A New Series of Cytotoxic Pyrazoline Derivatives as Potential Anticancer Agents that Induce Cell Cycle Arrest and Apoptosis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
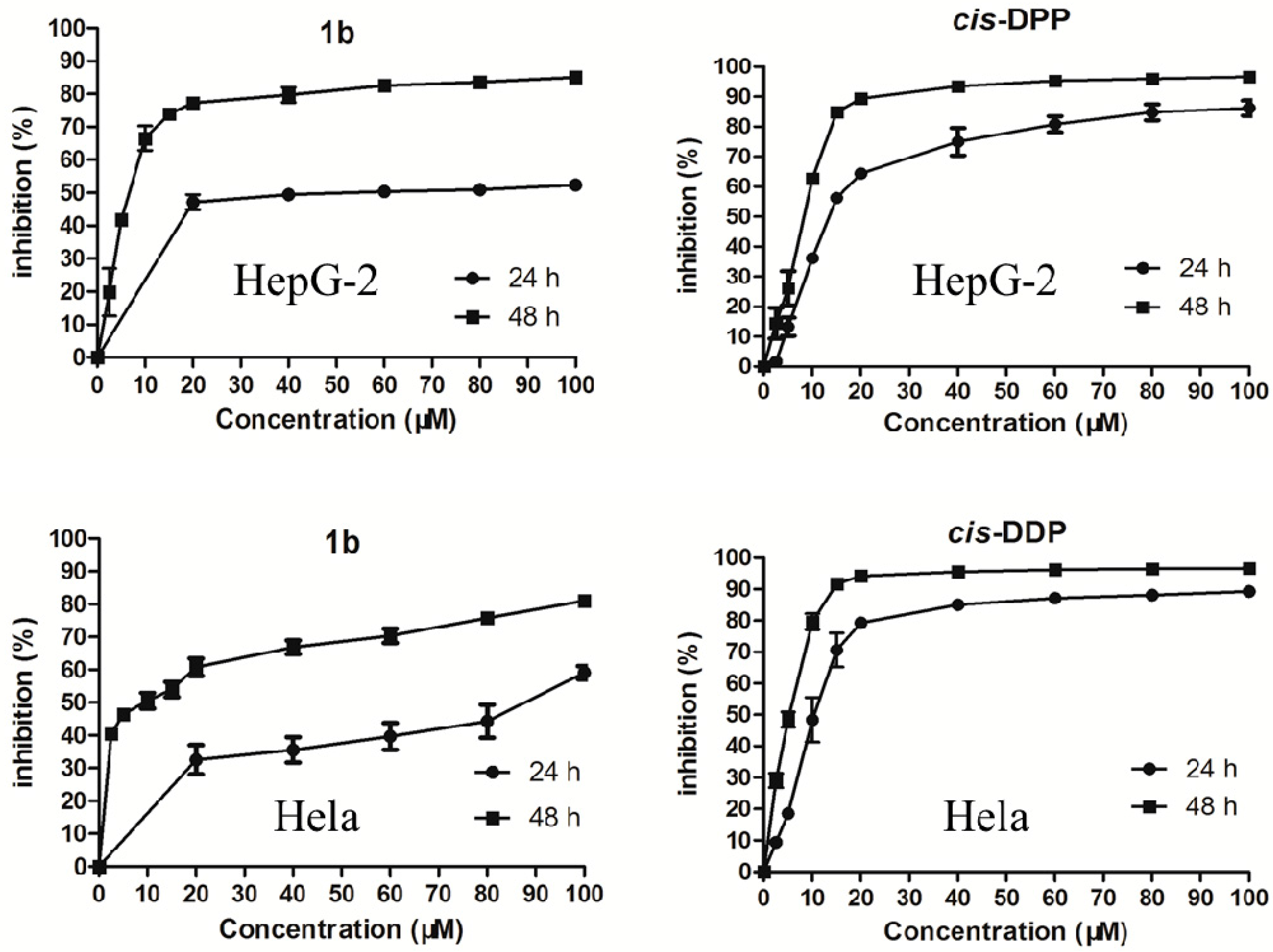
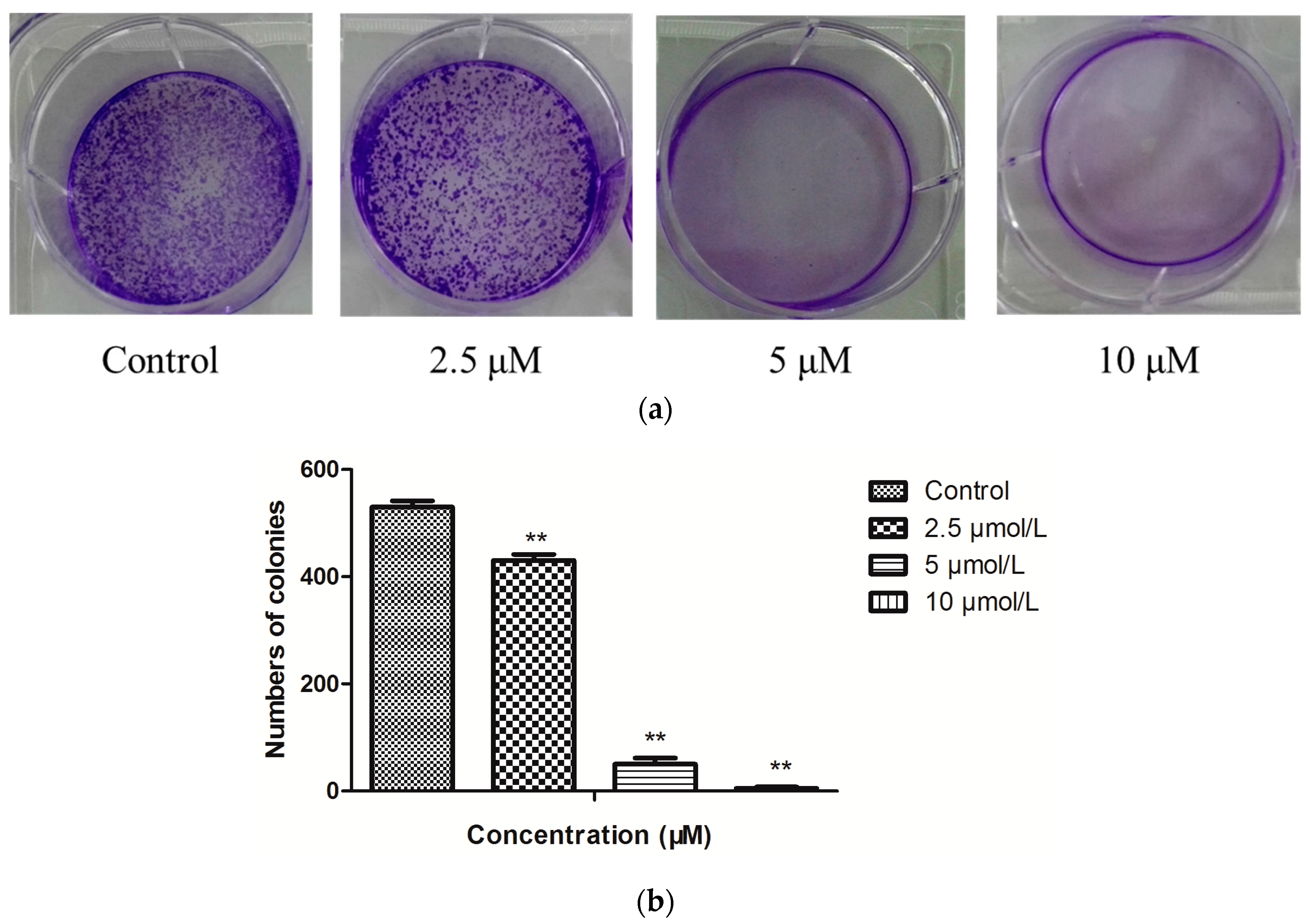
2.2. Antiproliferative Activity
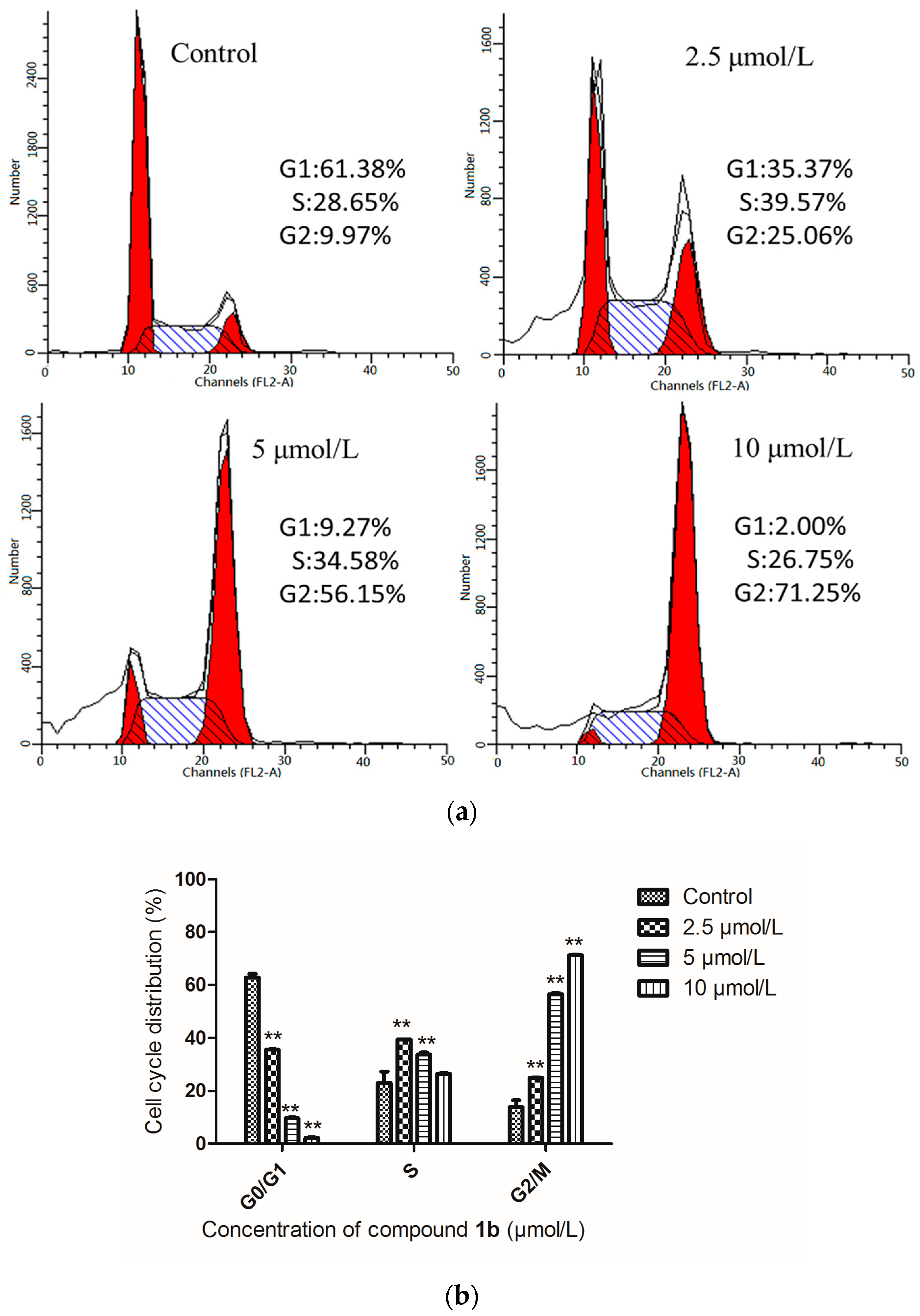
2.3. Influence of 1b on the HepG-2 Cell Cycle
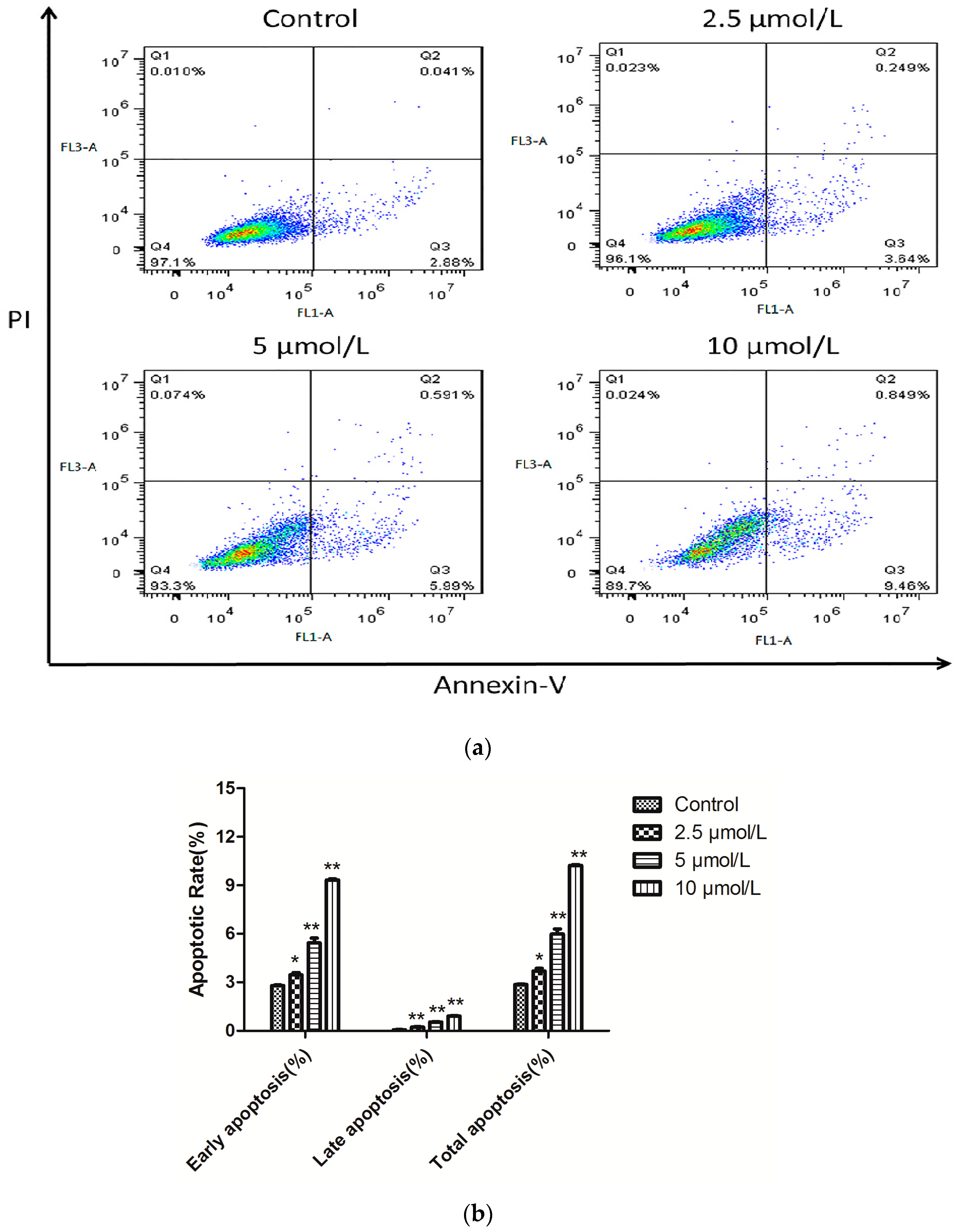
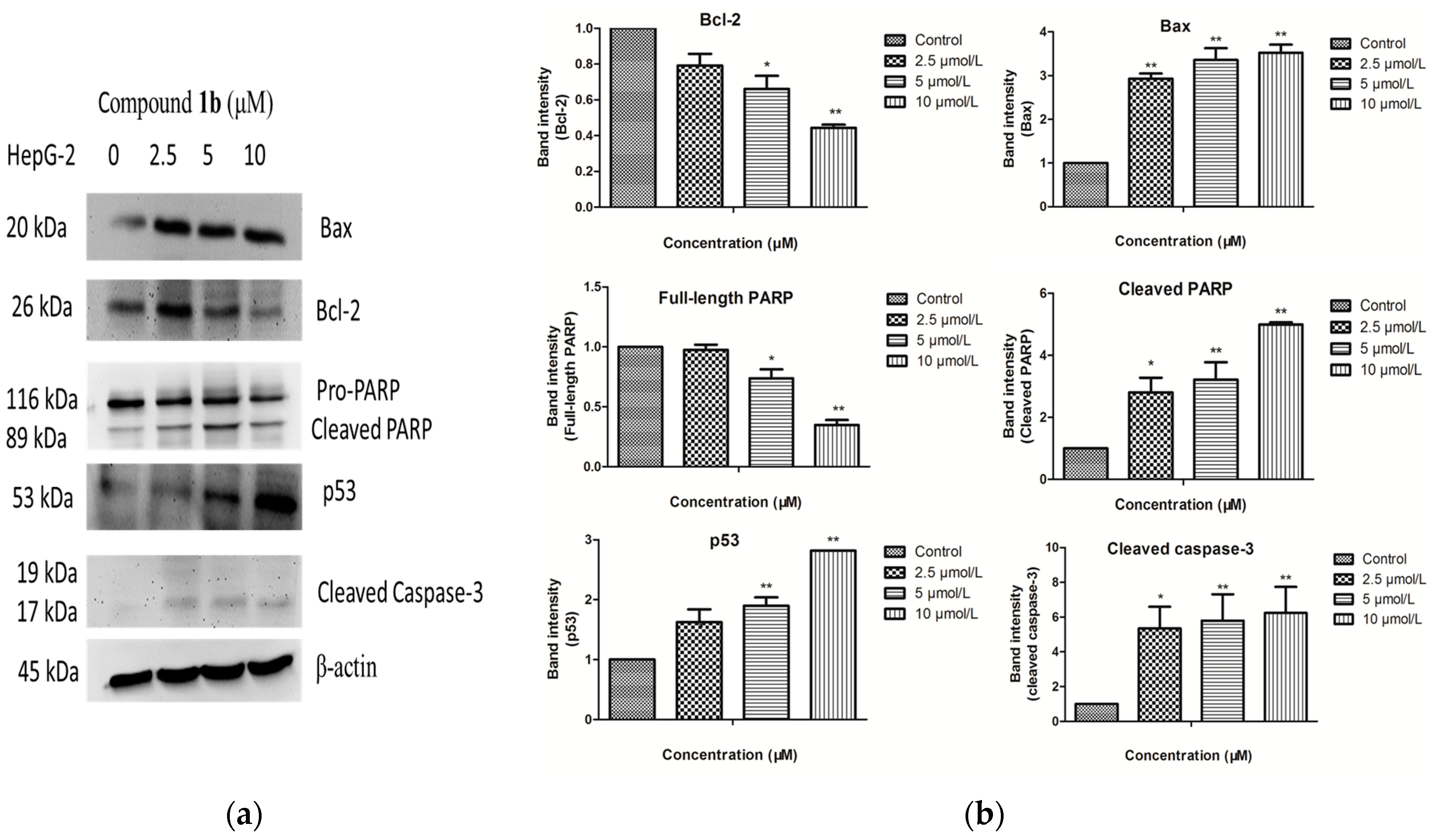
2.4. Induction of Apoptosis by 1b
2.5. Analysis of Targeted Cellular Pathways
3. Materials and Methods
3.1. General Information
3.2. Chemistry: General Procedure for the Synthesis of Chalcones
3.3. General Procedure for the Synthesis of 3,5-Disubstituted-4,5-dihydro-1H-pyrazole-1-carbothioamides 1b–12b
3.4. Biological Assays
3.4.1. MTT Assay and Clonogenic Survival Assay
3.4.2. Flow Cytometric Analysis of Cell Cycle Distribution
3.4.3. Cellular Apoptosis
3.4.4. Western Blot Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Seffrin, J.R.; Hill, D.; Burkart, W.; Magrath, I.; Badwe, R.A.; Ngoma, T.; Mohar, H.; Grey, N. It is time to include cancer and other noncommunicable disease in the millennium development goals. CA Cancer J. Clin. 2009, 59, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Nepali, K.; Sharma, S.; Sharma, M.; Bedi, P.M.S.; Dhar, K.L. Rational approaches, design strategies, structure activity relationship and mechanistic insights for anticancer hybrids. Eur. J. Med. Chem. 2014, 77, 422–487. [Google Scholar] [CrossRef] [PubMed]
