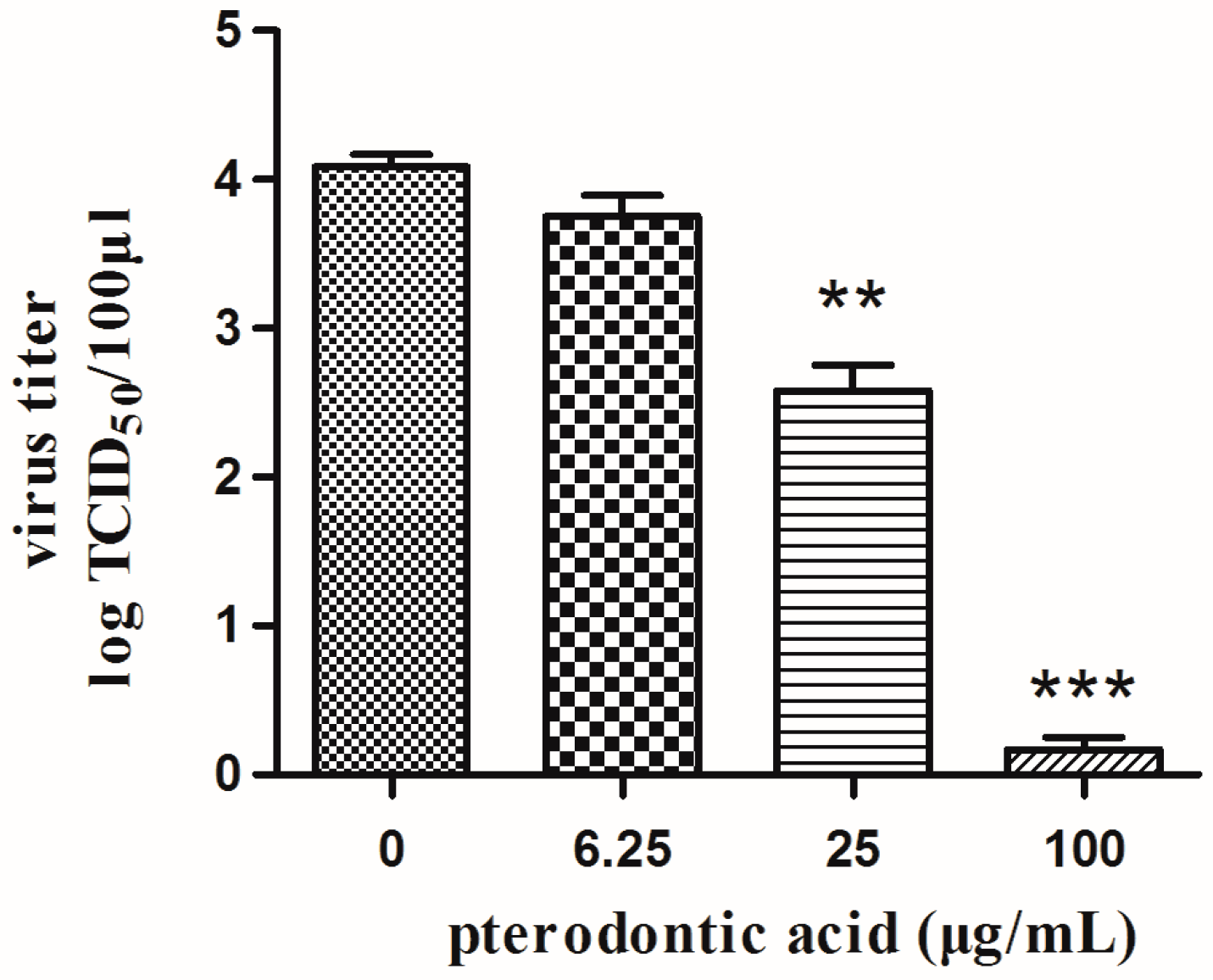
2.3. Anti-Viral Activity In Vitro
2.4. Activities on Neuraminidase
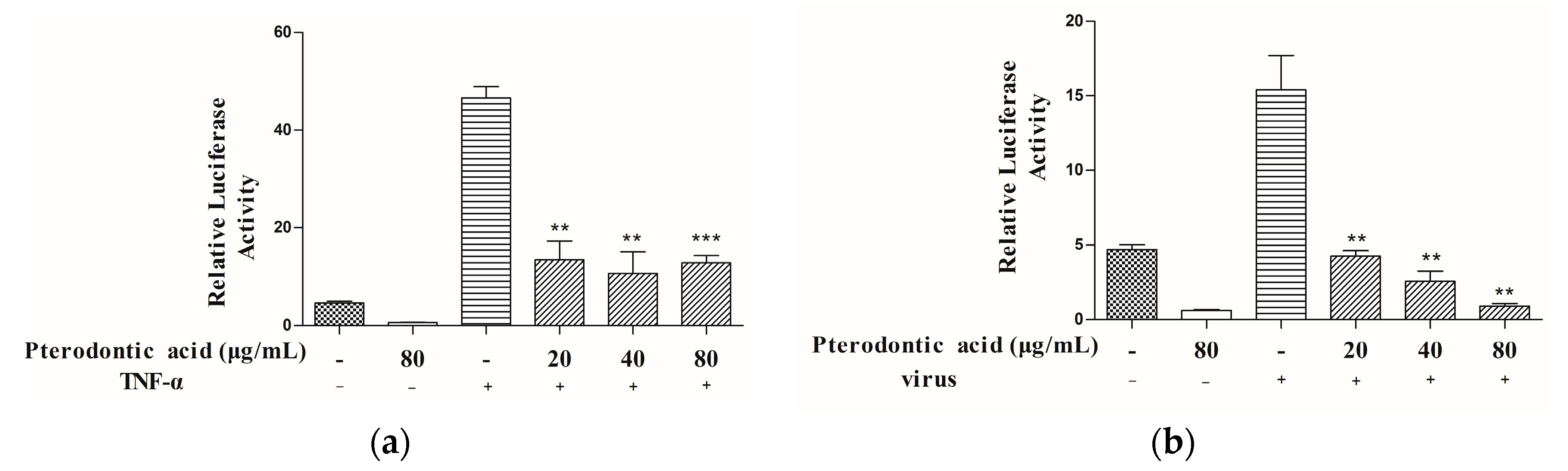
2.5. Suppression of NF-κB Signaling Pathway Activation
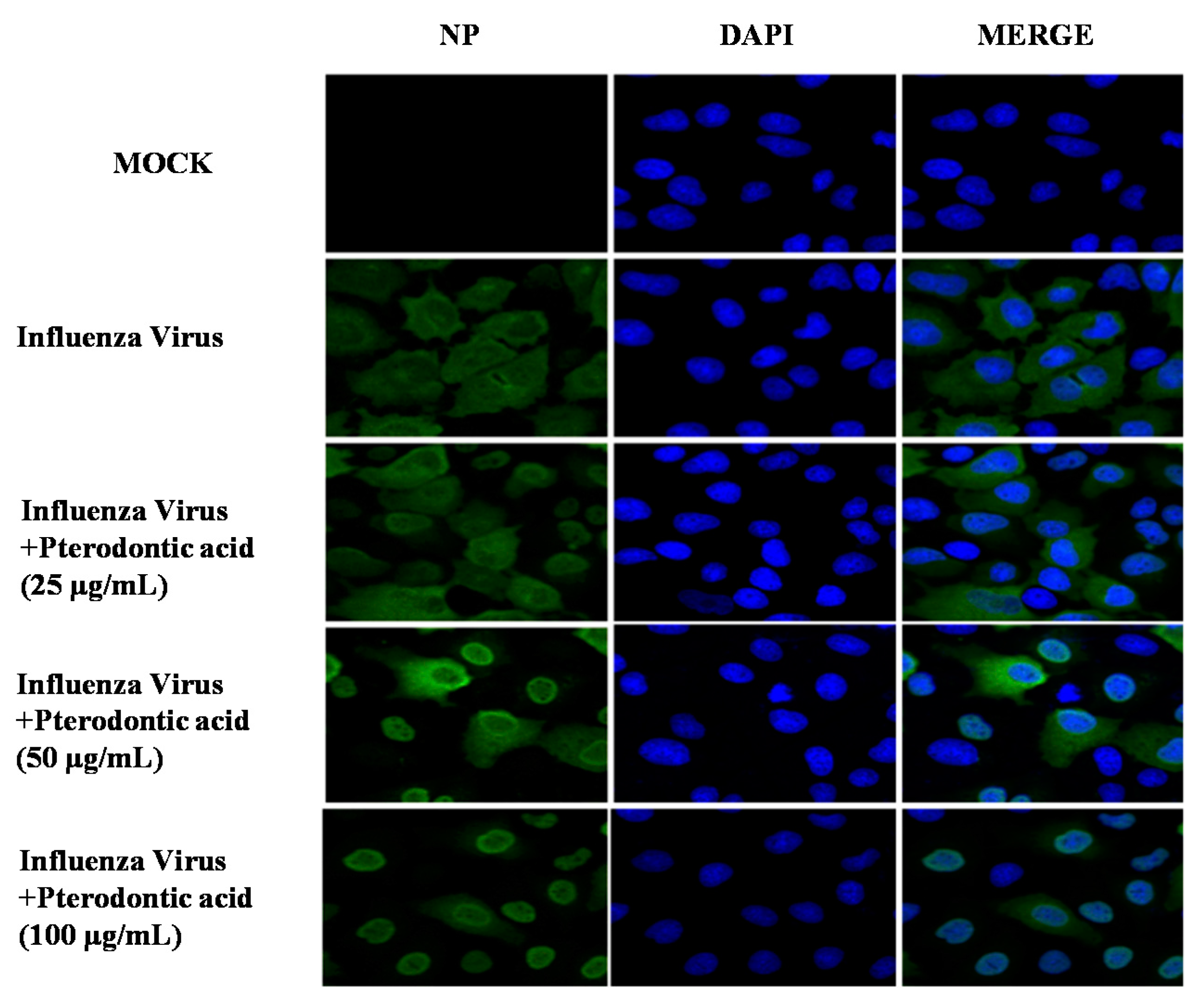
2.6. Inhibition of Viral RNP Export
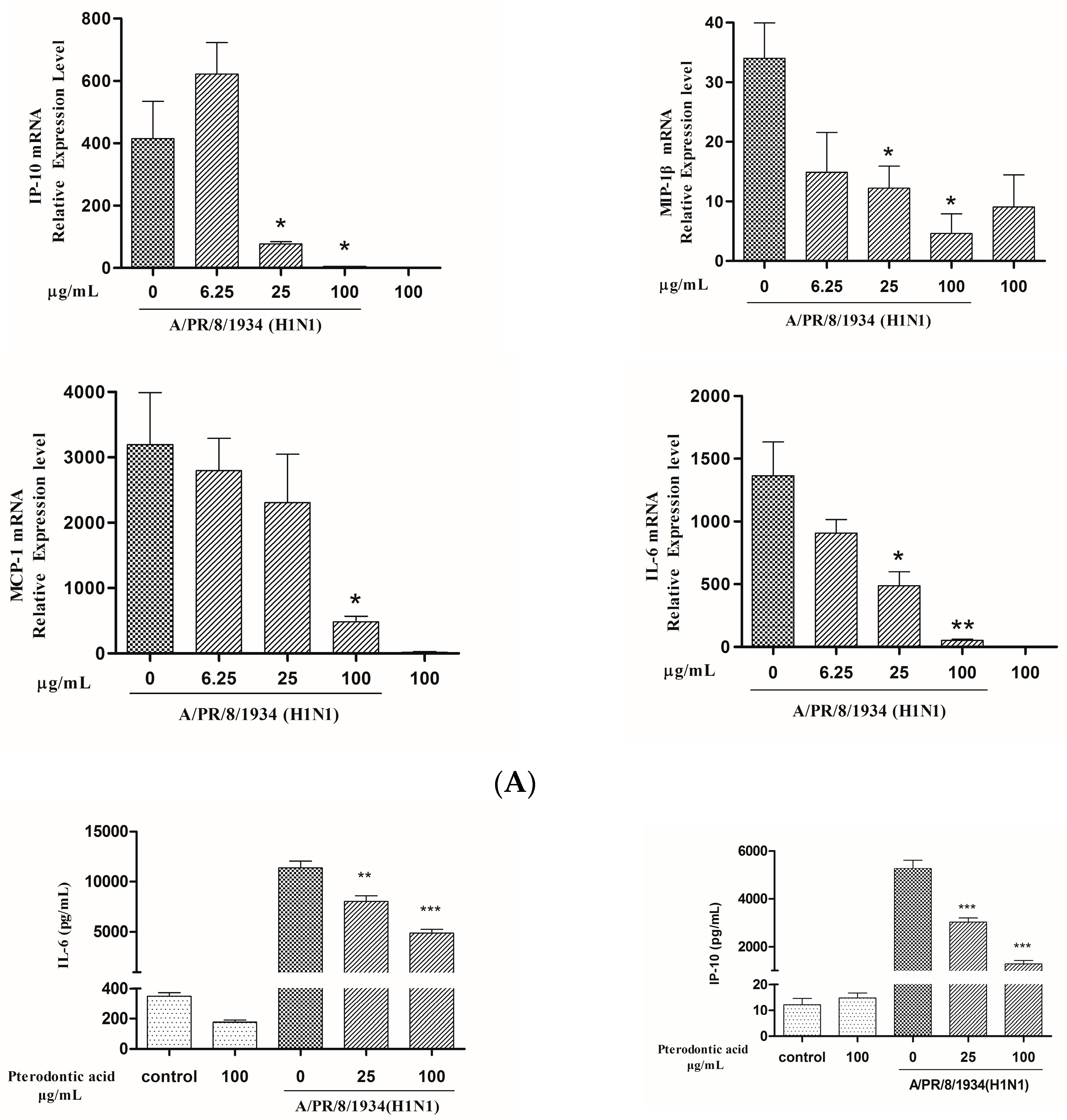
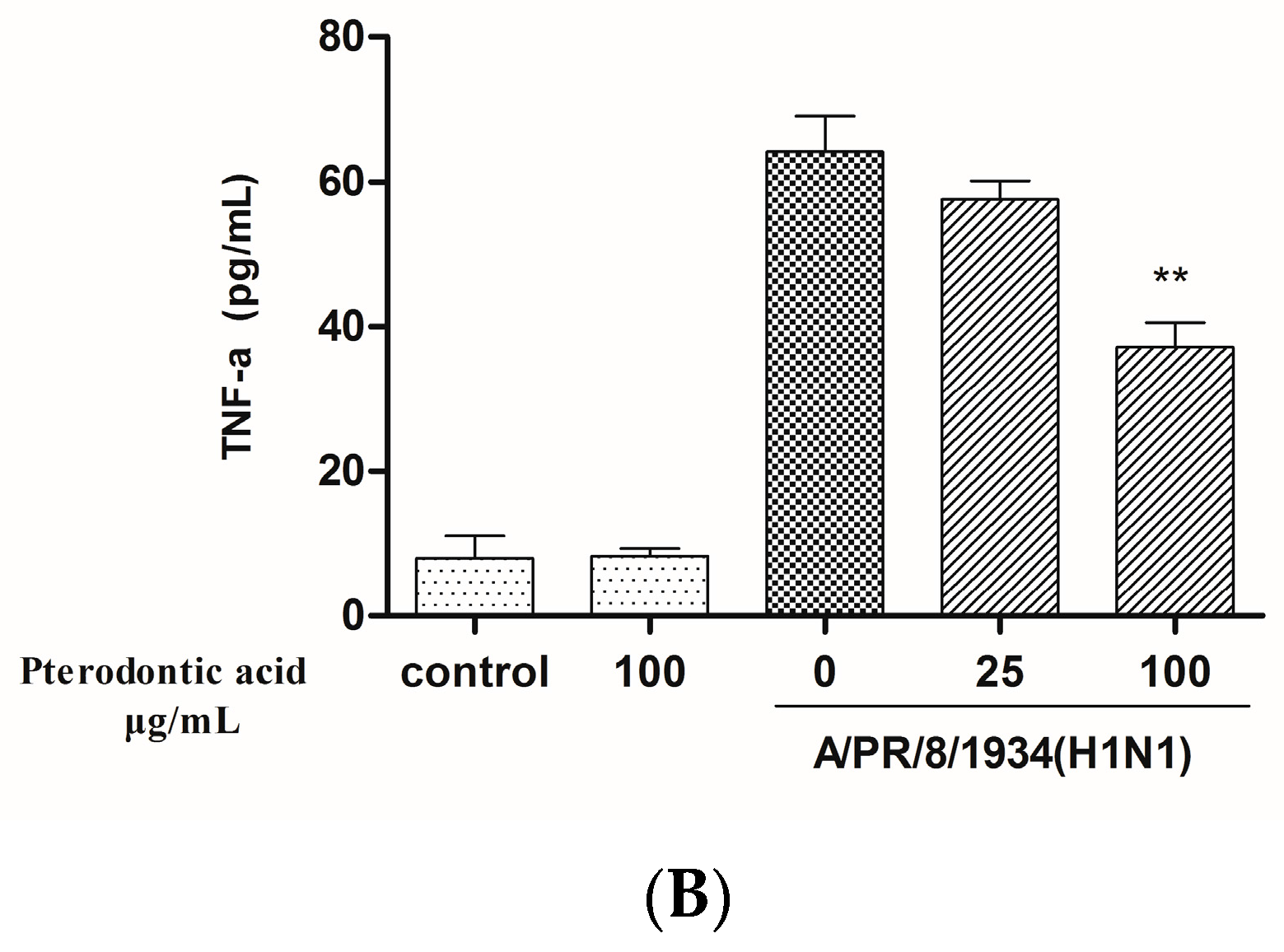
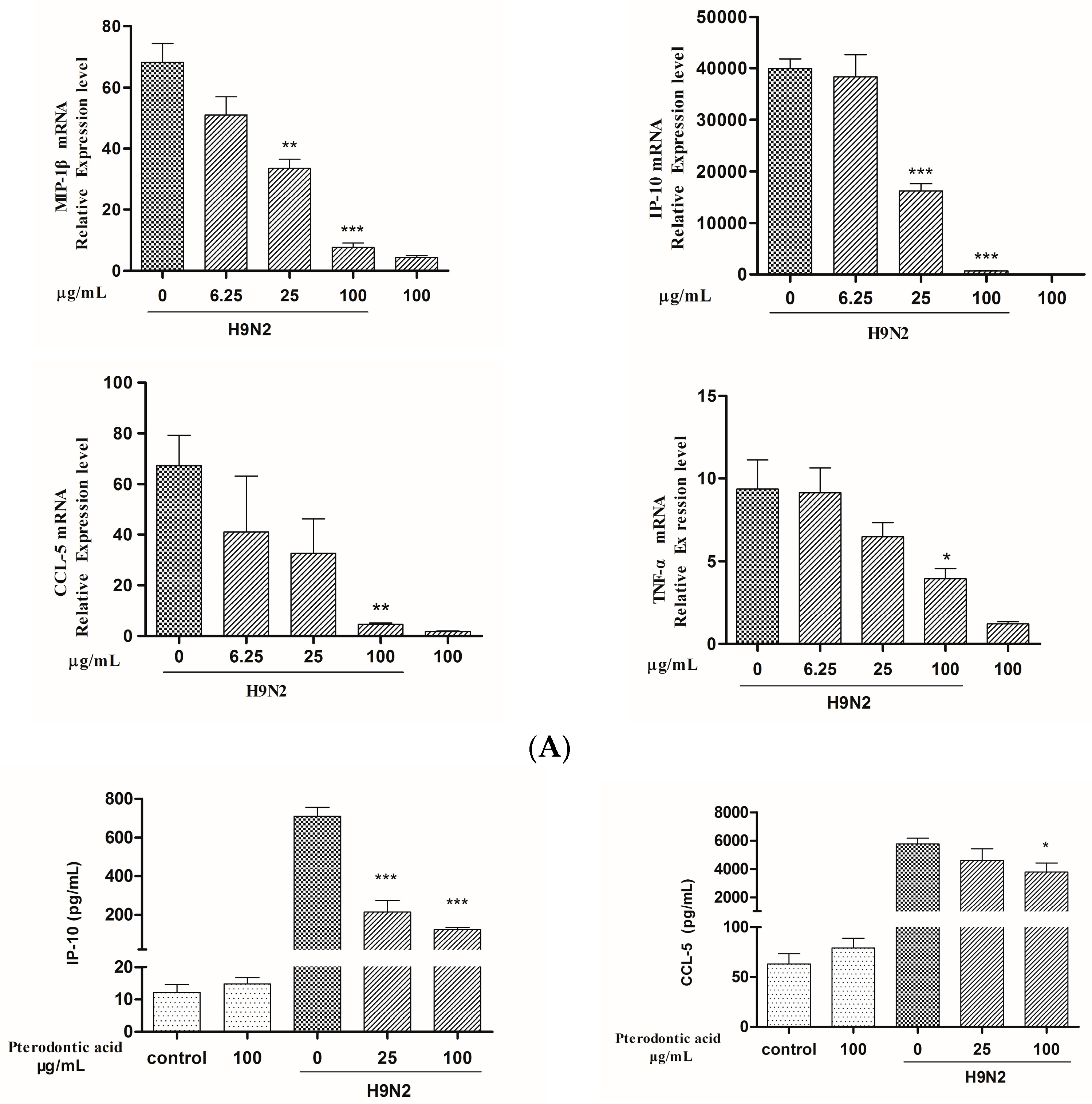
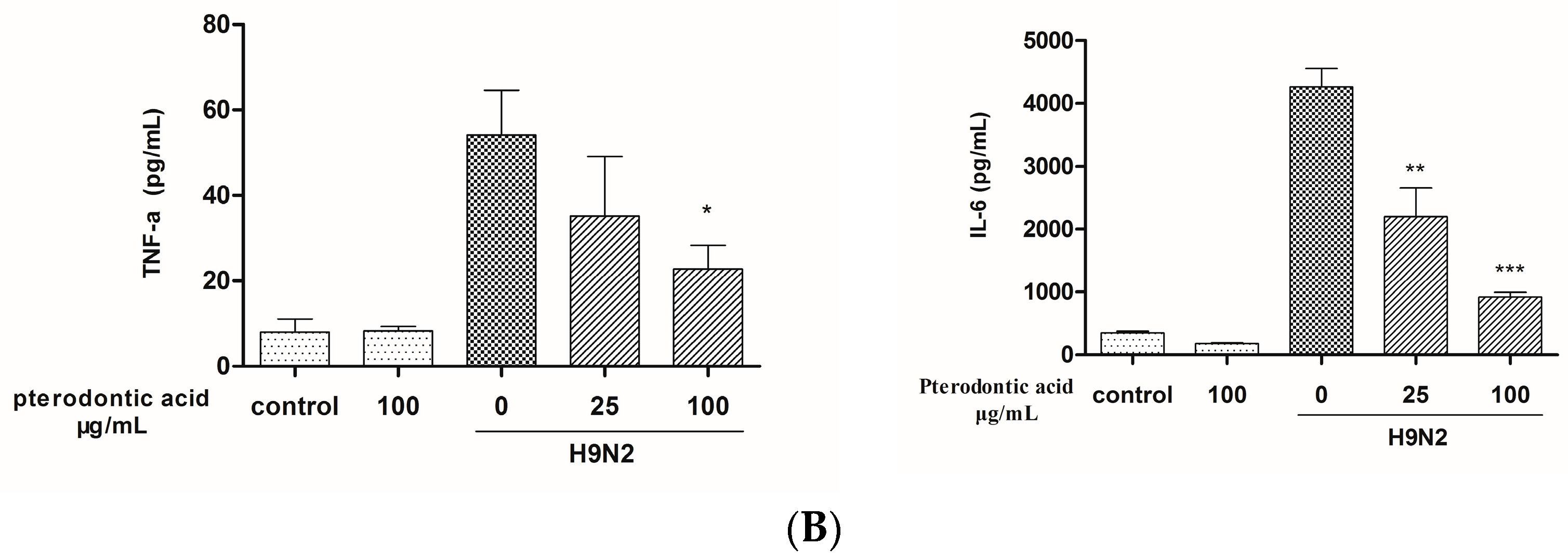
2.7. Impact on Influenza Associated Cytokines Expression in Infected A549 Cells
3. Discussion
4. Materials and Methods
4.1. Reagents and Materials
4.2. Plant Material and Preparation of Pterodontic Acid
4.3. In Vitro Cytotoxicity Assay
4.4. In Vitro Antiviral Assayand Progeny Virus Reduction Assay
4.5. NA Activity Assay
4.6. NF-κB Reporter Assay
4.7. Immunofluorescence Staining
4.8. mRNA Expression of Cytokines Assay by qRT-PCR
4.9. Measurement of Protein Level of Cytokine
4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| IL-6 | interleukin-6 |
| MCP-1 | monocytes chemotactic factor |
| IP-10 | interferon gamma-induced protein 10 |
| TNF-α | tumor necrosis factor alpha |
| NF-κB | Nuclear factor-κB |
| CCL-5 | Chemokine (C-C motif) ligand 5 |
| MIP-1β | Macrophage Inflammatory Proteins-1Beta |
| RNP | ribonucleoprotein |
| MTT | methylthiazolyltetrazolium |
| HEK-293 | Human embryonic kidney 293 cells |
