Facile and Green Synthesis of Saturated Cyclic Amines
Abstract
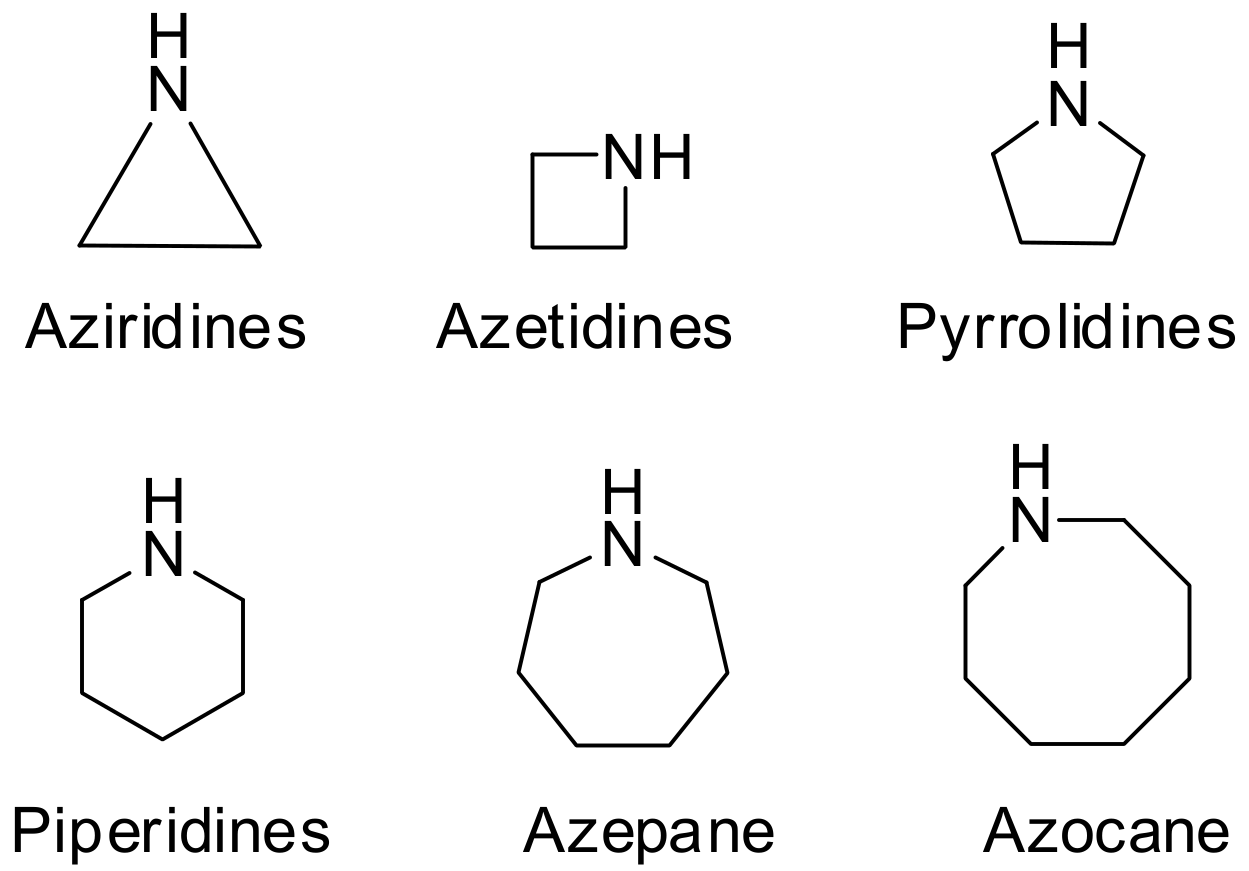
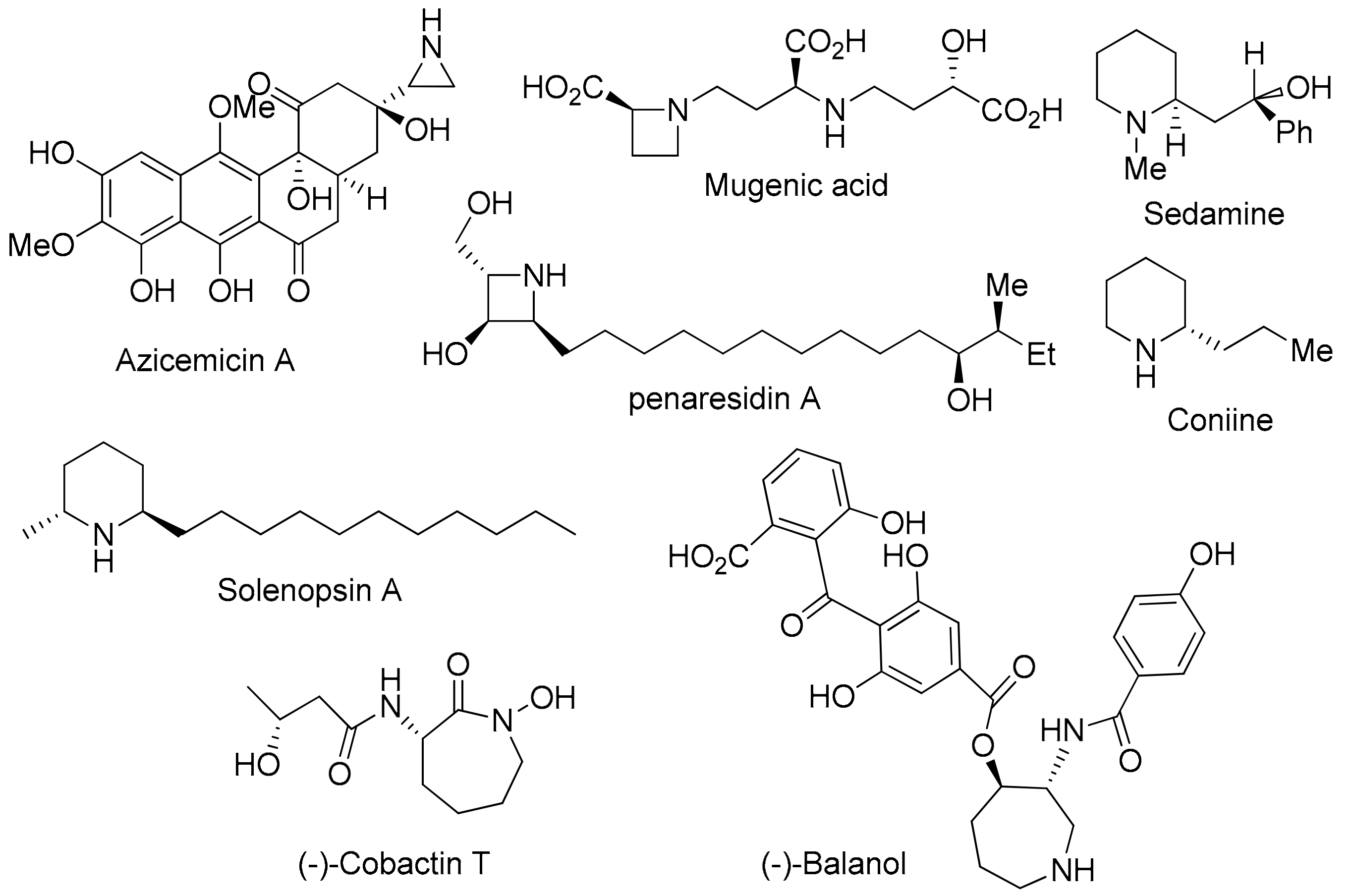
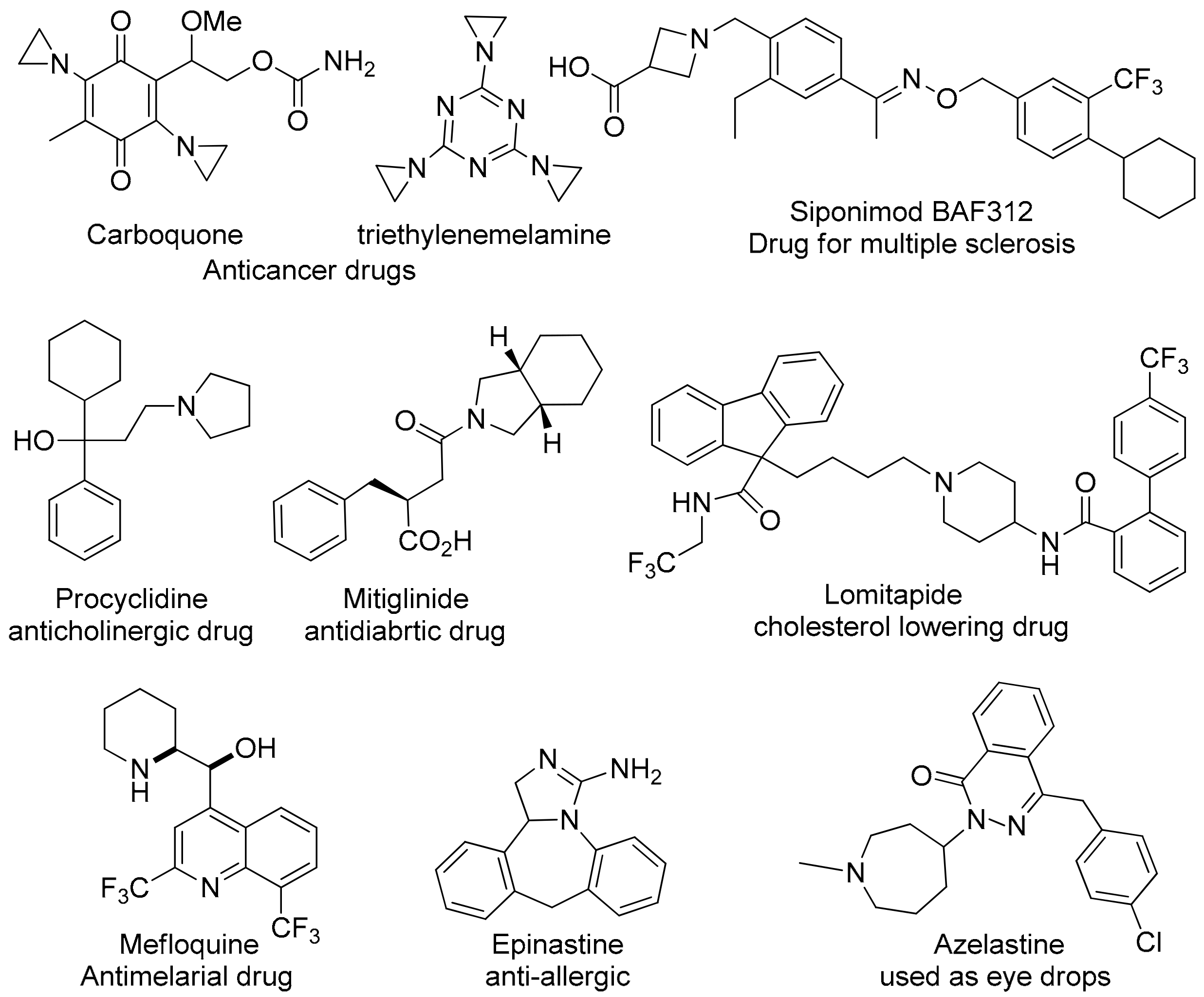
:1. Introduction
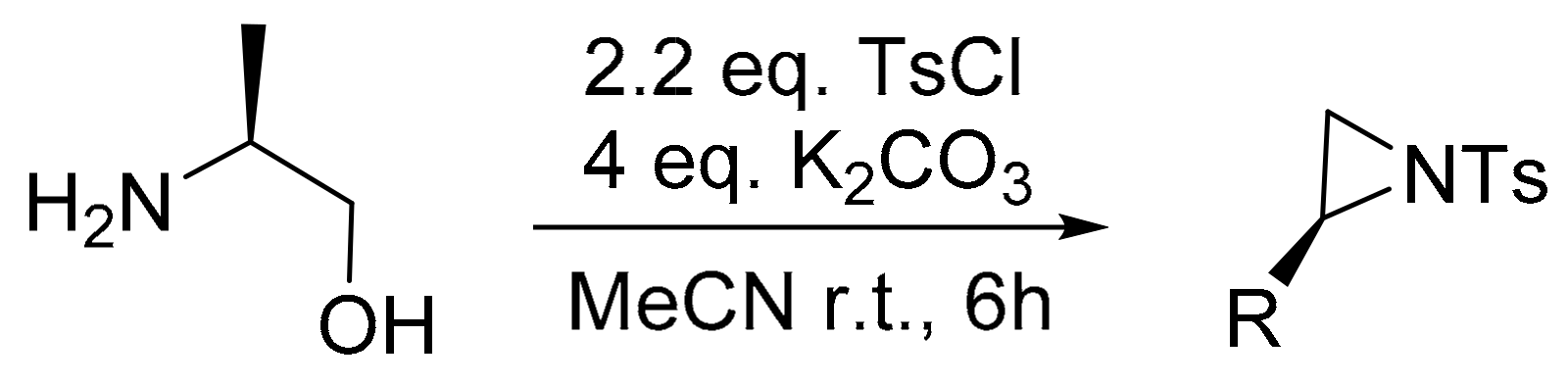
2. Green Methods for Synthesis of Aziridines
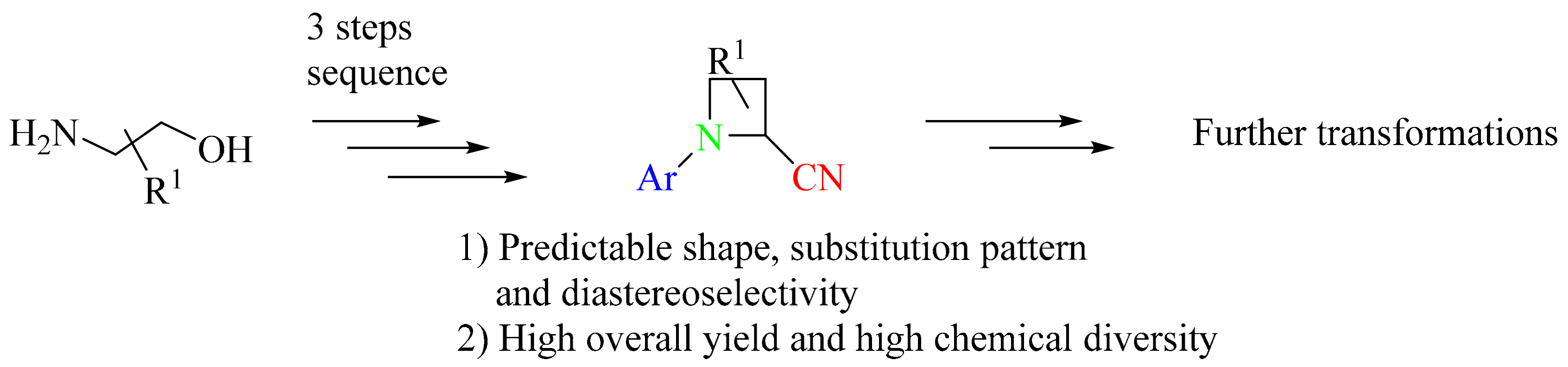
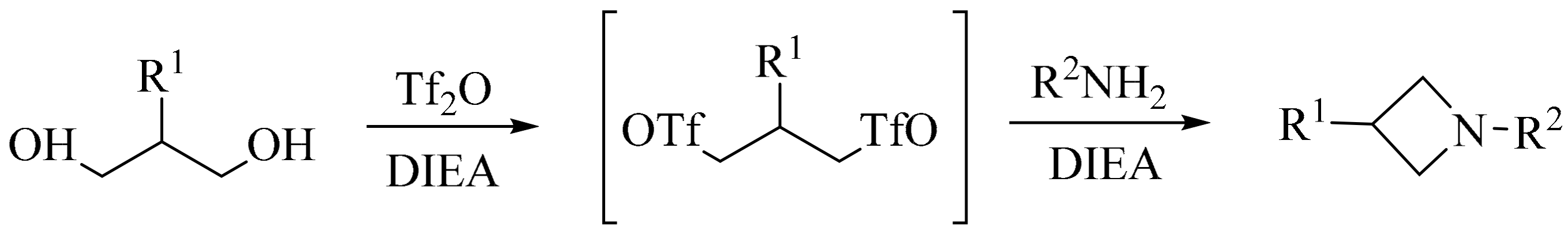
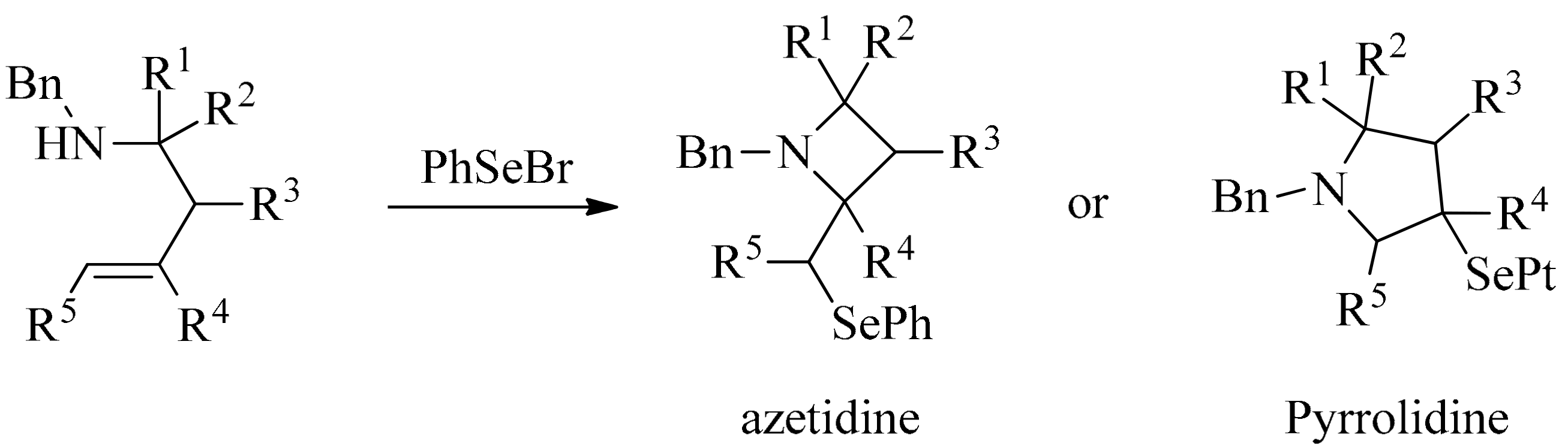
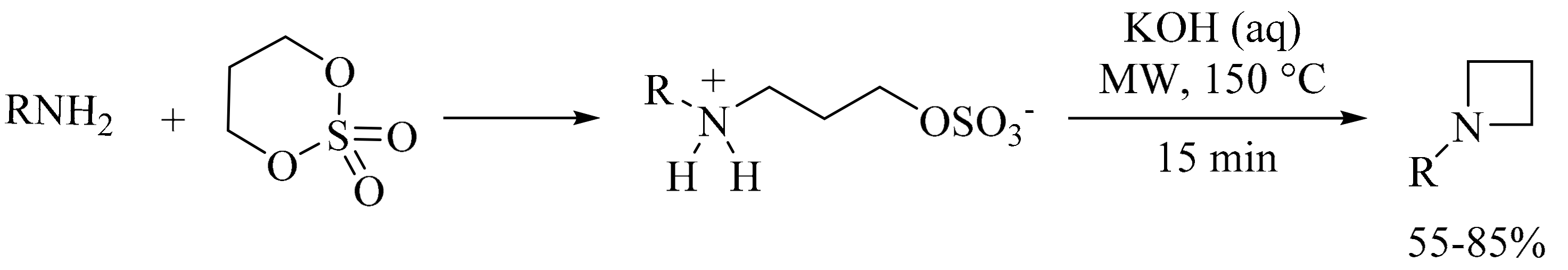
3. Green Synthesis of Azetidines
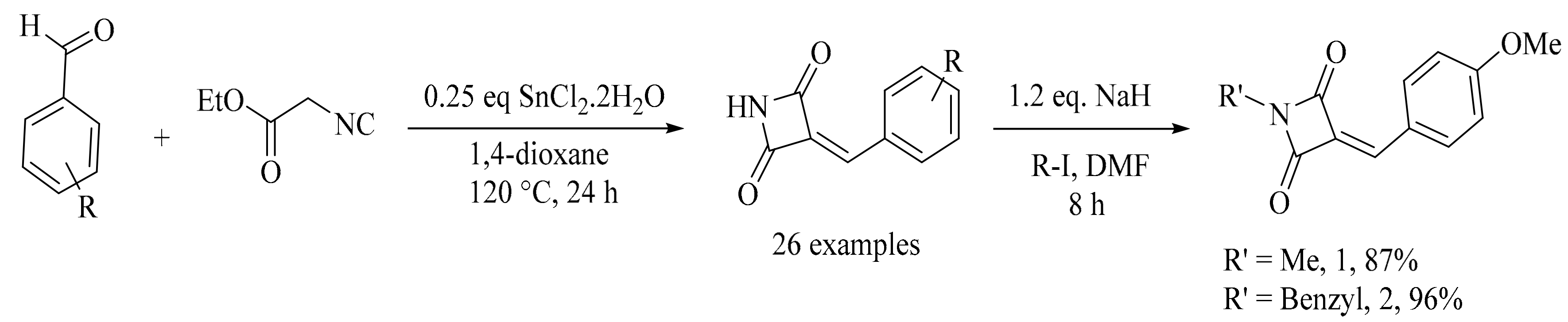
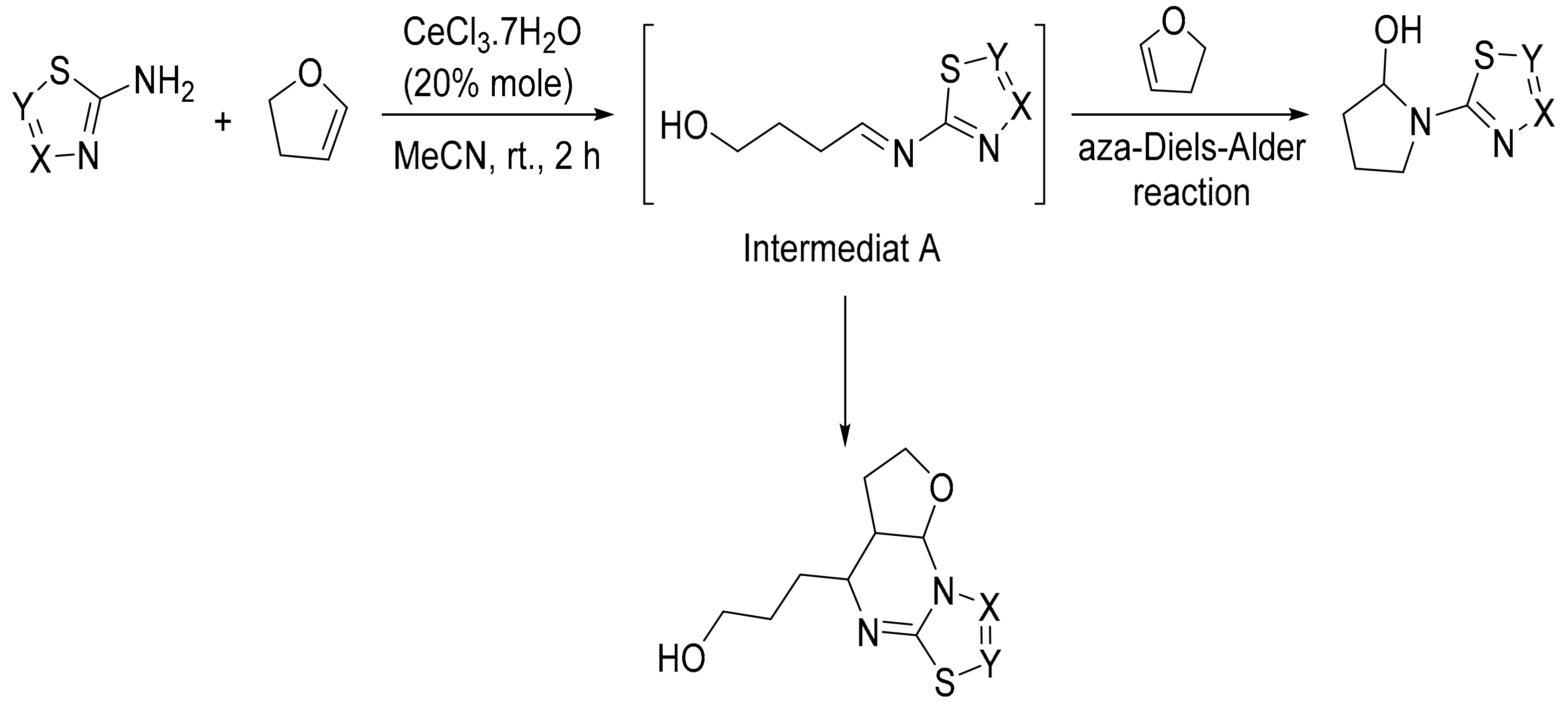
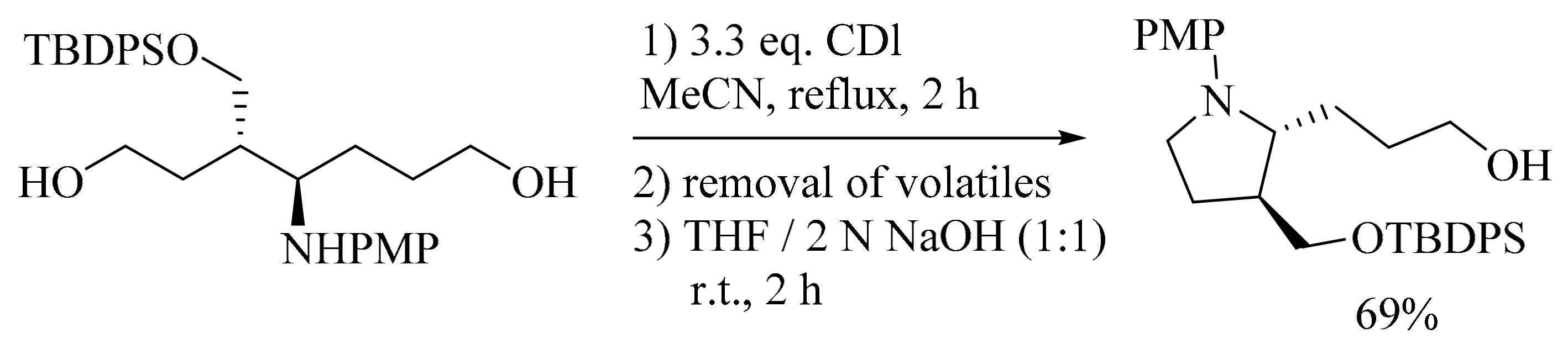
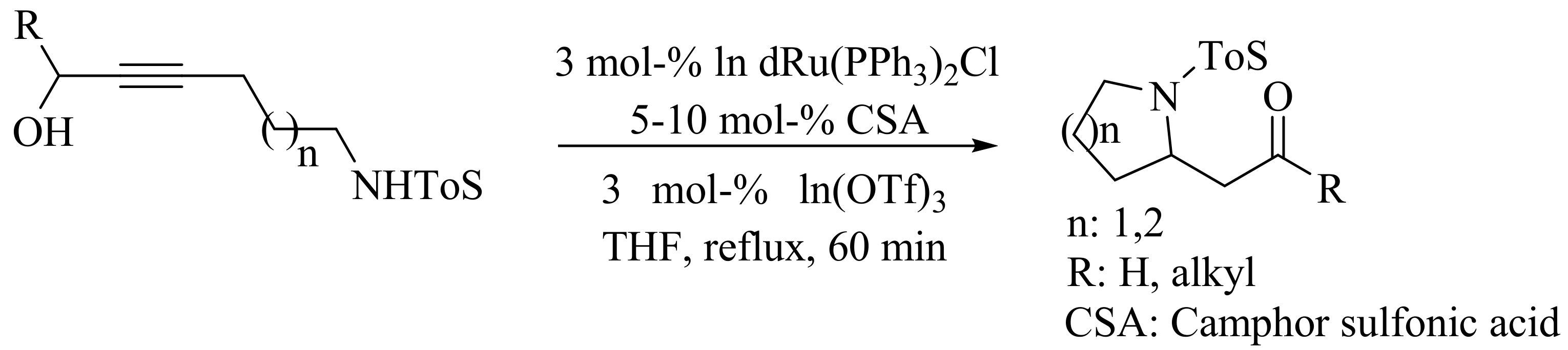
4. Green Synthesis of Pyrrolidines
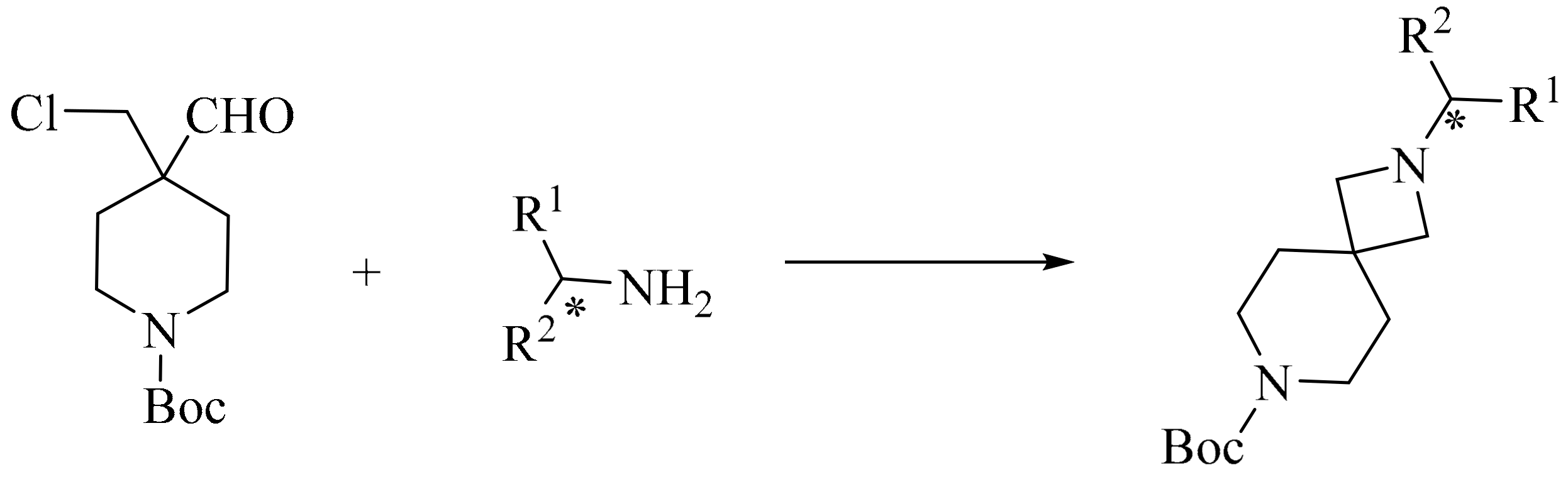
5. Green Synthesis of Piperidines
6. Green Synthesis of Azepanes
7. Green Synthesis of Azocanes
8. Conclusions
Author Contributions
Conflicts of Interest
References
- Bouyssi, D.; Monteiro, N.; Balme, G. Amines as key building blocks in Pd-assisted multicomponent processes. Beilstein J. Org. Chem. 2011, 7, 1387–1406. [Google Scholar] [CrossRef] [PubMed]
- Goethals, E.J.; Schacht, E.H.; Bruggeman, P.; Bossaer, P. Cationic Polymerization of Cyclic Amines. In Ring-Opening Polymerization; American Chemical Society: Washington, DC, USA, 1977; Volume 59, pp. 1–12. [Google Scholar]
- Noshadi, I.; Kanjilal, B.; Jafari, T.; Moharreri, E.; Khakpash, N.; Jiang, T.; Suib, S.L. Hydrophobic mesoporous adsorbent based on cyclic amine-divinylbenzene copolymer for highly efficient siloxane removal. RSC Adv. 2016, 6, 77310–77320. [Google Scholar] [CrossRef]
- Hunger, K. Industrial Dyes: Chemistry, Properties, Applications; Wiley: Kelkhem, Germany, 2007. [Google Scholar]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W.; Lai, C.A. Synthesis and characterization of protic ionic liquids containing cyclic amine cations and tetrafluoroborate anion. J. Iran. Chem. Soc. 2011, 8, 149–165. [Google Scholar] [CrossRef]
- Hall, H.K. Steric Effects on the base strengths of cyclic amines1. J. Am. Chem. Soc. 1957, 79, 5444–5447. [Google Scholar] [CrossRef]
