Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds
Abstract
1. Introduction
2. Results
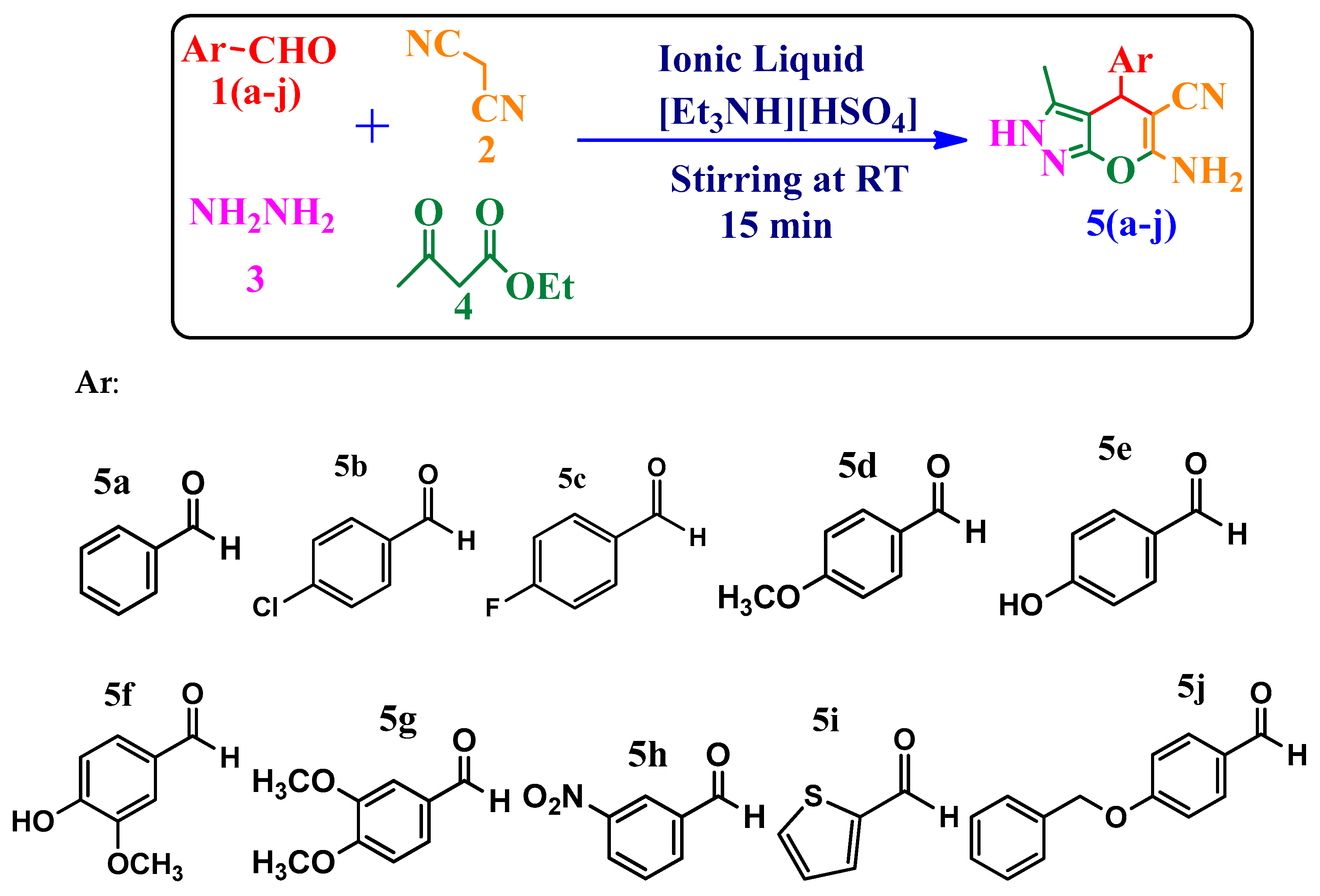
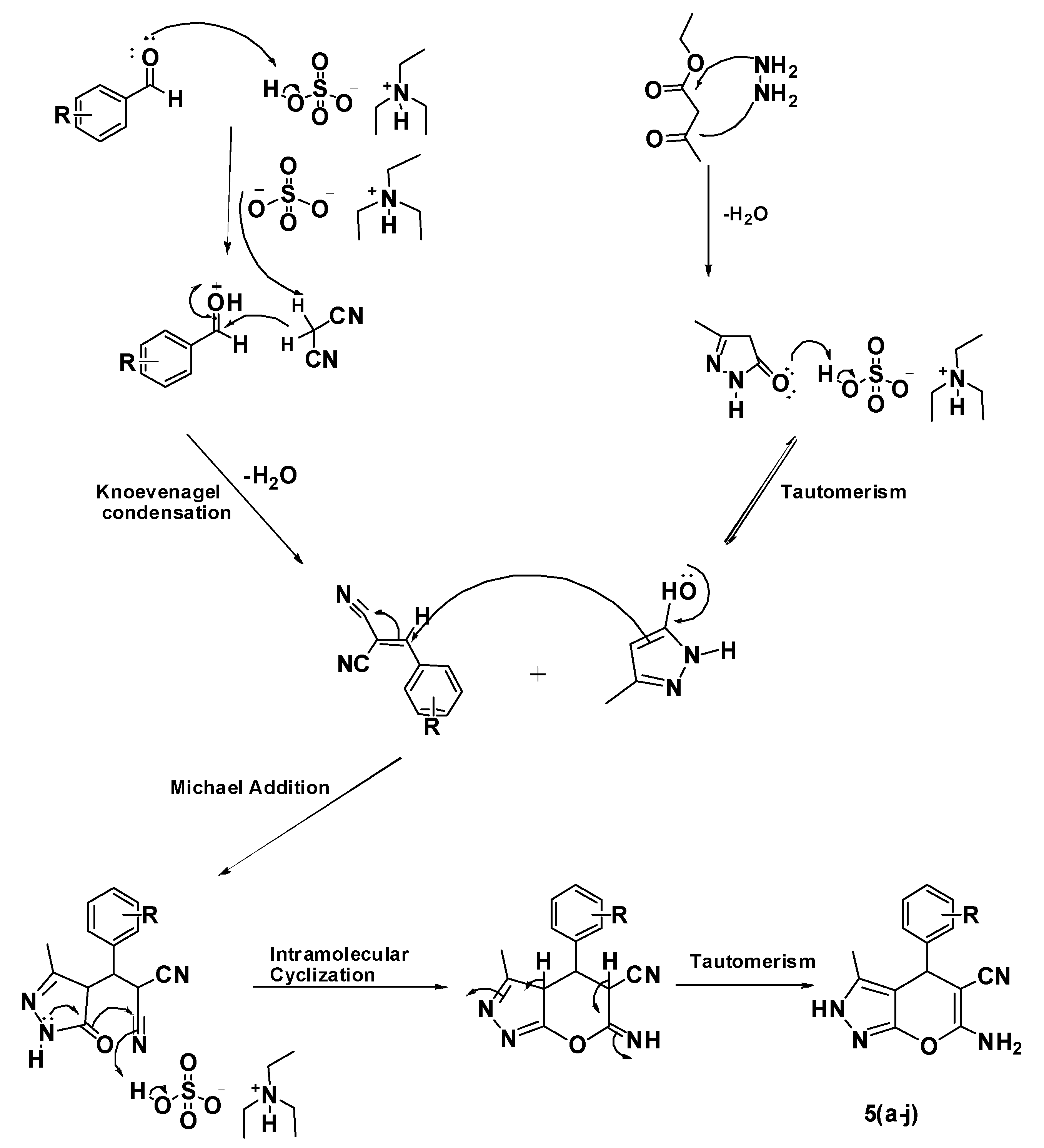
2.1. Chemistry
2.2. In Vitro Anticancer Activity
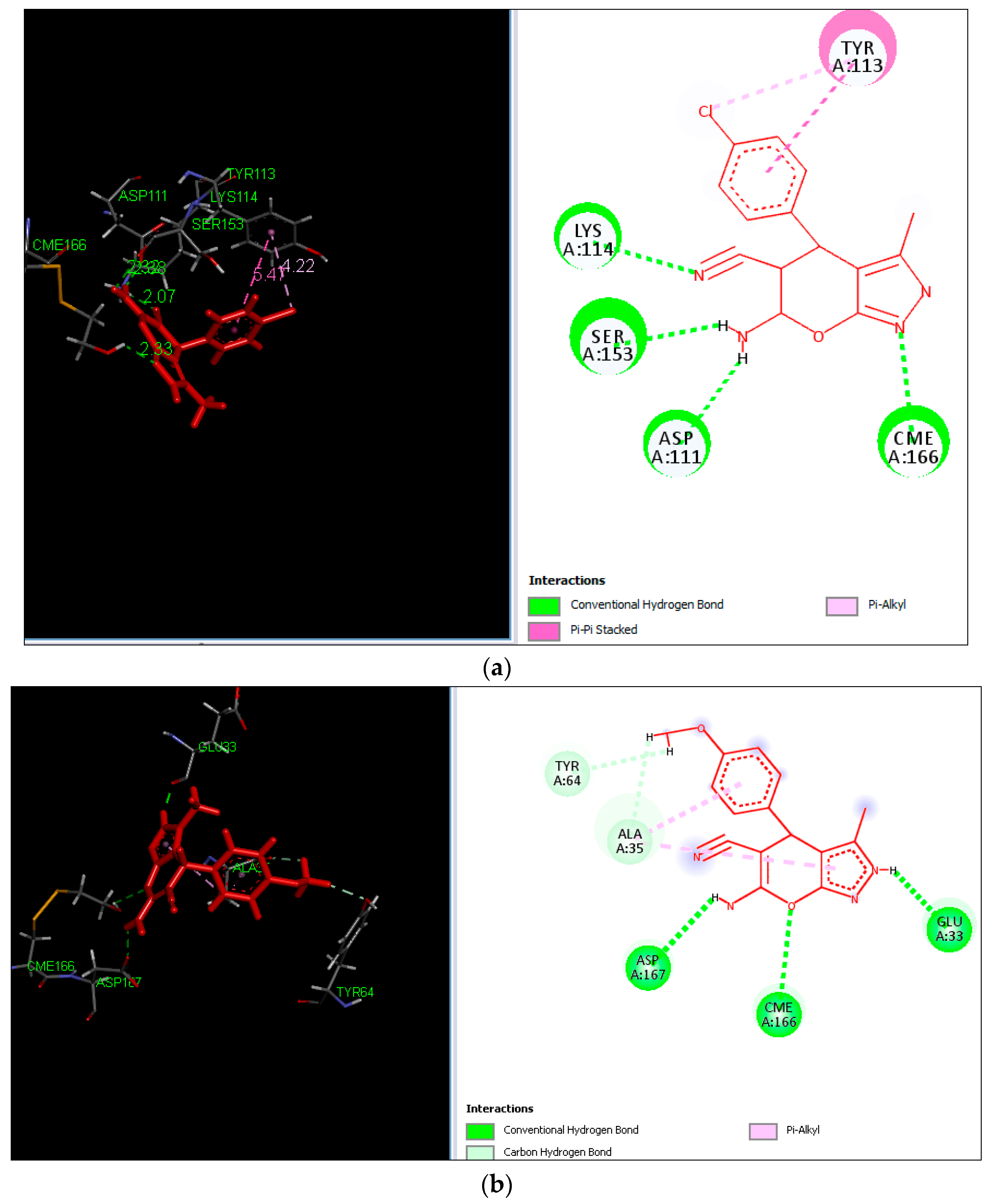
2.3. Molecular Docking
2.4. Prediction of ADMET Properties
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Synthesis of [Et3NH][HSO4]
4.3. Synthesis of 6-Amino-4-(substituted phenyl)-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles
4.4. Evaluation of In Vitro Anticancer Activity
4.5. Molecular Docking Study
4.6. In Silico ADMET Prediction
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Bertram, G.; Katzung, L. Basic and Clinical Pharmacology; McGraw-Hill Medical Publication Division: New York, NY, USA, 2010; Volume 11, pp. 1091–1116. [Google Scholar]
- Jemal, A.; Ward, E.M.; Johnson, C.J.; Cronin, K.A.; Ma, J.; Ryerson, A.B.; Mariotto, A.; Lake, A.J.; Wilson, R.; Sherman, R.L.; et al. Annual report to the nation on the status of cancer, 1975–2014, featuring survival. J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Karim, S.S.; Anwar, M.M.; Mohamed, N.A.; Nasr, T.; Elseginy, S.A. Design, synthesis, biological evaluation and molecular docking studies of novel benzofuran-pyrazole derivatives as anticancer agents. Bioorg. Chem. 2015, 63, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, H.H.; Srour, A.M.; Ismail, M.A.; Khater, M.A.; Serrya, R.A.; El-Manawaty, M.A. Design and synthesis of some new tri-substituted pyrazole derivatives as anticancer agents. Res. Chem. Intermed. 2016, 42, 6881–6892. [Google Scholar] [CrossRef]
- Fahmy, H.H.; Khalifa, N.M.; Ismail, M.M.; El-Sahrawy, H.M.; Nossier, E.S. Biological validation of novel polysubstituted pyrazole candidates with in vitro anticancer activities. Molecules 2016, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Rahmi, K.U. Design, synthesis, characterization, and anti-proliferative activity of novel pyrazole-3-carboxylic acid derivatives. Monatsh. Chem. 2015, 146, 1743–1749. [Google Scholar]
- Anam, A.; Abad, A.; Mohd, A.; Shamsuzzaman. Review: Biologically active pyrazole derivatives. New J. Chem. 2017, 41, 16–41. [Google Scholar]
- Lehmann, T.P. Cell-specific cytotoxic effect of pyrazole derivatives on breast cancer cell lines MCF7 and MDA-MB-231. J. Physiol. Pharmacol. 2017, 68, 201–207. [Google Scholar] [PubMed]
- El-Gamal, M.I.; Abdel-Maksoud, M.S.; Gamal El-Din, M.M.; Shin, J.S.; Lee, K.T.; Ho Yoo, K.; Oh, C.H. Synthesis, in vitro Anti-proliferative and Anti-inflammatory Activities, and Kinase Inhibitory effects of New 1,3,4-triarylpyrazole Derivatives. Anti-Cancer Agents Med. Chem. 2017, 17, 75–84. [Google Scholar]