| GFP | green fluorescent protein |
| HPAIV | highly pathogenic avian influenza virus |
| HSV-I | herpes simplex type I |
| HSV-II | herpes simplex type II |
| RSV | respiratory syncytial virus |
| NP | nucleoprotein |
References
- Shang, X.; Liabsuetrakul, T.; Sangsupawanich, P.; Xia, X.; He, P.; Cao, H.; McNeil, E. Efficacy and Safety of Laggera pterodonta in Children 3–24 Months with Acute Bronchiolitis: A Randomized Controlled Trial. Clin. Respir. J. 2015, 4, 1035–1042. [Google Scholar]
- Xia, X.L.; Sun, Q.M.; Wang, X.D.; Zhao, Y.J.; Yang, Z.F.; Huang, Q.H.; Jiang, Z.H.; Wang, X.H.; Zhang, R.P. Effect of Yunnan Herb Laggera pterodonta against Influenza a (H1N1) Virus in Vitro. China J. Chin. Mater. Med. 2015, 40, 3687–3692. [Google Scholar]
- Jiang, D.-F.; Ling, Z.-S.; Bai, C.-Y. The research progress of Dai Medicine Laggera pterodonta. Pharm. Clin. Chin. Mater. Med. 2013, 4, 53–61. [Google Scholar]
- Xiao, Y.; Zheng, Q.; Zhang, Q.; Sun, H.; Guéritte, F.; Zhao, Y. Eudesmane derivatives from Laggera pterodonta. Fitoterapia 2003, 74, 459–463. [Google Scholar] [CrossRef]
- Liu, Y.P.; Lai, R.; Yao, Y.G.; Zhang, Z.K.; Pu, E.T.; Cai, X.H.; Luo, X.D. Induced Furoeudesmanes: A Defense Mechanism Against Stress inLaggerapterodonta, a Chinese Herbal Plant. Org. Lett. 2013, 15, 4940–4943. [Google Scholar] [CrossRef] [PubMed]