- Rebucci, M.; Michiels, C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 2013, 85, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liang, Y.J.; He, H.; Fu, L. Synthesis and antitumor activity of ureas containing pyrimidinyl group. J. Med. Chem. 2011, 46, 429–432. [Google Scholar]
- Ramaswamy, B.; Mrozek, E.; Kuebler, J.P.; Bekaii-Saab, T.; Kraut, E.H. Phase II trial of pyrazoloacridine (NSC#366140) in patients with metastatic breast cancer. Investig. New Drug. 2011, 29, 347–351. [Google Scholar]
- Shaaban, M.R.; Mayhoub, A.S.; Farag, A.M. Recent advances in the therapeutic applications of pyrazolines. Expert. Opin. Ther. Pat. 2012, 22, 253–291. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bawa, S.; Drabu, S.; Kumar, R.; Gupta, H. Biological activities of pyrazoline derivatives–A recent development. Recent Pat. Anti-infect. Drug Discov. 2009, 4, 154–163. [Google Scholar] [CrossRef]
- Marella, A.; Rahmat Ali, M.; Tauquir Alam, M.; Saha, R.; Tanwar, O.; Akhter, M.; Shaquiquzzaman, M.; Mumtaz Alam, M. Pyrazolines: A biological review. Mini. Rev. Med. Chem. 2013, 13, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Pal, D.; Saha, S.; Singh, S. Importance of pyrazole moiety in the field of cancer. Int. J. Pharm. Pharm. Sci. 2012, 4, 98–104. [Google Scholar]