- Elguero, J.; Goya, P.; Jagerovic, N.; Silva, A.M.S. Pyrazoles as drugs: Facts and fantasies. In Targets in Heterocyclic Systems; Attanasi, O.A., Spinelli, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2002; Volume 6, pp. 52–98. [Google Scholar]
- Singh, S.K.; Reddy, P.G.; Rao, K.S.; Lohray, B.B.; Misra, P.; Rajjak, S.A.; Rao, Y.K. Venkatewarlu, A. Polar substitutions in the benzenesulfonamide ring of celecoxib afford a potent 1,5-diarylpyrazole class of COX-2 inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Qvortrup, K.; Jensen, J.F.; Sørensen, M.S.; Kouskoumvekaki, I.; Petersen, R.K.; Taboureau, O.; Kristiansen, K.; Nielsen, T.E. Synthesis and biological evaluation of dihydropyrano[2,3-c]pyrazoles as a new class of PPARγ partial agonists. PLoS ONE 2017, 12, e0162642. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kaur, A.; Mankotia, S.; Singh, H.; Singh, A.; Singh, J.; Gupta, M.; Sharma, S.; Nepali, K.; Singh Bedi, P.M. Synthesis, screening and docking of fused pyrano[3,2-d]pyrimidine derivatives as xanthine oxidase inhibitor. Eur. J. Med. Chem. 2017, 131, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.L.; Liu, D.; Zhang, Z.J.; Shan, S.; Han, X.; Srinivasula, S.M.; Croce, C.M.; Alnemri, E.S.; Huang, Z. Structure-based discovery of an organic compound that bind Bc1-2 protein and induces apoptosis of tumor cells. Proc. Natl. Acad. Sci. USA 2000, 97, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.R.; Khaireldin, N.Y.; Fahmyb, A.F.; El-Sayeda, A.A.F. Facile synthesis of fused nitrogen containing heterocycles as anticancer agents. Der. Pharm. Chem. 2010, 2, 400–417. [Google Scholar]
- Erugu, Y.; Sangepu, B.; Varre, K.; Pamanji, R.; Bomma, Y.; Janapala, V.R.; Srinivasarao, V.; Tigulla, P.; Jetti, V.R. Design, an efficient ecofriendly synthesis of spirooxindole derivatives and their anticancer activity supported by molecular docking studies. World J. Pharm. Pharm. Sci. 2014, 3, 1895–1914. [Google Scholar]
- Soad, K.S.; Magda, F.M.; Ahmed, F.D.; Ahmed, H.M.E.; Ismail, A.A. Molecular docking simulation and anticancer assessment on human breast carcinoma cell line using novel bis(1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile)andis(1,4-dihydropyrazolo[4′,3′:5,6]pyrano[2,3-b]pyridine-6-carbonitrile) derivatives. Bioorg. Chem. 2017. [Google Scholar] [CrossRef]
- Pantsar, T.; Singha, P.; Tapio, J.N.; Koshevoy, I.; Leppänen, J.; Poso, A.; Juha, M.A.N.; Pasonen-Seppänen, S.; Savinainen, J.R.; Laitinen, T.; et al. Design, synthesis, and biological evaluation of 2,4-dihydropyrano[2,3-c]pyrazole derivatives as autotaxin inhibitors. Eur. J. Pharm. Sci. 2017. [Google Scholar] [CrossRef]
- Adibi, H.; Hosseinzadeh, L.; Farhadi, S.; Ahmadi, F. Synthesis and cytotoxic evaluation of6-amino-4-aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-carbonitrile derivatives using borax with potential anticancer effects. J. Rep. Pharm. Sci. 2013, 2, 116–124. [Google Scholar]