- Geiss, G.K.; Salvatore, M.; Tumpey, T.M.; Carter, V.S.; Wang, X.; Basler, C.F.; Taubenberger, J.K.; Bumgarner, R.E.; Palese, P.; Katze, M.G.; et al. Cellular Transcriptional Profiling in Influenza a Virus-Infected Lung Epithelial Cells: The Role of the Nonstructural NS1 Protein in the Evasion of the Host Innate Defense and Its Potential Contribution to Pandemic Influenza. Proc. Natl. Acad. Sci. USA 2002, 99, 10736–10741. [Google Scholar] [CrossRef] [PubMed]
- Smith James, R.; Ariano, R.E.; Toovey, S. The Use of Antiviral Agents for the Management of Severe Influenza. Crit. Care Med. 2010, 38, e43–e51. [Google Scholar] [CrossRef] [PubMed]
- Regoes, R.R.; Bonhoeffer, S. Emergence of Drug-Resistant Influenza Virus: Population Dynamical Considerations. Science 2006, 312, 389–391. [Google Scholar] [CrossRef] [PubMed]
- Kobasa, D.; Jones, S.M.; Shinya, K.; Kash, J.C.; Copps, J.; Ebihara, H.; Hatta, Y.; Kim, J.H.; Halfmann, P.; Hatta, M.; et al. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature 2007, 445, 319–323. [Google Scholar] [CrossRef] [PubMed]
- De Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.; Chau, T.N.; Hoang, D.M.; Chau, N.V.; Khanh, T.H.; Dong, V.C.; et al. Fatal outcome of human influenza A (H5N1) is associatedwith high viral load and hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef] [PubMed]