- Fahmy, H.H.; Khalifa, N.M.; Ismail, M.M.F.; El-Sahrawy, H.M.; Nossier, E.S. Biological validation of novel polysubstituted pyrazole candidate with in vitro anticancer activities. Molecules 2016, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Nitulescu, G.M.; Draghici, C.; Missir, A.V. Synthesis of new pyrazole derivatives and their anticancer evaluation. Eur. J. Med. Chem. 2010, 45, 4914–4919. [Google Scholar] [CrossRef] [PubMed]
- Balbi, A.; Anzaldi, M.; Maccio, C.; Aiello, C.; Mazzei, M.; Gangemi, R.; Castagnola, P.; Miele, M.; Rosano, C.; Viale, M. Synthesis and biological evaluation of novel pyrazole derivatives with anticancer activity. Eur. J. Med. Chem 2011, 46, 5293–5309. [Google Scholar] [CrossRef] [PubMed]
- Strocchi, E.; Fornari, F.; Minguzzi, M.; Gramantieri, L.; Milazzo, M.; Rebuttini, V.; Breviglieri, S.; Camaggi, C.M.; Locatelli, E.; Bolondi, L.; et al. Design, synthesis and biological evaluation of pyrazole derivatives as potential multi-kinase inhibitors in hepatocellular carcinoma. Eur. J. Med. Chem. 2012, 48, 391–401. [Google Scholar] [CrossRef] [PubMed]
- Kucukoglu, K.; Oral, F.; Aydin, T.; Yamali, C.; Algul, O.; Sakagami, H.; Gulcin, I.; Supuran, C.T.; Gul, H.I. Synthesis, cytotoxicity and carbonic anhydrase inhibitory activities of new pyrazolines. J. Enzyme. Inhib. Med. Chem. 2016, 31, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Lobo, P.L.; Poojary, B.; Kumsi, M.; Chandra, V.; Kumari, N.S.; Chandrashekar, K.R. Synthesis, antimicrobial and antioxidant activity of 2-[1-{3,5-diaryl-4,5-dihydro-1H-pyrazolenyl}]-4-(4-nitrophenyl)-[1,3]-thiazoles. Med. Chem. Res. 2012, 22, 1689–1699. [Google Scholar] [CrossRef]