- Kamel, M.M. Convenient synthesis, characterization, cytotoxicity and toxicity of pyrazole derivatives. Acta Chim. Slov. 2015, 62, 136–151. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.V.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.G. Ultrasound mediated one-pot, three component synthesis, docking and ADME prediction of novel 5-amino-2-(4-chlorophenyl)-7-substitutedphenyl-8,8a-dihydro-7H-(1,3,4)thiadiazolo(3,2)pyrimidine-6-carbonitrile derivatives as anticancer agents. Molecules 2016, 21, 894. [Google Scholar] [CrossRef] [PubMed]
- Nikalje, A.G.; Tiwari, S.V.; Tupe, J.G.; Vyas, V.K.; Qureshi, G. Ultrasound assisted-synthesis and biological evaluation of piperazinylprop-1-en-2-yloxy-2h-chromen-2-ones as cytotoxic agents. Lett. Drug Des. Discov. 2017, 14. [Google Scholar] [CrossRef]
- Tiwari, S.V.; Siddiqui, S.; Seijas, J.A.; Vazquez-Tato, M.P.; Sarkate, A.P.; Lokwani, D.K.; Nikalje, A.G. Microwave-assisted facile synthesis, anticancer evaluation and docking study of N-((5-(substituted methylene amino)-1,3,4-thiadiazol-2-yl)methyl)benzamide derivatives. Molecules 2017, 22, 995. [Google Scholar] [CrossRef] [PubMed]
- Junek, H.; Aigner, H. Synthesenmit Nitrilen, XXXV. Reaktionen von Tetracyanathylen mit Heterocyclen. Eur. J. Inorg. Chem. 1973, 106, 914–921. [Google Scholar]
- Sharanin, Y.A.; Sharanina, L.G.; Puzanova, V.V. Nitrile cyclization reactions. VII. Synthesis of 6-amino-4-aryl-3-methyl-5-cyano-1H,4H-pyrazolo[3,4-B]pyrans. Z. Chem. Inform. 1983, 19, 2609–2615. [Google Scholar] [CrossRef]
- Sharanin, Y.A.; Sharanina, L.G.; Puzanova, V.V. Nitrile cyclization reactions. VII. synthesis of 6-amino-4-aryl-3-methyl-5-cyano-1H,4H-pyrazolo(3,4-B) pyrans. J. Org. Chem. USSR (Engl. Transl.) 1983, 19, 221. [Google Scholar] [CrossRef]
- Peng, Y.; Song, G.; Dou, R. Surface cleaning under combined microwave and ultrasound irradiation: Flash synthesis of 4H-pyrano[2,3-c]pyrazoles in aqueous media. Green Chem. 2006, 8, 573–575. [Google Scholar] [CrossRef]
- Vasuki, G.; Kumaravel, K. Rapid four component reaction in water: Synthesis of pyranopyrazoles. Tetrahedron Lett. 2008, 49, 5636–5638. [Google Scholar] [CrossRef]
- Lehmann, F.; Holm, S.L.; Laufer, M.S. Three-component combinatorial synthesis of novel dihydropyrano[2,3-c]pyrazoles. J. Comb. Chem. 2008, 10, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Ghods, A.; Derikvand, F.; Bakhtiari, K.; Bammoharram, F.F. H14 [NaP 5W 30 O 110] catalyzed one-pot three-component synthesis of dihydropyrano[2,3-c]pyrazole and pyrano[2,3-d]pyrimidine derivatives. J. Iran. Chem. Soc. 2010, 7, 615–620. [Google Scholar] [CrossRef]
- Reddy, M.B.M.; Jayashankara, V.P.; Pasha, M.A. Glycine-catalyzed efficient synthesis of pyranopyrazoles via one-pot multicomponent reaction. Synth. Commun. 2010, 40, 2930–2934. [Google Scholar] [CrossRef]