- Walsha, K.B.; Teijaroa, J.R.; Wilkerb, P.R.; Jatzekb, A.; Fremgena, D.M.; Dasb, S.C.; Watanabec, T.; Hattab, M.; Shinyad, K.; Sureshb, M.; et al. Suppression of cytokine storm with a sphingosine analog provides protection against pathogenic influenza virus. Proc. Natl. Acad. Sci. USA 2011, 108, 12018–12023. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ooi, L.S.; Wang, H.; But, P.P.; Ooi, V.E. Antiviral Activities of Medicinal Herbs Traditionally Used in Southern Mainland China. Phytother. Res. 2004, 18, 718–722. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wang, Y.; Liu, Y.; Zhang, R.; Li, X.; Su, W.; Long, F.; Luo, X.; Peng, T. Inhibition of enterovirus 71 replication by chrysosplenetin and penduletin. Eur. J. Pharm. Sci. 2011, 44, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, B.; Lu, J.; Chen, Q.; Ti, H.; Huang, W.; Li, J.; Yang, Z.; Jiang, Z.; Wang, X. Inhibition of influenza virus via a sesquiterpene fraction isolated from Laggera pterodonta by targeting the NF-κB and p38 pathways. BMC Complement. Altern. Med. 2017, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Z.; Li, Y.F.; Yu, X.; Mei, Z.N. Terpenoids and Flavonoids From Laggera pterodonta. Yao Xue Xue Bao 2007, 42, 511–515. [Google Scholar] [PubMed]
- Nimmerjahn, F.; Dudziak, D.; Dirmeier, U.; Hobom, G.; Riedel, A.; Schlee, M.; Staud, L.M.; Rosenwald, A.; Behrends, U.; Bornkamm, G.W.; et al. Active NF-κB signalling is a prerequisite for influenza virus infection. J. Gen. Virol. 2004, 85, 2347–2356. [Google Scholar] [CrossRef] [PubMed]