- Mushtaque, M.; Avecilla, F.; Haque, A.; Perwez, A.; Khan, M.S.; Rizvi, M.M.A. Experimental and theoretical studies of a pyrazole-thiazolidin-2,4-di-one hybrid. J. Mol. Struct. 2017, 1141, 417–427. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Gzella, A.; Lesyk, R. Synthesis of new 4-thiazolidinone-, pyrazoline-, and isatin-based conjugates with promising antitumor activity. J. Med. Chem. 2012, 55, 8630–8641. [Google Scholar] [CrossRef] [PubMed]
- Ismail, N.S.M.; Ali, G.M.E.; Ibrahim, D.A.; Elemetwali, A.M. Medicinal attributes of pyrazolo[1,5-a] pyrimidine based scaffold derivatives targeting kinases as anticancer agents. Fut. J. Pharm. Sci. 2016, 2, 60–70. [Google Scholar] [CrossRef]
- Beswick, M.C.; Brough, P.A.; Drysdale, M.J.; Dymock, B.W. 3-(2-Hydroxy-phenyl)-1H-pyrazole-4-carboxylic acid Amide Derivatives as HSP90 Inhibitors for the Treatment of Cancer. U.S. Patent 7,803,831, 28 September 2010. [Google Scholar]
- Abadi, A.H.; Eissa, A.A.H.; Hassan, G.S. Synthesis of novel 1,3,4-trisubstituted pyrazole derivatives and their evaluation as antitumor and antiangiogenic agents. Chem. Pharm. Bull. 2003, 51, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Manna, F.; Chimenti, F.; Fioravantia, R.; Bolasco, A.; Secci, D.; Chimenti, P.; Ferlini, C.; Scambia, G. Synthesis of some Pyrazole Derivatives and Preliminary Investigation of Their Affinity Binding to P-glycoprotein. Bioorg. Med. Chem. Lett. 2005, 15, 4632–4635. [Google Scholar] [CrossRef] [PubMed]