- Shi, Y.; Chu, W.; Wang, Y.; Wang, S.; Du, J.; Zhang, J.; Li, S.; Zhou, G.; Qin, X.; Zhang, C. Synthesis, characterization and cytotoxicity of the Au(III) complexes with cyclic amine-based dithiocarbamate ligands. Inorg. Chem. Commun. 2013, 30, 178–181. [Google Scholar] [CrossRef]
- Zhang, X. Transition Metal-Catalyzed Reactions Based on Chiral Amine Oxazolinyl Ligands. U.S. Patent 6,255,493, 3 July 2001. [Google Scholar]
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in drugs. J. Med. Chem. 2014, 57, 5845–5859. [Google Scholar] [CrossRef] [PubMed]
- Ismail, F.M.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef] [PubMed]
- Shiota, T.; Kataoka, K.-I.; Imai, M.; Tsutsumi, T.; Sudoh, M.; Sogawa, R.; Morita, T.; Hada, T.; Muroga, Y.; Takenouchi, O. Cyclic Amine Derivatives and Their Use as Drugs. WO 1999025686 A1, 27 May 1999. [Google Scholar]
- Urch, C.J.; Salmon, R.; Lewis, T.; Godfrey, C.R.A.; Clough, M.S. Bicyclic Amines as Insecticides. WO 1996037494 A1, 28 November 1996. [Google Scholar]
- Zambach, W.; Hueter, O.F.; Wenger, J.; Goeghova, M.; Pitterna, T.; Maienfisch, P.; Muehlebach, M. Spiroheterocyclic Pyrrolidine Dione Derivatives Useful as Pesticides. WO 2009049851 A1, 23 April 2009. [Google Scholar]
- Duke, S.O.; Cantrell, C.L.; Meepagala, K.M.; Wedge, D.E.; Tabanca, N.; Schrader, K.K. Natural toxins for use in pest management. Toxins 2010, 2, 1943–1962. [Google Scholar] [CrossRef] [PubMed]
- Fokas, D.; Coffen, D.L.; Ryan, W.J. Spiro [Pyrrolidine-2,3′-oxindole] Compounds and Methods of Use. WO 1999012904 A1, 8 March 1999. [Google Scholar]
- Wan, X.W.; Peng, Y.Q.; Gan, H.J.; Shi, Z.L.; Xing, G. Mixed Weedicide as Well as Preparation Method and Application Thereof. CN 104351234 A, 18 February 2015. [Google Scholar]
- Turner, N.J.; Truppo, M.D. Biocatalytic routes to nonracemic chiral amines. In Chiral Amine Synthesis; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Kohls, H.; Steffen, M.F.; Hohne, M. Recent achievements in developing the biocatalytic toolbox for chiral amine synthesis. Curr. Opin. Chem. Biol. 2014, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Nugent, T.C. Chiral Amine Synthesis: Methods, Developments and Applications; John Wiley & Sons: Bremen, Germany, 2010. [Google Scholar]