- Kanagaraj, K.; Pitchumani, K. Solvent-free multicomponent synthesis of pyrano-pyrazoles: Per-6-amino-β-cyclodextrin as a remarkable catalyst and host. Tetrahedron Lett. 2010, 51, 3312–3316. [Google Scholar] [CrossRef]
- Samant, S.D.; Patil, N.R.; Kshirsagar, S.W. Mg-Al Hydrotalcite as a first heterogeneous basic catalyst for the synthesis of 4H-pyrano[2,3-c]pyrazoles through a four-component reaction. Synth. Commun. 2011, 41, 1320–1325. [Google Scholar]
- Babaie, M.; Sheibani, H. Nanosized magnesium oxide as a highly effective heterogeneous base catalyst for the rapid synthesis of pyranopyrazoles via a tandem four-component reaction. Arab. J. Chem. 2011, 4, 159–162. [Google Scholar] [CrossRef]
- Mecadon, H.; Rohman, M.R.; Kharbangar, I.; Laloo, B.M.; Kharkongor, I.; Rajbangshi, M.; Myrboh, B. L-Proline as an efficient catalyst for the multi-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-F]pyrazole-5-carbonitriles in water. Tetrahedron Lett. 2011, 52, 3228–3231. [Google Scholar] [CrossRef]
- Mecadon, H.; Rohman, M.R.; Rajbangshi, M.; Myrboh, B. γ-Alumina as a recyclable catalyst for the four-component synthesis of 6-amino-4-alkyl/aryl-3-methyl-2,4-dihydropyrano[2,3-c]pyrazole-5-carbonitriles in aqueous medium. Tetrahedron Lett. 2011, 52, 2523–2525. [Google Scholar] [CrossRef]
- Kiyani, H.; Samimi, H.A.; Ghorbani, F.; Esmaieli, S. One-pot, four-component synthesis of pyrano[2,3-c]pyrazoles catalyzed by sodium benzoate in aqueous medium. Curr. Chem. Lett. 2013, 2, 197–206. [Google Scholar] [CrossRef]
- Bihani, M.; Bora, P.P.; Bez, G.; Askari, H. Amberlyst A21 catalyzed chromatography free method for multicomponent synthesis of dihydropyrano[2,3-F]pyrazoles in ethanol. ACS Sustain. Chem. Eng. 2013, 1, 440–447. [Google Scholar] [CrossRef]
- Wu, M.; Feng, Q.; Wan, D.; Ma, J. CTACl as catalyst for four-component, one-pot synthesis of pyranopyrazole derivatives in aqueous medium. Synth. Commun. 2013, 43, 1721–1726. [Google Scholar] [CrossRef]
- Yakaiah, S.; Buchappa, G.; Durgaprasad, K.; Ravibabu, K.; Aparna, P. Ionic liquid catalyzed one-pot three component synthesis of dihydropyrano[2,3-c]pyrazole under green condtion. Asian J. Chem. 2016, 28, 2441–2445. [Google Scholar] [CrossRef]
- Chaudhari, M.A.; Gujar, J.B.; Kawade, D.S.; Jogdand, N.R.; Shingare, M.S. A highly efficient and sustainable synthesis of dihydropyrano[2,3-c]pyrazoles using polystyrene-supported p-toluenesulfonic acid as reusable catalyst. Cogent Chem. 2015, 1, 1063830. [Google Scholar] [CrossRef]
- Brahmachari, G.; Banerjee, B. Facile and one-pot access to diverse and densely functionalized2-amino-3-cyano-4h-pyrans and pyran-annulated heterocyclic scaffolds via an eco-friendly multicomponent reaction at room temperature using urea as a novel organo-catalyst. ACS Sustain. Chem. Eng. 2014, 2, 411–422. [Google Scholar] [CrossRef]