- Atamanyuk, D.; Zimenkovsky, B.; Lesyk, R. Synthesis and anticancer activity of novel thiopyrano[2,3-d]thiazole-based compounds containing norbornane moiety. J. Sulfur. Chem. 2008, 29, 151–162. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.C.; Li, Q.N.; Yu, X.Y.; Wang, J.Z.; Zheng, J.H. The Synthesis and Evaluation of Novel Hydroxyl Substituted Chalcone Analoges with in Vitro Anti-Free Radicals Pharmacological Activity and in Vivo Anti-Oxidation Activity in a Free Radical-Injury Alzheimer’s Model. Molecules 2013, 18, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Guo, H.X. The effect of substituted groups in benzene ring on the condensation reaction of acetophenones with benzaldehydes. Chin. J. Synth. Chem. 1999, 7, 422–426. [Google Scholar]
- Altintop, M.D.; Ozdemir, A.; Zitouni, G.T.; Llgın, S.; Atlı, O.; Demirel, R.; Kaplancıklı, Z.A. A novel series of thiazolyl-pyrazoline derivatives: Synthesis and evaluation of antifungal activity, cytotoxicity and genotoxicity. Eur. J. Med. Chem. 2015, 92, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zheng, J.H.; Wang, H. A Dihydropyrazole Derivative and Its Preparation Method and Application. C.N. Patent 106,496,123, 15 March 2017. [Google Scholar]