- Bieber, L.; De Araújo, M. Short and efficient synthesis of optically active N-tosyl aziridines from 2-amino alcohols. Molecules 2002, 7, 902–906. [Google Scholar] [CrossRef]
- Lohray, B.B.; Gao, Y.; Sharpless, K.B. One pot synthesis of homochiral aziridines and aminoalcohols from homochiral 1,2-cyclic sulfates. Tetrahedron Lett. 1989, 30, 2623–2626. [Google Scholar] [CrossRef]
- Hayashi, Y.; Urushima, T.; Sakamoto, D.; Torii, K.; Ishikawa, H. One-pot synthesis of chiral aziridines by a domino reaction by using desulfonylative formation on the N-tosyl imine of chloroacetaldehyde with an asymmetric mannich reaction as a key step. Chem. Eur. J. 2011, 17, 11715–11718. [Google Scholar] [CrossRef] [PubMed]
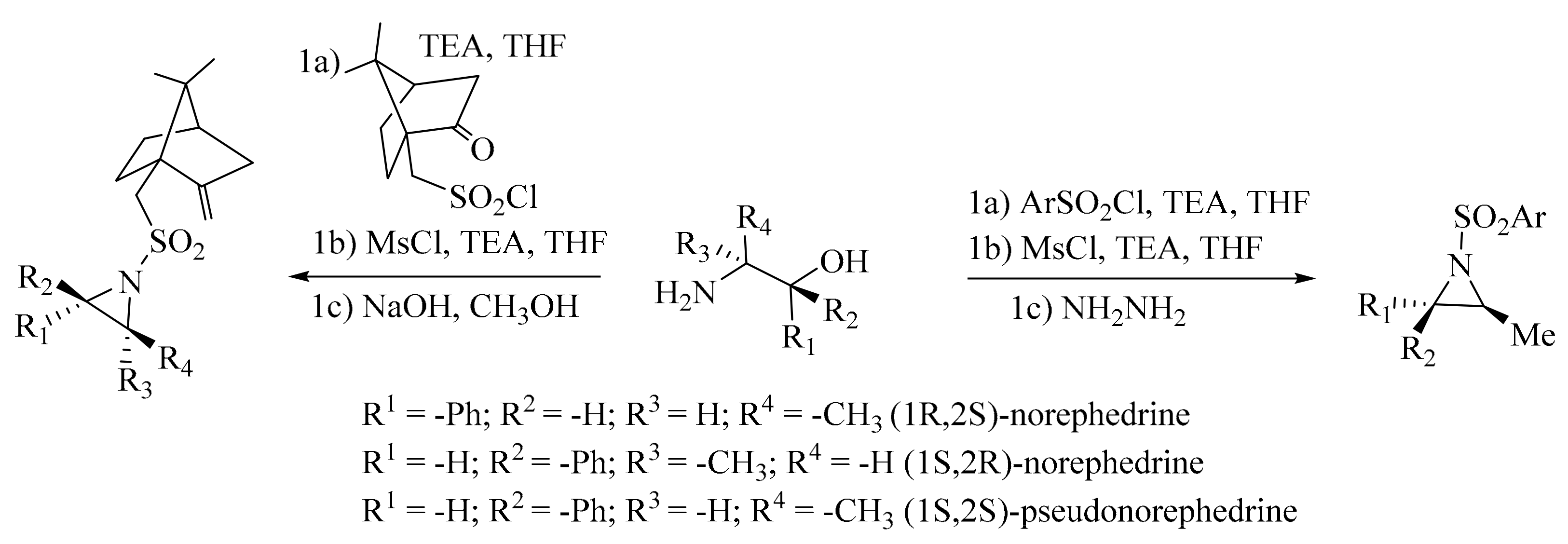
- Groeper, J.A.; Eagles, J.B.; Hitchcock, S.R. A facile, one-pot synthesis of Ephedra-based aziridines. Tetrahedron Asymmetry 2009, 20, 1969–1974. [Google Scholar] [CrossRef]
- Yadav, L.D.S.; Kapoor, R. Organocatalytic stereoselective aziridination of imines via ammonium ylides. Synlett 2009, 2009, 3123–3126. [Google Scholar] [CrossRef]
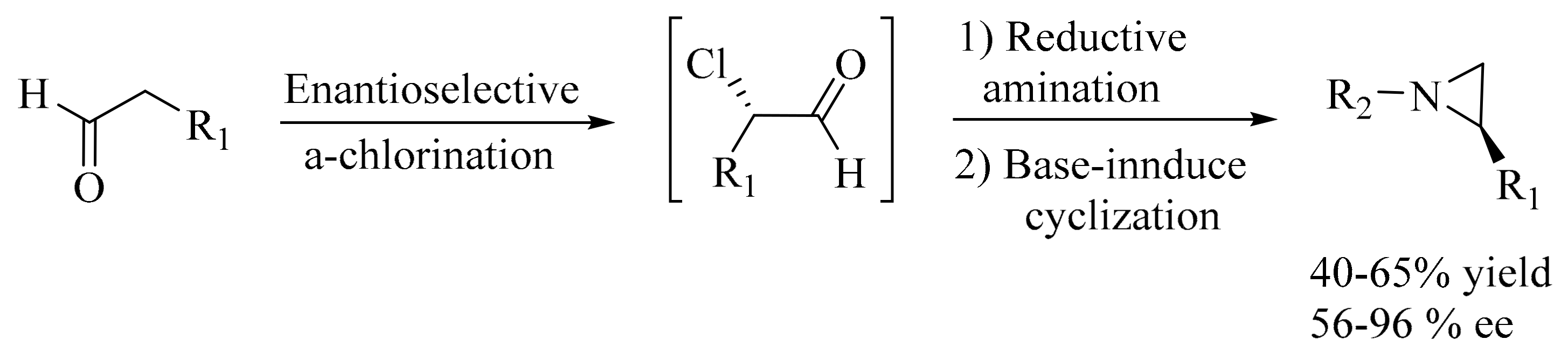
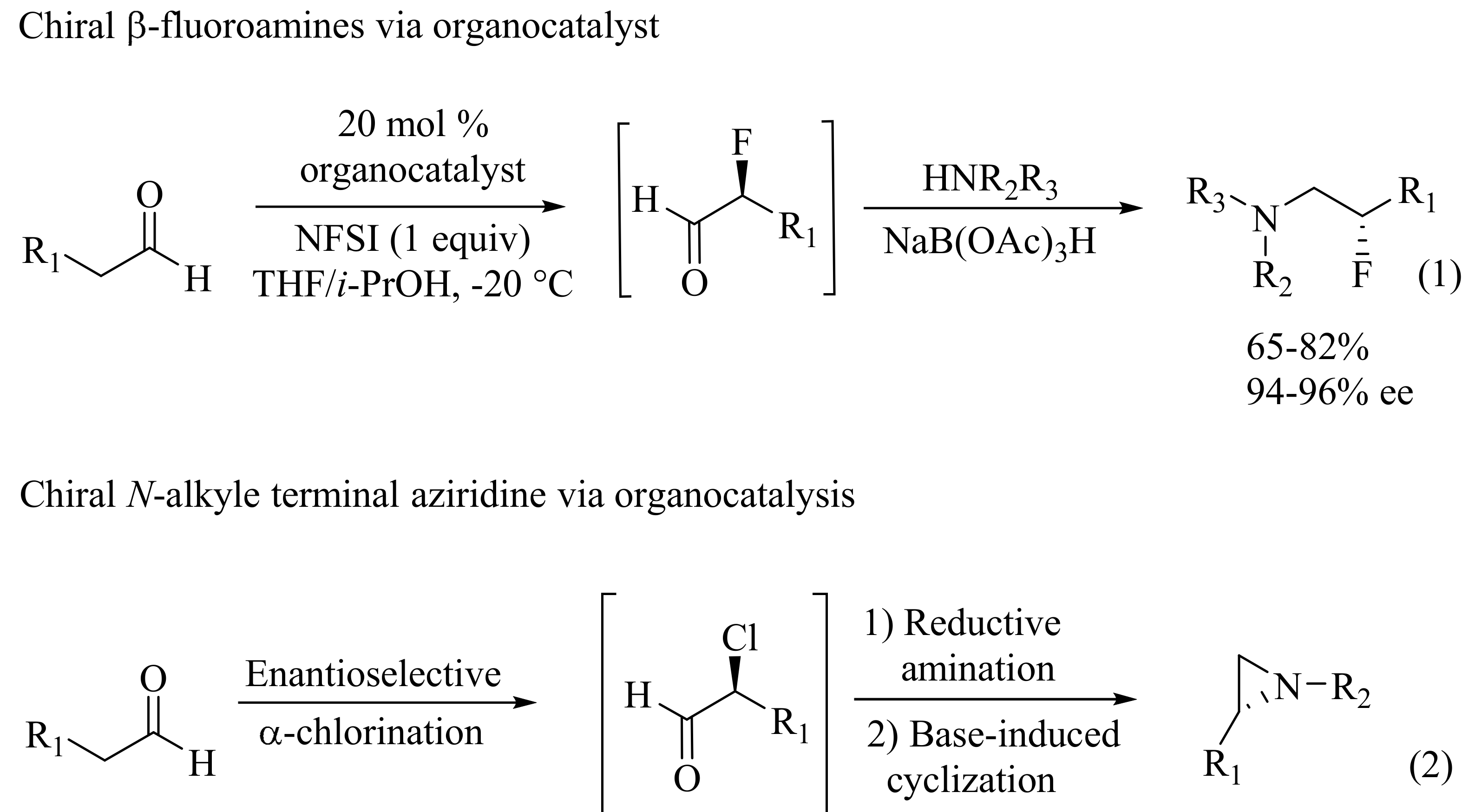
- Fadeyi, O.O.; Schulte, M.L.; Lindsley, C.W. General access to chiral N-alkyl terminal aziridines via organocatalysis. Org. Lett 2010, 12, 3276–3278. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Talukdar, A.; Cushman, M. Regioselective synthesis of N-β-hydroxyethylaziridines by the ring-opening reaction of epoxides with aziridine generated in situ. Org. Lett. 2006, 8, 1085–1087. [Google Scholar] [CrossRef] [PubMed]
- Kano, D.; Minakata, S.; Komatsu, M. Novel organic-solvent-free aziridination of olefins: Chloramine-T-I2 system under phase-transfer catalysis conditions. J. Chem. Soc. Perkin Trans. 1 2001, 23, 3186–3188. [Google Scholar] [CrossRef]
- Kiyokawa, K.; Kosaka, T.; Minakata, S. Metal-free aziridination of styrene derivatives with iminoiodinane catalyzed by a combination of iodine and ammonium iodide. Org. Lett. 2013, 15, 4858–4861. [Google Scholar] [CrossRef] [PubMed]