- Mandha, S.R.; Siliverib, S.; Allaa, M.; Bommenaa, V.R.; Bomminenib, M.R.; Balasubramanian, S. Eco-friendly synthesis and biological evaluation of substituted pyrano[2,3-c]pyrazoles. Bioorg. Med. Chem. Lett. 2012, 22, 5272–5278. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, Z.N.; Khan, K. [Et3NH][HSO4]-catalyzed efficient, eco-friendly, and sustainable synthesis of quinoline derivatives via knoevenagel condensation. ACS Sustain. Chem. Eng. 2014, 2, 1187–1194. [Google Scholar] [CrossRef]
- Hailes, H.C. Reaction solvent selection: The potential of water as a solvent for organic transformations. Org. Process Res. Dev. 2007, 11, 114–120. [Google Scholar] [CrossRef]
- El-Tamany, E.S.; El-Shahed, F.A.; Mohamed, B.H. Synthesis and biological activity of some pyrazole derivatives. J. Serb. Chem. Soc. 1999, 64, 9–18. [Google Scholar]
- Lei, Z.; Dai, C.; Chen, B. Gas solubility in ionic liquids. Chem. Rev. 2014, 114, 1289–1326. [Google Scholar] [CrossRef] [PubMed]
- Fedorov, M.V.; Kornyshev, A.A. Ionic liquids at electrified interfaces. Chem. Rev. 2014, 114, 2978–3036. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, C.; Li, H.; Wang, Y. Preparation of dialkoxypropanes in simple ammonium ionic liquids. Green Chem. 2006, 8, 1076–1079. [Google Scholar] [CrossRef]
- Wang, C.; Guo, L.; Li, H.; Wang, Y.; Weng, J.; Wu, L. Preparation of simple ammonium ionic liquids and their application in the cracking of dialkoxypropanes. Green Chem. 2006, 8, 603–607. [Google Scholar] [CrossRef]
- Weng, J.; Wang, C.; Li, H.; Wang, Y. Novel quaternary ammonium ionic liquids and their use as dual solvent-catalysts in the hydrolytic reaction. Green Chem. 2006, 8, 96–99. [Google Scholar] [CrossRef]
- Ganeshpure, P.A.; George, G.; Das, J. Brønsted acidic ionic liquids derived from alkylamines as catalysts andmediums for Fischer esterification: Study of structure-activity relationship. J. Mol. Catal. A Chem. 2008, 279, 182–186. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, W.; Li, H.; Guo, L. Solvent-free synthesis of unsaturated ketones by Saucy-Marbet reaction with simple ammonium ionic liquid as the catalyst. Green Chem. 2009, 11, 843–847. [Google Scholar] [CrossRef]
- Rajendran, A.; Raghupathy, D.; Priyadarshini, M.A. Domino Green Synthesis of Bis (indolyl) methane catalyzed by ionic liquid [Et3NH][HSO4]. Int. J. ChemTech. Res. 2011, 3, 298–302. [Google Scholar]
- Kermani, E.T.; Khabazzadeh, H.; Jazinizadeh, T. Friedländer synthesis of poly-substituted quinolines in the presence of triethylammonium hydrogen sulfate [Et3NH][HSO4] as a highly efficient, and cost effective acidic ionic liquid catalyst. J. Heterocycl. Chem. 2011, 48, 1192–1196. [Google Scholar] [CrossRef]
- Suryawanshi, N.S.; Jain, P.; Singhal, M.; Khan, I. Mannich synthesis under ionic liquid [Et3NH][HSO4]. Catal. J. Appl. Chem. 2012, 1, 18–23. [Google Scholar] [CrossRef]