- Qin, Y.J.; Li, Y.J.; Jiang, A.Q.; Yang, M.R.; Zhu, Q.Z.; Dong, H.; Zhu, H.L. Design, synthesis and biological evaluation of novel pyrazoline-containing derivatives as potential tubulin assembling inhibitors. Eur. J. Med. Chem. 2015, 94, 447–457. [Google Scholar] [CrossRef] [PubMed]
- Henry, C.M.; Hollville, E.; Martin, S.J. Measuring apoptosis by microscopy and flow cytometry. Methods 2013, 61, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C.; Wu, C.L.; Lee, C.C.; Chen, C.L.; Yu, D.S.; Huang, H.S. Structure-based hybridization, synthesis and biological evaluation of novel tetracyclic heterocyclic azathioxanthone analogues as potential antitumor agents. Eur. J. Med. Chem. 2015, 103, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Li, W.; Wang, Y.Y.; Zhong, Q.Q.; Wang, S.Q.; Wang, X.N.; Ren, D.M.; Lou, H.X. Antiproliferative activities of Garcinia bracteata extract and its active ingredient, isobractatin, against human tumor cell lines. Arch. Pharm. Res. 2014, 37, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Li, D.H.; Hu, X.; Han, T.; Xu, S.; Zhou, T.; Wang, Z.; Cheng, K.; Li, Z.; Hua, H.; Xiao, W.; et al. Synthesis, Biological Activity and Apoptotic Properties of NO-Donor/Enmein-Type ent-Kauranoid Hybrids. Int. J. Mol. Sci. 2016, 17, 747. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.; Yu, Y.; Wang, Z.; Zuo, Y.; Li, S.; Yang, D.; Hu, S.; Xiang, M.; Xu, Z.; et al. Metformin inhibits the growth of nasopharyngeal carcinoma cells and sensitizes the cells to radiation via inhibition of the DNA damage repair pathway. Oncol. Rep. 2014, 32, 2596–2604. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Compounds | R2 | R3 | R4 | R5 | R6 | Mol. Formula |
|---|---|---|---|---|---|---|
| 1b | OCH3 | OCH3 | OCH3 | H | F | C19H20FN3O3S |
| 2b | OCH3 | OCH3 | OCH3 | H | Cl | C19H20ClN3O3S |
| 3b | OCH3 | OCH3 | OCH3 | H | Br | C19H20BrN3O3S |
| 4b | OCH3 | OCH3 | OCH3 | H | CH3 | C20H23N3O3S |
| 5b | Br | OH | OCH3 | H | Cl | C17H15BrClN3O2S |
| 6b | Br | OH | OCH3 | H | Br | C17H15Br2N3O2S |
| 7b | Br | OH | OCH3 | OH | H | C17H16BrN3O3S |
| 8b | OCH3 | OH | OCH3 | OH | H | C18H19N3O4S |
| 9b | Br | OH | OCH2CH3 | OH | H | C16H15N3OS |
| 10b | OCH3 | OCH3 | OCH2CH3 | OH | H | C19H21N3O4S |
| 11b | OCH3 | OCH3 | OCH3 | H | OCH3 | C20H23N3O4S |
| 12b | Br | OH | OCH3 | H | CH3 | C18H18BrN3O2S |
| Compounds | (IC50 μM) 1 | ||||
|---|---|---|---|---|---|
| HepG-2 | Hela | A549 | NIH/3T3 Cell Line | SI 3 | |
| 1b | 6.78 ± 1.44 | 7.63 ± 1.35 | >100 | >100 | >14.75 |
| 2b | 16.02 ± 2.55 | 9.37 ± 1.47 | >50 | >100 | >6.24 |
| 3b | 35.55 ± 2.56 | 16.93 ± 1.99 | >100 | >100 | >2.81 |
| 4b | >50 | >50 | >100 | >100 | - |
| 5b | 33.06 ± 3.54 | 15.78 ± 2.33 | 44.12 ± 3.02 | 26.28 ± 1.81 | 0.79 |
| 6b | 52.99 ± 2.81 | 20.20 ± 1.54 | 50.99 ± 2.11 | 23.52 ± 1.39 | 0.44 |
| 7b | >50 | 11.44 ± 2.64 | >50 | >100 | - |
| 8b | >50 | >50 | >100 | >100 | - |