- Stephan, L.; Oliver, P. Influenza viruses and the NF-κB signaling pathway—Towards a novel concept of antiviral therapy. Biol. Chem. 2008, 389, 1307–1312. [Google Scholar]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, W.J.; Ehrhardt, C.; Pleschka, S.; Berberich-Siebelt, F.; Wolff, T.; Walczak, H.; Planz, O.; Ludwig, S. NF-κB-dependent induction of tumor necrosis factor-relatedapoptosis-inducing ligand (TRAIL) and Fas/FasL is crucial forefficient influenza virus propagation. J. Biol. Chem. 2004, 279, 30931–30937. [Google Scholar] [CrossRef] [PubMed]
- Wurzer, W.J.; Planz, O.; Ehrhardt, C.; Giner, M.; Silberzahn, T.; Pleschka, S.; Ludwig, S. Caspase 3 activation isessential for efficient influenza virus propagation. EMBO J. 2003, 22, 2717–2728. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.T.; Gary, S.F. NF-κB: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar]
- Trine, H.M.; Søren, R.P. Molecular Pathways in Virus-Induced Cytokine Production. Microbiol. Mol. Biol. Rev. 2001, 65, 131–150. [Google Scholar]
- Julkunen, I.; Sareneva, T.; Pirhonen, J.; Ronni, T.; Melén, K.; Matikainen, S. Molecular pathogenesis of influenza A virus infection and virus-induced regulation of cytokine gene expression. Cytokine Growth Factor Rev. 2001, 12, 171–180. [Google Scholar] [CrossRef]