- Khabazzadeh, H.; Kermani, E.T.; Jazinizadeh, T. An efficient synthesis of 3,4-dihydropyrimidin-2(1H)-ones catalyzed by molten [Et3NH][HSO4]. Arab. J. Chem. 2012, 5, 485–488. [Google Scholar] [CrossRef]
- Zhou, Z.; Deng, X. [Et3NH][HSO4] Catalyzed efficient and green synthesis of 1,8-dioxo-octahydroxanthenes. J. Mol. Catal. A Chem. 2013, 367, 99–102. [Google Scholar] [CrossRef]
- Malla, A.M.; Parveen, M.; Ahmad, F.; Azaz, S.; Alam, M. [Et3NH][HSO4]-catalyzed eco-friendly and expeditious synthesis of thiazolidine and oxazolidine derivatives. RSC Adv. 2015, 5, 19552–19569. [Google Scholar] [CrossRef]
- Han, X.X.; Du, H.; Hung, C.T.; Liu, L.L.; Wu, P.H.; Ren, D.H.; Huang, S.J.; Liu, S.B. Syntheses of novel halogen-free Brønsted—Lewis acidic ionic liquid catalysts and their applications for synthesis of methyl caprylate. Green Chem. 2015, 17, 499–508. [Google Scholar] [CrossRef]
- Subhedar, D.D.; Shaikh, M.H.; Arkile, M.A.; Yeware, A.; Sarkar, D.; Shingate, B.B. Facile synthesis of 1,3-thiazolidin-4-ones as antitubercular agents. Bioorg. Med. Chem. Lett. 2016, 26, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Prasanna, P.; Perumal, S.; Menéndez, J.C. Chemodivergent, multicomponent domino reactions in aqueous media: L-proline catalyzed assembly of densely functionalized 4H-pyrano[2,3-c]pyrazoles and bispyrazolylpropanoates from simple, acyclic starting materials. Green Chem. 2013, 15, 1292–1299. [Google Scholar] [CrossRef]
- Tiwari, S.V.; Nikalje, A.G.; Lokwani, D.K.; Sarkate, A.P.; Jamir, K. Synthesis, biological evaluation, molecular docking study and acute oral toxicity study of coupled Imidazolyl-Pyrimidine derivatives. Lett. Drug Des. Discov. 2017, 14. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Nimbalkar, U.D.; Nikalje, A.G.; Netankar, P.D.; Lingampalle, D.L. Ionic liquid mediated synthesis of 5-arylidine-2,4-thiazolidinedionesand antibacterial evaluation. J. Med. Chem. Drug Discov. 2015, 1, 331–342. [Google Scholar]
- Shaikh, M.H.; Subhedar, D.D.; Khan, F.A.K.; Sangshetti, J.N.; Shingate, B.B. [Et3NH][HSO4]-catalyzed one-pot, solvent-free synthesis and biological evaluation of a-amino phosphonates. Res. Chem. Intermed. 2016, 2, 5115–5131. [Google Scholar] [CrossRef]
- Lin, C.M.; Ho, H.H.; Pettit, G.R.; Hamel, E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: Studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry 1989, 28, 6984–6991. [Google Scholar] [CrossRef] [PubMed]
- Lewis, S.A.; Gilmartin, M.E.; Hall, J.L.; Cowan, N.J. Three expressed sequences within the human betatubulin multigene family each define a distinct isotype. J. Mol. Biol. 1985, 182, 11–20. [Google Scholar] [CrossRef]