- Borkin, D.; Carlson, A.; Török, B. K-10-catalyzed highly diastereoselective synthesis of aziridines. Synlett 2010, 2010, 745–748. [Google Scholar]
- Quinodoz, P.; Drouillat, B.; Wright, K.; Marrot, J.; Couty, F. N-Arylazetidines: Preparation through anionic ring closure. J. Org. Chem. 2016, 81, 2899–2910. [Google Scholar] [CrossRef] [PubMed]
- Kern, N.; Felten, A.-S.; Weibel, J.-M.; Pale, P.; Blanc, A. Robust synthesis of N-sulfonylazetidine building blocks via ring contraction of α-bromo N-sulfonylpyrrolidinones. Org. Lett. 2014, 16, 6104–6107. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Nadir, U.K. A facile synthesis of 1-arenesulfonylazetidines through reaction of 1-arenesulfonylaziridines with dimethylsulfoxonium methylide generated under microwave irradiation. Synlett 2008, 2008, 108–110. [Google Scholar] [CrossRef]
- Ju, Y.; Varma, R.S. Aqueous N-heterocyclization of primary amines and hydrazines with dihalides: microwave-assisted syntheses of N-azacycloalkanes, isoindole, pyrazole, pyrazolidine, and phthalazine derivatives. J. Org. Chem. 2006, 71, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Cheng, D.; Li, J.; Guo, H.; Yan, J. Copper-catalyzed highly efficient multicomponent reactions: Synthesis of 2-(sulfonylimino)-4-(alkylimino)azetidine derivatives. Org. Lett. 2007, 9, 1585–1587. [Google Scholar] [CrossRef] [PubMed]
- Hillier, M.C.; Chen, C.Y. A one-pot preparation of 1,3-disubstituted azetidines. J. Org. Chem. 2006, 71, 7885–7887. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.S.; Supekar, M.V.; Burate, P.A.; Rupanwar, B.D.; Shelke, A.M.; Suryavanshi, G. An efficient Sn(ii)-catalyzed one-pot synthesis of a 3-substituted azetidine-2,4-dione framework. Org. Biomol. Chem. 2017, 15, 2385–2391. [Google Scholar] [CrossRef] [PubMed]
- Orr, S.T.M.; Cabral, S.; Fernando, D.P.; Makowski, T. One-pot synthesis of chiral azetidines from chloroaldehyde and chiral amines. Tetrahedron Lett. 2011, 52, 3618–3620. [Google Scholar]
- Franck, X.; Leleu, S.; Outurquin, F. Regioselective synthesis of azetidines or pyrrolidines by selenium-induced cyclization of secondary homoallylic amines. Tetrahedron Lett. 2010, 51, 4437–4440. [Google Scholar] [CrossRef]