- Schmolke, M.; Viemann, D.; Roth, J.; Ludwig, S. Essential impact of NF-κB signaling on the H5N1 influenza A virus-induced transcriptome. J. Immunol. 2009, 183, 5180–5189. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.C.; Beck, M.A.; Kuziel, W.A.; Henderson, F.; Maeda, N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 2000, 156, 1951–1959. [Google Scholar] [CrossRef]
- Pinto, R.; Herold, S.; Cakarova, L.; Hoegner, K.; Lohmeyer, J.; Planz, O.; Pleschka, S. Inhibition of influenza virus-induced NF-kappaB and Raf/MEK/ERK activationcan reduce both virus titers and cytokine expression simultaneouslyin vitroandin vivo. Antivir. Res. 2011, 92, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, B.; Li, C.; Chen, Q.; Wang, Y.; Li, Z.; Chen, T.; Yang, C.; Jiang, Z.; Zhong, N.; et al. Lariciresinol-4-O-β-d-glucopyranoside from the root of Isatis indigotica inhibits influenza A virus-induced pro-inflammatory response. J. Ethnopharmacol. 2015, 174, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Reed, L.J.; Muench, H. A simple method of estimating fifty percent end points. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |
| Influenza Virus Type and Strain | ||||||||
|---|---|---|---|---|---|---|---|---|