- Nicoletti, M.I.; Valoti, G.; Giannakakou, P.; Zhan, Z.; Kim, J.H.; Lucchini, V.; Landoni, F.; Mayo, J.G.; Giavazzi, R.; Fojo, T. Expression of beta-tubulin isotypes in human ovarian carcinoma xenografts and in a sub-panel of human cancer cell lines from the NCI-Anticancer Drug Screen: Correlation with sensitivity to microtubule active agents. Clin. Cancer Res. 2001, 7, 2912–2922. [Google Scholar] [PubMed]
- McKean, P.G.; Vaughan, S.; Gull, K. The extended tubulin superfamily. J. Cell Sci. 2001, 114, 2723–2733. [Google Scholar] [PubMed]
- Perez, E.A. Microtubule inhibitors: Differentiating tubulin-inhibiting agents based on mechanisms of action, clinical activity, and resistance. Mol. Cancer Ther. 2009, 8, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Kavallaris, M. Cancer. Nat. Rev. 2010, 10, 1–12. [Google Scholar]
- Chaudhary, A.; Sharma, P.P.; Bhardwaj, G.; Jain, V.; Bharatam, P.V.; Shrivastav, B.; Roy, R.K. Synthesis, biological evaluation, and molecular modeling studies of novel heterocyclic compounds as anti-proliferative agents. Med. Chem. Res. 2013, 12, 5654–5669. [Google Scholar] [CrossRef]
- Huang, Y.; Hickey, R.P.; Yeh, J.L.; Liu, D.; Dadak, A.; Young, L.H.; Johnson, R.S.; Giordano, F.J. Cardiacmyocyte-specific HIF-1alpha deletion alters vascularization, energy availability, calcium flux, and contractility in the normoxic heart. FASEB J. 2004, 18, 1138–1140. [Google Scholar] [PubMed]
- Lagorce, D.; Sperandio, H.; Miteva, M.; Villoutreix, B.O. FAF-Drugs2: Free ADME/tox filtering tool to assist drug discovery and chemical biology project. BMC Biol. Inform. 2008, 9, 396. [Google Scholar] [CrossRef] [PubMed]
- Sangshetti, J.; Khan, F.; Chouthe, R.; Damale, M.; Shinde, D. Synthesis, docking and ADMET prediction of novel 5-((5-substituted-1-H-1,2,4-triazol-3-yl)methyl)-4,5,6,7-tetrahydrothieno[3,2-c]pyridine as antifungal agents. Chin. Chem. Lett. 2014, 25, 1033–1038. [Google Scholar] [CrossRef]
- Lipinski, C.; Lombardo, F.; Dominy, B. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Abraham, M.H.; Lee, J.; Hersey, A.; Luscombe, C.N.; Beek, G.; Sherborne, B.; Cooper, I. Rate-limited steps of human oral absorption and QSAR studies. Pharm. Res. 2002, 19, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Rohde, B.; Selzer, P. Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties. J. Med. Chem. 2000, 43, 3714–3747. [Google Scholar] [CrossRef] [PubMed]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |

| Solvent | Temperature (°C) | Time (min) | Yield (%) |
|---|---|---|---|
| Polyethylene glycol (PEG) | 80 | 60 | 72 |
| Deep eutectic solvent of cholinechloride:urea | 80 | 20 | 92 |
| Ionic liquid (N-methylpyridiniumtosylate) | 120 | 75 | 62 |
| Ionic liquid (triethylammonium hydrogen sulphate[Et3NH][HSO4]) | R.T. | 15 | 94 |
| [Et3NH][HSO4] mol % | Time (min) | Yield (%) |
|---|---|---|
| - | 60 | 00 |
| 5 | 50 | 50 |
| 10 | 45 | 65 |
| 15 | 15 | 70 |
| 20 | 15 | 94 |