- Burkett, B.A.; Ting, S.Z.; Gan, G.C.S.; Chai, C.L.L. Microwave-assisted synthesis of azetidines in aqueous media. Tetrahedron Lett. 2009, 50, 6590–6592. [Google Scholar] [CrossRef]
- Yi, X.; Barry, B.-D.; Liao, P.; Bi, X. Synthesis of N-Sulfonylazetidin-2-imines via the Copper(I) oxide catalyzed multicomponent reaction of alkynes, sulfonyl azides and diimines under solvent-free conditions. Synthesis 2012, 44, 1323–1328. [Google Scholar] [CrossRef]
- Xu, F.; Simmons, B.; Reamer, R.A.; Corley, E.; Murry, J.; Tschaen, D. Chlorination/cyclodehydration of amino alcohols with SOCl2: An old reaction revisited. J. Org. Chem. 2008, 73, 312–315. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.; Sharma, K.; Kumar, D. Ionic liquid mediated 1,3-dipolar cycloaddition of azomethine ylides: A facile and green synthesis of novel dispiro heterocycles. Tetrahedron Lett. 2012, 53, 1993–1997. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, Q.; Zi, G.; Hou, G. Enantioselective direct synthesis of free cyclic amines via intramolecular reductive amination. Org. Lett. 2017, 19, 4215–4218. [Google Scholar] [CrossRef] [PubMed]
- Almansour, A.I.; Arumugam, N.; Kumar, R.S.; Periyasami, G.; A. Ghabbour, H.; Fun, H.K. A novel one-pot green synthesis of dispirooxindolo-pyrrolidines via 1,3-dipolar cycloaddition reactions of azomethine ylides. Molecules 2015, 20, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Ugarriza, I.; Uria, U.; Reyes, E.; Carrillo, L.; Vicario Jose, L. Organocatalytic enantioselective [3 + 2] cycloaddition of azomethine ylides and acrolein. Asymmetric Catal. 2015, 2, 26–36. [Google Scholar] [CrossRef]
- Varma, P.P.; Mahadevan, K.M.; Khader, A.; Hulikal, V. One pot synthesis of 2-hydroxy pyrrolidine derivatives. Org. Commun. 2011, 4, 52. [Google Scholar]
- Cui, Z.; Yu, H.-J.; Yang, R.-F.; Gao, W.-Y.; Feng, C.-G.; Lin, G.-Q. Highly enantioselective arylation of N-tosylalkylaldimines catalyzed by rhodium-diene complexes. J. Am. Chem. Soc. 2011, 133, 12394–12397. [Google Scholar] [CrossRef] [PubMed]