| A/PR/8/34 (H1N1) | A/California/04/2009 (H1N1) | A/GZ/GIRD07/09 (H1N1) | A/Aichi/2/68 (H3N2) | A/Duck/Guangdong/1994 (H7N3) | A/Duck/Guangdong/2009 (H6N2) | A/HK/Y280/97 (H9N2) | B/Lee/1940 (FluB) | |
| Pterodontic acid IC50 (μg/mL) | 37.14 | 21.75 | 9.47 | >278.9 | >278.9 | >278.9 | >278.9 | >278.9 |
| SI | 7.51 | 12.82 | 29.45 | <1 | <1 | <1 | <1 | <1 |
| Ribavirin IC50 (μg/mL) | 8.84 | 4.42 | 25 | 39.68 | 19.84 | 50 | 35.36 | 12.5 |
| NA Inhibitory Effect (IC50 (μg/mL)) | |||
|---|---|---|---|
| A/PR/8/34 (H1N1) | A/California/04/2009 (H1N1) | A/GZ/GIRD02/09 (H1N1) | |
| pterodontic acid | - | 668.1 | - |
| Oseltamivir | 3.12 × 10−4 | 2.54 × 10−4 | 3.41 × 10−4 |
| Gene | Primes and Probe | Sequence (5′→3′) |
|---|---|---|
| IL-6 | Forward | CGGGAACGAAAGAGAAGCTCTA |
| Reverse | CGCTTGTGGAGAAGGAGTTCA | |
| Probe | TCCCCTCCAGGAGCCCAGCT | |
| IP-10 | Forward | GAAATTATTCCTGCAAGCCAATTT |
| Reverse | TCACCCTTCTTTTTCAT-TGTAGCA | |
| Probe | TCCACGTGTTGAGATCA | |
| MIP-1β | Forward | AAAACCTCTTTGCCACCAATACC |
| Reverse | GAGAGCAGAAGGCAGCTACTAG | |
| Probe | TGAAGCTCTGCGTGACTGTCCTGTCT | |
| MCP-1 | Forward | CAAGCAGAAGTGGGTTCAGGAT |
| Reverse | AGTGAGTGTTCAAGTCTTCGGAGTT | |
| Probe | CATGGACCACCTGGACAAGCAAACC | |
| CCL-5 | Forward | CAGCAGTCGTCTTTGTCACC |
| Reverse | GTTGATGTACTCCCGAACCC | |
| Probe | CGCCAAGTGTGTGCCAACCC | |
| TNF-α | Forward | AACATCCAACCTTCCCAAACG |
| Reverse | GACCCTAAGCCCCCAATTCTC | |