| 25 | 15 | 85 |
| Run | Time (min) | Yield (%) |
|---|---|---|
| 1 | 15 | 94 |
| 2 | 15 | 82 |
| 3 | 15 | 78 |
| 4 | 15 | 75 |
| Compounds | GI50 Against Cancer Cell Line in µM | Total Score -Logki | Crash Score | Polar Score | |||
|---|---|---|---|---|---|---|---|
| MAD-MB-231 | HeLa | SK-MEL-2 | K-562 | ||||
| 5b | 0.74 | >100 | <0.1 | 11.2 | 6.4039 | −0.9801 | 2.0598 |
| 5d | 25.76 | >100 | <0.1 | 6.41 | 5.4734 | −1.3871 | 4.449 |
| 5g | 2.02 | >100 | 2.92 | 40.73 | 4.6919 | −0.4209 | 5.4948 |
| 5h | 49.84 | >100 | 0.12 | 4.57 | 4.5247 | −0.184 | 3.7291 |
| 5j | 66.65 | >100 | <0.1 | 9.47 | 4.6555 | −0.7369 | 3.5549 |
| ADR | <0.1 | 0.03 | <0.1 | <0.1 | NA | NA | NA |
| CL2 | NA | NA | NA | NA | 5.1969 | −0.8748 | 3.5552 |
| Lig_ID | %ABS (<100%) | MW (<500) | LogP (<5) | PSA (<150) | n-RotB (<10) | n-RigB (<25) | HBD (<5) | HBA (<10) | RatioH/C (<1) | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 78.64 | 252.3 | 2.64 | 87.72 | 1 | 17 | 2 | 2 | 0.357 | Non Toxic |
| 5b | 78.64 | 286.7 | 3.29 | 87.72 | 1 | 17 | 2 | 2 | 0.428 | Non Toxic |
| 5c | 78.64 | 270.3 | 2.78 | 87.72 | 1 | 17 | 2 | 2 | 0.428 | Non Toxic |
| 5d | 75.55 | 282.3 | 2.65 | 96.95 | 2 | 17 | 2 | 3 | 0.4 | Non Toxic |
| 5e | 71.76 | 268.3 | 2.34 | 108 | 1 | 17 | 3 | 3 | 0.428 | Non Toxic |
| 5f | 68.57 | 298.3 | 2.35 | 117.2 | 2 | 17 | 3 | 4 | 0.466 | Non Toxic |
| 5g | 72.37 | 312.3 | 2.65 | 106.2 | 3 | 17 | 2 | 4 | 0.437 | Non Toxic |
| 5h | 62.93 | 297.3 | 3.07 | 127.7 | 2 | 18 | 2 | 4 | 0.571 | Non Toxic |
| 5i | 78.64 | 258.3 | 2.7 | 116 | 1 | 16 | 2 | 3 | 0.5 | Non Toxic |
| 5j | 75.55 | 358.4 | 4.22 | 96.95 | 4 | 23 | 2 | 3 | 0.5 | Non Toxic |
| CL2 | 75 | 252.3 | 2.64 | 87.72 | 1 | 17 | 2 | 2 | 0.357 | Non Toxic |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nimbalkar, U.D.; Seijas, J.A.; Vazquez-Tato, M.P.; Damale, M.G.; Sangshetti, J.N.; Nikalje, A.P.G. Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds. Molecules 2017, 22, 1628. https://doi.org/10.3390/molecules22101628
Nimbalkar UD, Seijas JA, Vazquez-Tato MP, Damale MG, Sangshetti JN, Nikalje APG. Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds. Molecules. 2017; 22(10):1628. https://doi.org/10.3390/molecules22101628
Chicago/Turabian StyleNimbalkar, Urja D., Julio A. Seijas, Maria Pilar Vazquez-Tato, Manoj G. Damale, Jaiprakash N. Sangshetti, and Anna Pratima G. Nikalje. 2017. "Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds" Molecules 22, no. 10: 1628. https://doi.org/10.3390/molecules22101628
APA StyleNimbalkar, U. D., Seijas, J. A., Vazquez-Tato, M. P., Damale, M. G., Sangshetti, J. N., & Nikalje, A. P. G. (2017). Ionic Liquid-Catalyzed Green Protocol for Multi-Component Synthesis of Dihydropyrano[2,3-c]pyrazoles as Potential Anticancer Scaffolds. Molecules, 22(10), 1628. https://doi.org/10.3390/molecules22101628