- Gandon, V.; Bertus, P.; Szymoniak, J. Conversion of imines into C,N-dimagnesiated compounds and trapping with electrophiles. One-pot access to 1-azaspirocyclic framework. Synthesis 2002, 2002, 1115–1120. [Google Scholar] [CrossRef]
- De Figueiredo, R.M.; Fröhlich, R.; Christmann, M. N,N’-Carbonyldiimidazole-mediated cyclization of amino alcohols to substituted azetidines and other N-heterocycles. J. Org. Chem. 2006, 71, 4147–4154. [Google Scholar] [CrossRef] [PubMed]
- Carson, C.A.; Kerr, M.A. Diastereoselective synthesis of pyrrolidines via the Yb (OTf) 3 catalyzed three-component reaction of aldehydes, amines, and 1,1-cyclopropanediesters. J. Org. Chem. 2005, 70, 8242–8244. [Google Scholar] [CrossRef] [PubMed]
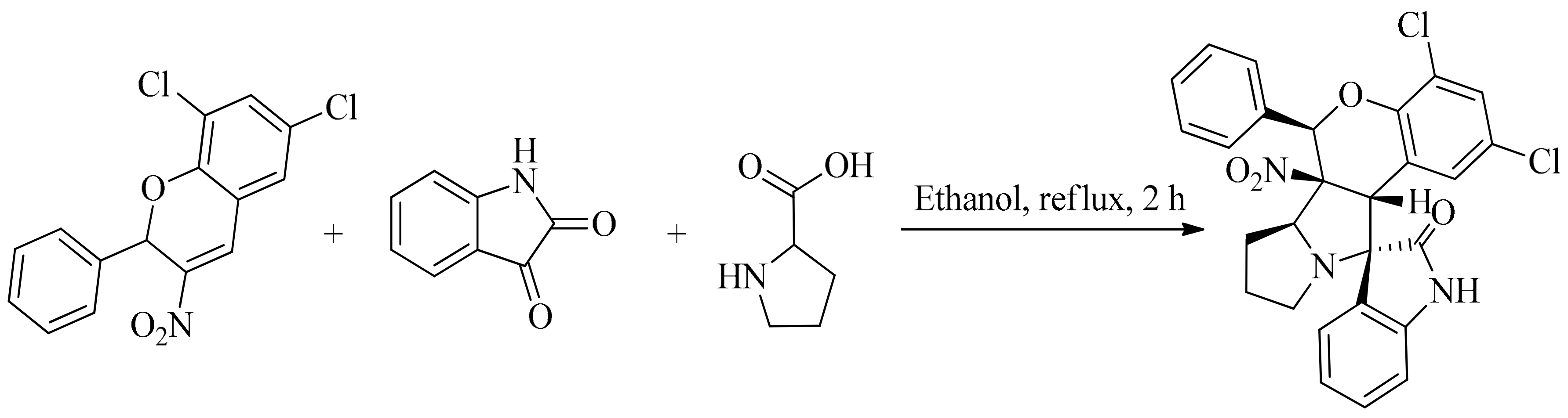
- Nayak, S.; K. Mishra, S.; Bhakta, S.; Panda, P.; Baral, N.; Mohapatra, S.; S. Purohit, C.; Satha, P. Green synthesis of spirooxindole-pyrrolidine/piperidine fused nitrochromane: One pot three component stereo and regioselective cycloaddition. Lett. Org. Chem. 2016, 13, 11–21. [Google Scholar] [CrossRef]
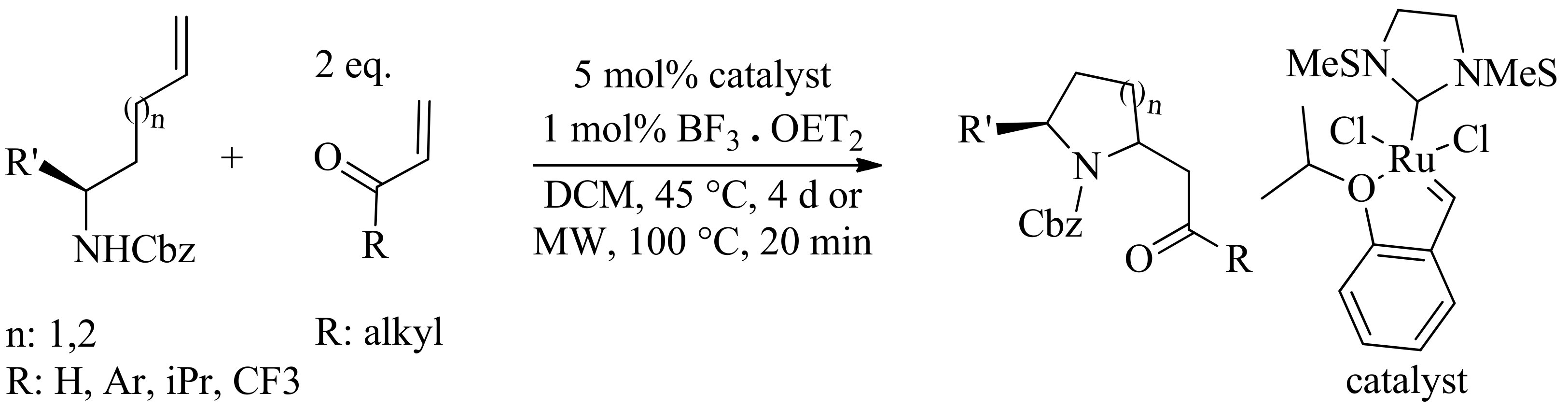
- Trost, B.M.; Maulide, N.; Livingston, R.C. A ruthenium catalyzed, atom-economical synthesis of nitrogen heterocycles. J. Am. Chem. Soc. 2008, 130, 16502–16503. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Liu, L.-P.; Tang, J. Gold (I)-catalyzed domino ring-opening ring-closing hydroamination of methylenecyclopropanes (MCPs) with sulfonamides: Facile preparation of pyrrolidine derivatives. Org. Lett. 2006, 8, 4043–4046. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-B.; Tang, Y.; Sun, X.-L. Scandium triflate catalyzed cycloaddition of imines with 1,1-cyclopropanediesters: Efficient and diastereoselective synthesis of multisubstituted pyrrolidines. Org. Biomol. Chem. 2006, 4, 299–301. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.M.; Nicewicz, D.A. Anti-markovnikov hydroamination of alkenes catalyzed by an organic photoredox system. J. Am. Chem. Soc. 2013, 135, 9588–9591. [Google Scholar] [CrossRef] [PubMed]
- Fustero, S.; Jiménez, D.; Sánchez-Roselló, M.; del Pozo, C. Microwave-assisted tandem cross metathesis intramolecular aza-Michael reaction: An easy entry to cyclic β-amino carbonyl derivatives. J. Am. Chem. Soc. 2007, 129, 6700–6701. [Google Scholar] [CrossRef] [PubMed]