| Probe | CCCCCTCCTTCAGACACCCTCAACC | |
| GAPDH | Forward | GAAGGTGAAGGTCGGAGTC |
| Reverse | GAAGATGGTGATGGGATTTC | |
| Probe | CAAGCTTCCCGTTCTCAGCC |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, W.; Li, J.; Chen, Q.; Jiang, Z.; Zhang, R.; Wang, X.; Yang, Z.; Pan, X. Pterodontic Acid Isolated from Laggera pterodonta Inhibits Viral Replication and Inflammation Induced by Influenza A Virus. Molecules 2017, 22, 1738. https://doi.org/10.3390/molecules22101738
Guan W, Li J, Chen Q, Jiang Z, Zhang R, Wang X, Yang Z, Pan X. Pterodontic Acid Isolated from Laggera pterodonta Inhibits Viral Replication and Inflammation Induced by Influenza A Virus. Molecules. 2017; 22(10):1738. https://doi.org/10.3390/molecules22101738
Chicago/Turabian StyleGuan, Wenda, Jing Li, Qiaolian Chen, Zhihong Jiang, Rongping Zhang, Xinhua Wang, Zifeng Yang, and Xiping Pan. 2017. "Pterodontic Acid Isolated from Laggera pterodonta Inhibits Viral Replication and Inflammation Induced by Influenza A Virus" Molecules 22, no. 10: 1738. https://doi.org/10.3390/molecules22101738
APA StyleGuan, W., Li, J., Chen, Q., Jiang, Z., Zhang, R., Wang, X., Yang, Z., & Pan, X. (2017). Pterodontic Acid Isolated from Laggera pterodonta Inhibits Viral Replication and Inflammation Induced by Influenza A Virus. Molecules, 22(10), 1738. https://doi.org/10.3390/molecules22101738