| 9b | 32.25 ± 2.83 | 7.74 ± 1.63 | >100 | >100 | >3.10 |
| 10b | >50 | 22.31 ± 2.78 | >100 | >100 | - |
| 11b | >50 | >50 | >100 | >100 | - |
| 12b | 19.69 ± 2.45 | 14.82 ± 2.33 | 50.04 ± 3.10 | 27.11 ± 2.02 | 1.38 |
| cis-DDP 2 | 7.57 ± 0.98 | 5.28 ± 0.66 | 29.48 ± 3.30 | 4.88 ± 1.93 | 0.64 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zheng, J.; Xu, W.; Chen, C.; Wei, D.; Ni, W.; Pan, Y. A New Series of Cytotoxic Pyrazoline Derivatives as Potential Anticancer Agents that Induce Cell Cycle Arrest and Apoptosis. Molecules 2017, 22, 1635. https://doi.org/10.3390/molecules22101635
Wang H, Zheng J, Xu W, Chen C, Wei D, Ni W, Pan Y. A New Series of Cytotoxic Pyrazoline Derivatives as Potential Anticancer Agents that Induce Cell Cycle Arrest and Apoptosis. Molecules. 2017; 22(10):1635. https://doi.org/10.3390/molecules22101635
Chicago/Turabian StyleWang, Hong, Jinhong Zheng, Weijie Xu, Cheng Chen, Duncan Wei, Wenxiu Ni, and Ying Pan. 2017. "A New Series of Cytotoxic Pyrazoline Derivatives as Potential Anticancer Agents that Induce Cell Cycle Arrest and Apoptosis" Molecules 22, no. 10: 1635. https://doi.org/10.3390/molecules22101635
APA StyleWang, H., Zheng, J., Xu, W., Chen, C., Wei, D., Ni, W., & Pan, Y. (2017). A New Series of Cytotoxic Pyrazoline Derivatives as Potential Anticancer Agents that Induce Cell Cycle Arrest and Apoptosis. Molecules, 22(10), 1635. https://doi.org/10.3390/molecules22101635