- Oura, I.; Shimizu, K.; Ogata, K.; Fukuzawa, S.-I. Highly endo-selective and enantioselective 1,3-dipolar cycloaddition of azomethine ylide with α-enones catalyzed by a silver (I)/ThioClickFerrophos complex. Org. Lett. 2010, 12, 1752–1755. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Soni, P.; Sharma, J.; Bhardwaj, S.; Halve, A. One-pot multi-component green synthesis of highly substituted piperidines. Curr. Chem. Lett. 2017, 6, 135–142. [Google Scholar] [CrossRef]
- Khan, A.T.; Lal, M.; Khan, M.M. Synthesis of highly functionalized piperidines by one-pot multicomponent reaction using tetrabutylammonium tribromide (TBATB). Tetrahedron Lett. 2010, 51, 4419–4424. [Google Scholar] [CrossRef]
- Guérinot, A.; Serra-Muns, A.; Gnamm, C.; Bensoussan, C.; Reymond, S.; Cossy, J. FeCl3-catalyzed highly diastereoselective synthesis of substituted piperidines and tetrahydropyrans. Org. lett. 2010, 12, 1808–1811. [Google Scholar] [CrossRef] [PubMed]
- LaLonde, R.L.; Sherry, B.D.; Kang, E.J.; Toste, F.D. Gold (I)-catalyzed enantioselective intramolecular hydroamination of allenes. J. Am. Chem. Soc. 2007, 129, 2452–2453. [Google Scholar] [CrossRef] [PubMed]
- Métro, T.-X.; Pardo, D.G.; Cossy, J. Highly enantioselective synthesis of β-amino alcohols: A catalytic version. J. Org. Chem. 2007, 72, 6556–6561. [Google Scholar] [CrossRef] [PubMed]
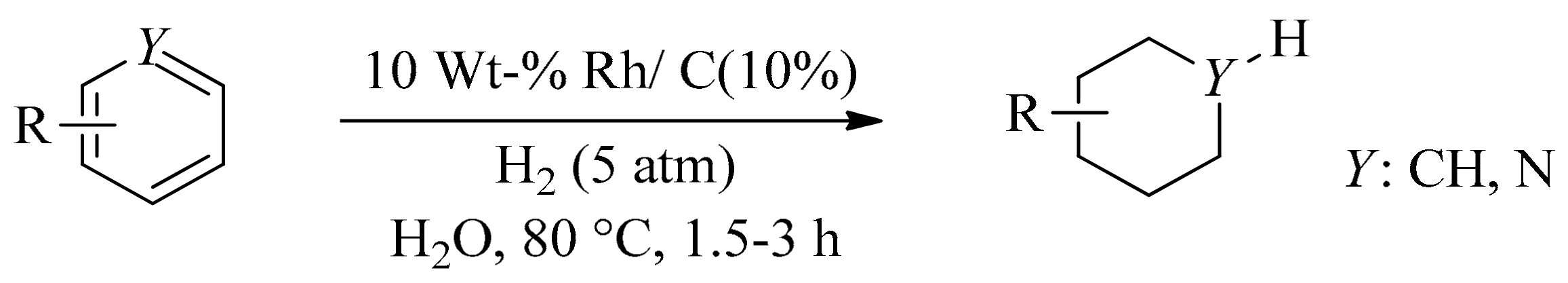
- Maegawa, T.; Akashi, A.; Sajiki, H. A mild and facile method for complete hydrogenation of aromatic nuclei in water. Synlett 2006, 2006, 1440–1442. [Google Scholar] [CrossRef]
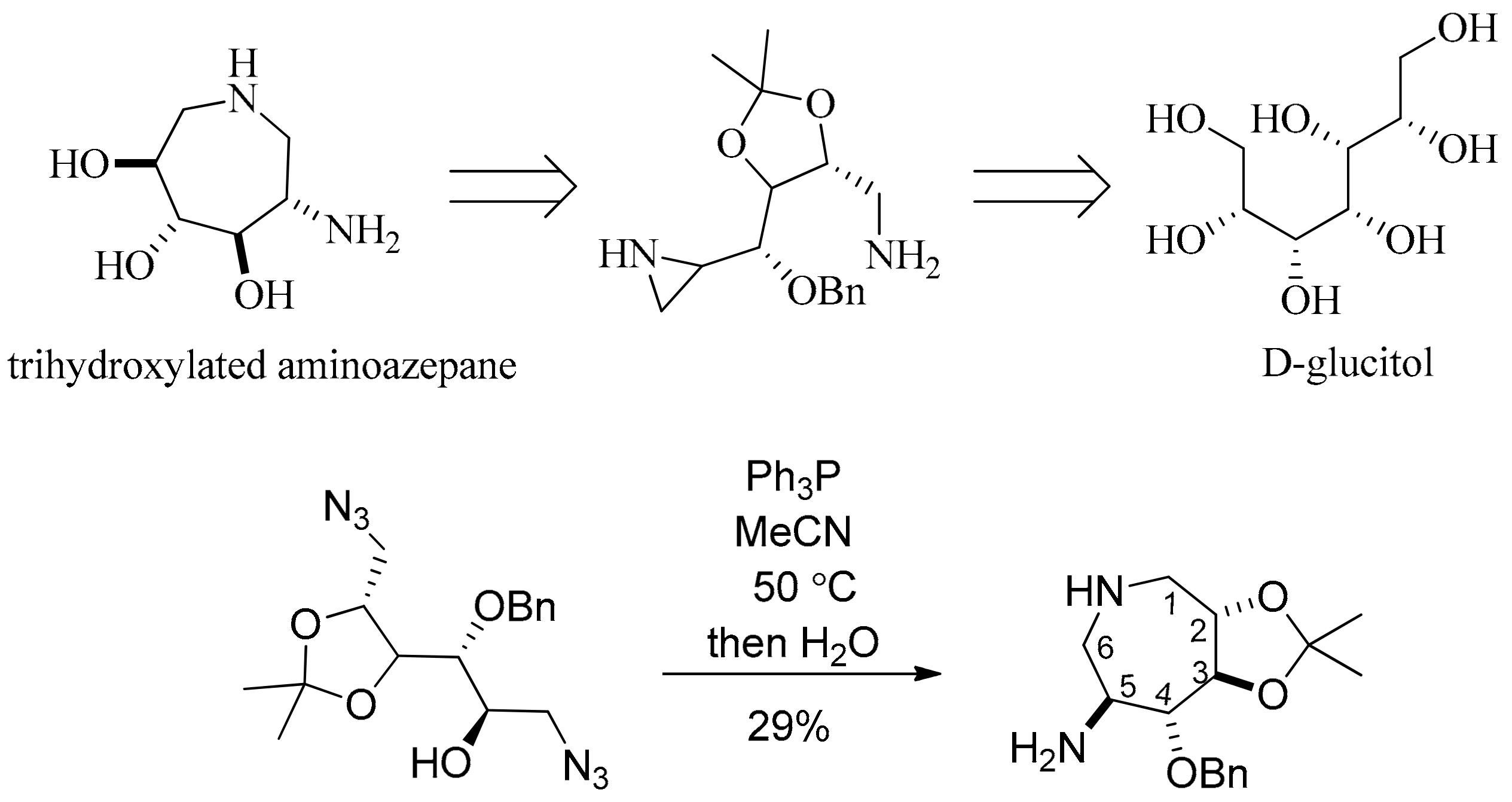
- Valle, M.S.; Braga, R.M. Synthesis of a trihydroxylated aminoazepane from d-glucitol by an intramolecular aziridine ring opening. Synlett 2008, 2008, 2874–2876. [Google Scholar]
- Calder, E.D.; Grafton, M.W.; Sutherland, A. One-pot multi-reaction processes: Synthesis of natural products and drug-like scaffolds. Synlett 2014, 25, 1068–1080. [Google Scholar] [CrossRef]
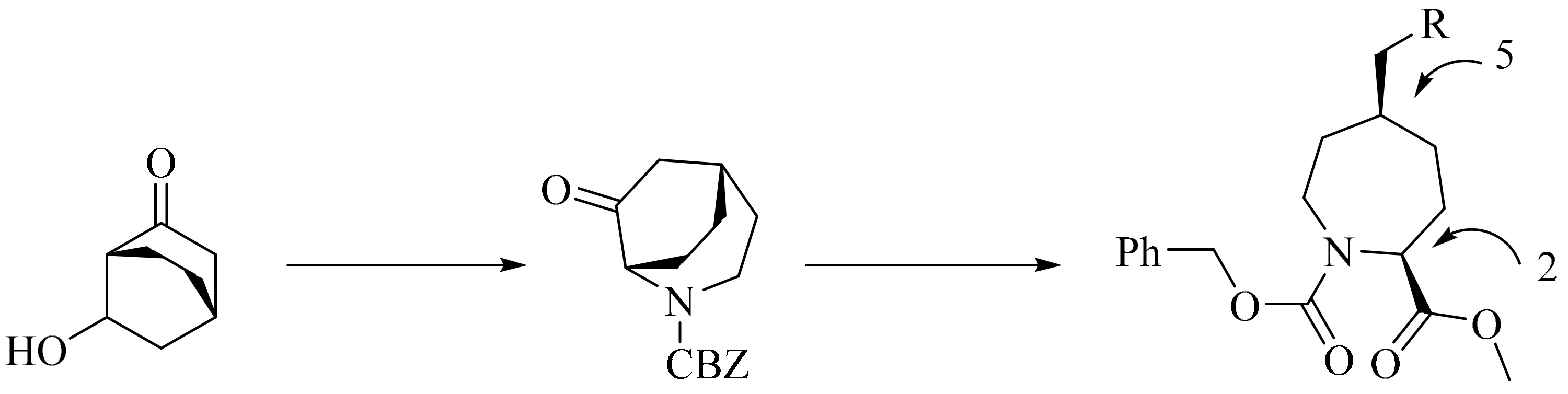
- Wishka, D.G.; Bédard, M.; Brighty, K.E.; Buzon, R.A.; Farley, K.A.; Fichtner, M.W.; Kauffman, G.S.; Kooistra, J.; Lewis, J.G.; O’Dowd, H. An asymmetric synthesis of (2S,5S)-5-Substituted azepane-2-carboxylate derivatives. J. Org. Chem. 2011, 76, 1937–1940. [Google Scholar] [CrossRef] [PubMed]
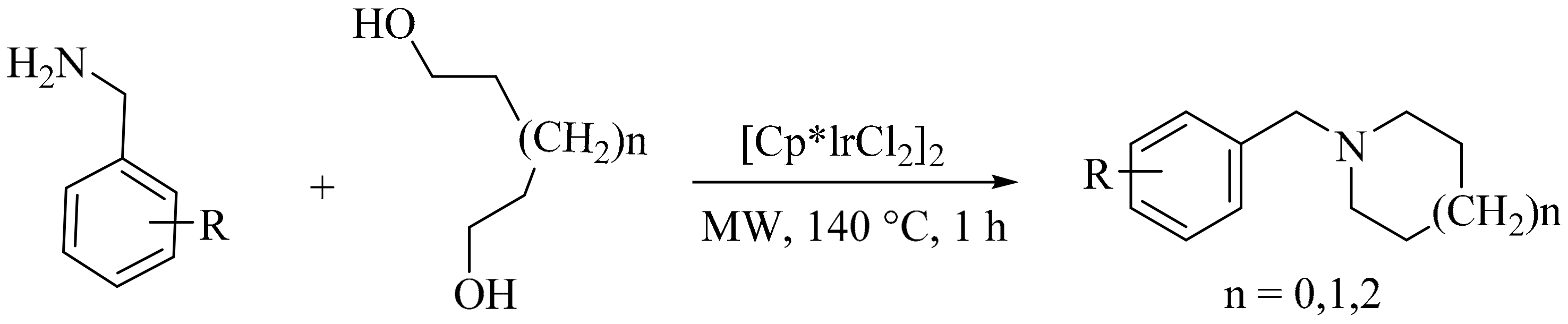
- Zhang, W.; Dong, X.; Zhao, W. Microwave-assisted solventless reaction of iridium-catalyzed alkylation of amines with alcohols in the absence of base. Org. Lett. 2011, 13, 5386–5389. [Google Scholar] [CrossRef] [PubMed]
- Sacher, J.R.; Weinreb, S.M. Construction of the azocane (azacyclooctane) moiety of the Lycopodium alkaloid lycopladine H via an intramolecular hydroaminomethylation strategy. Org. Lett. 2012, 14, 2172–2175. [Google Scholar] [CrossRef] [PubMed]

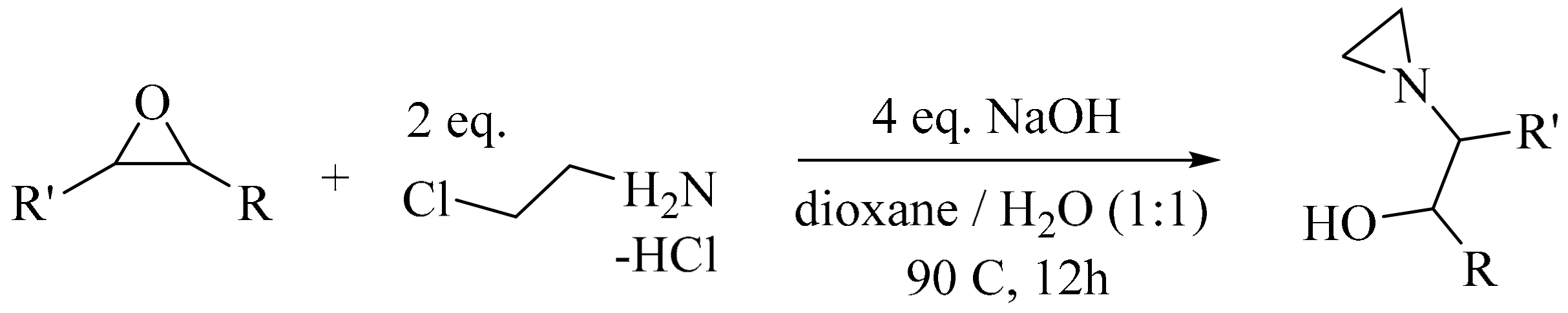
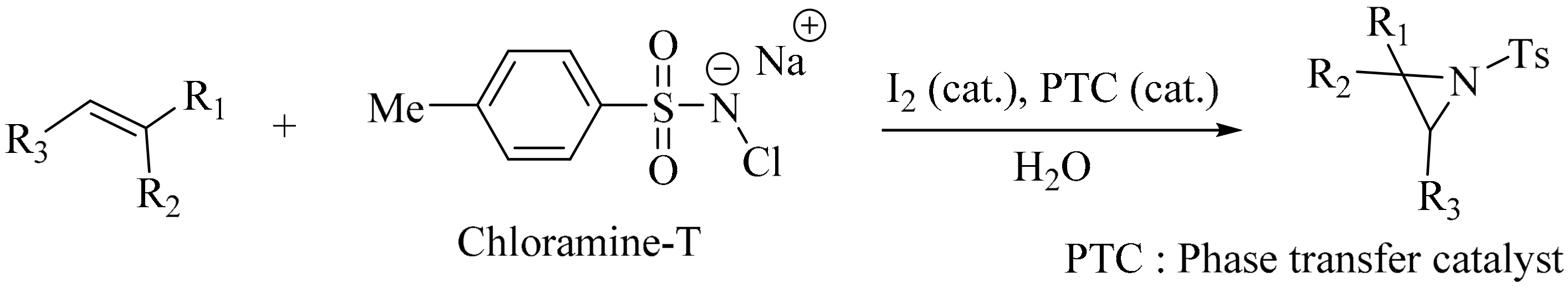

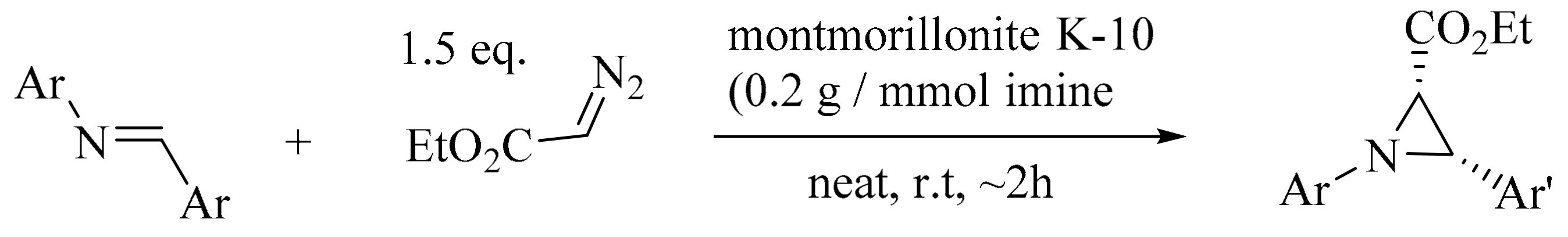


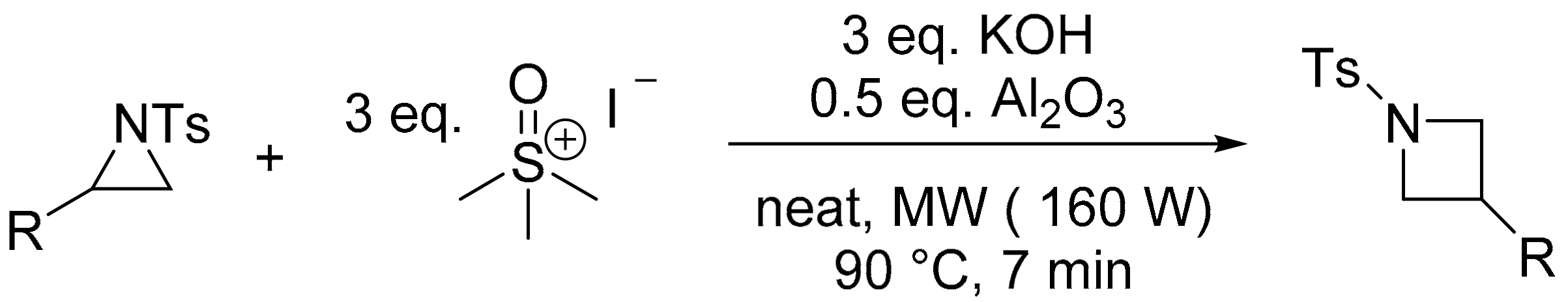

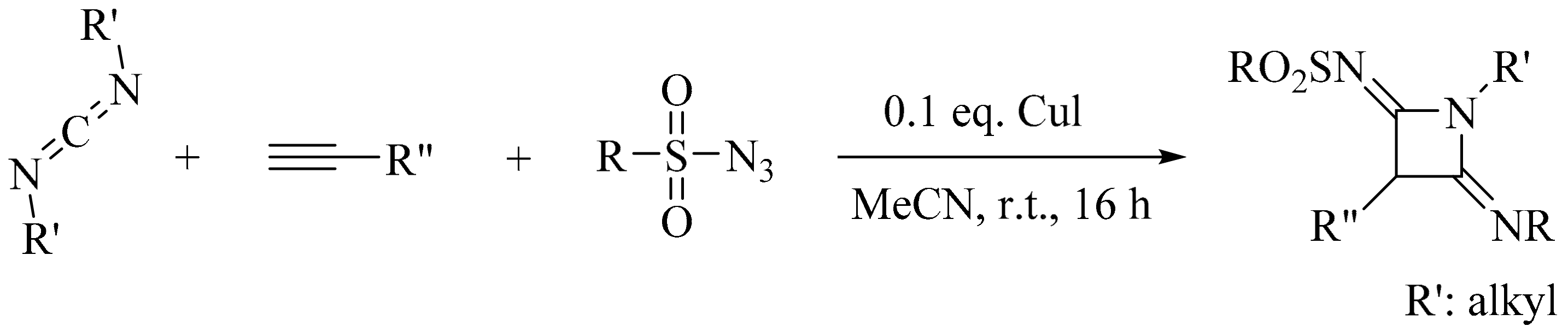







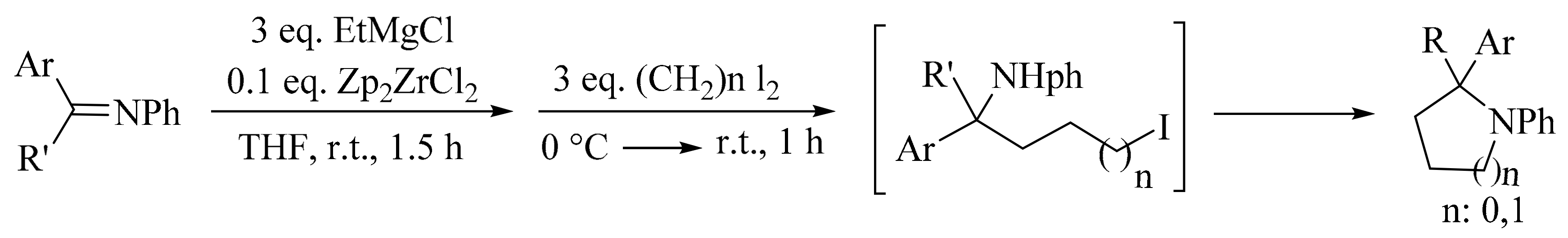


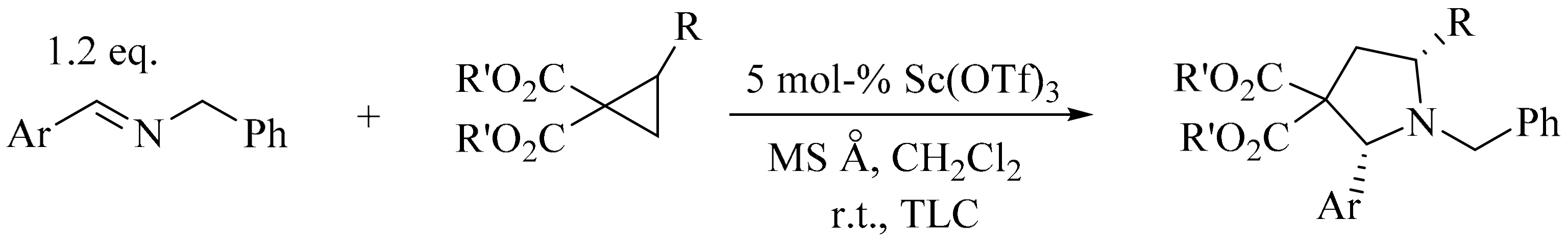




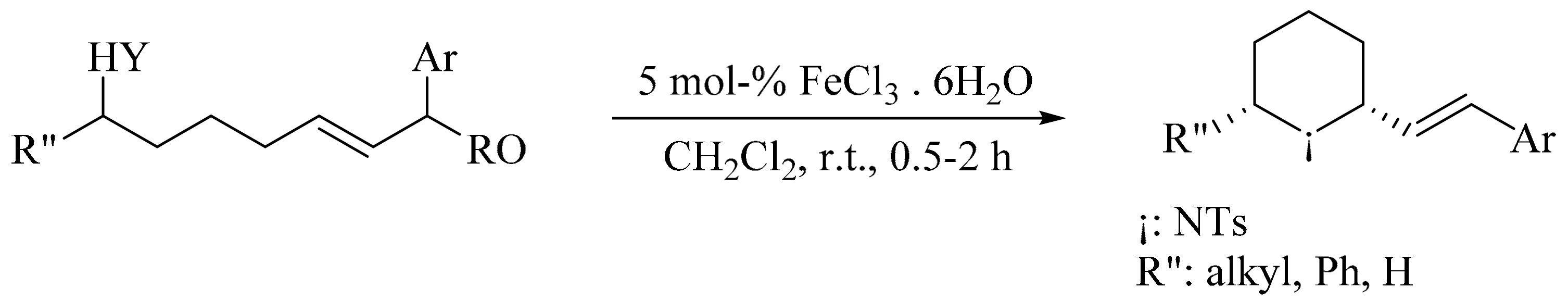




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hameed, A.; Javed, S.; Noreen, R.; Huma, T.; Iqbal, S.; Umbreen, H.; Gulzar, T.; Farooq, T. Facile and Green Synthesis of Saturated Cyclic Amines. Molecules 2017, 22, 1691. https://doi.org/10.3390/molecules22101691
Hameed A, Javed S, Noreen R, Huma T, Iqbal S, Umbreen H, Gulzar T, Farooq T. Facile and Green Synthesis of Saturated Cyclic Amines. Molecules. 2017; 22(10):1691. https://doi.org/10.3390/molecules22101691
Chicago/Turabian StyleHameed, Arruje, Sadia Javed, Razia Noreen, Tayyaba Huma, Sarosh Iqbal, Huma Umbreen, Tahsin Gulzar, and Tahir Farooq. 2017. "Facile and Green Synthesis of Saturated Cyclic Amines" Molecules 22, no. 10: 1691. https://doi.org/10.3390/molecules22101691
APA StyleHameed, A., Javed, S., Noreen, R., Huma, T., Iqbal, S., Umbreen, H., Gulzar, T., & Farooq, T. (2017). Facile and Green Synthesis of Saturated Cyclic Amines. Molecules, 22(10), 1691. https://doi.org/10.3390/molecules22101691



