Pharmacokinetics, Tissue Distribution, and Elimination of Three Active Alkaloids in Rats after Oral Administration of the Effective Fraction of Alkaloids from Ramulus Mori, an Innovative Hypoglycemic Agent
Abstract
:1. Introduction
2. Results
2.1. Pharmacokinetic Studies of Three Alkaloids
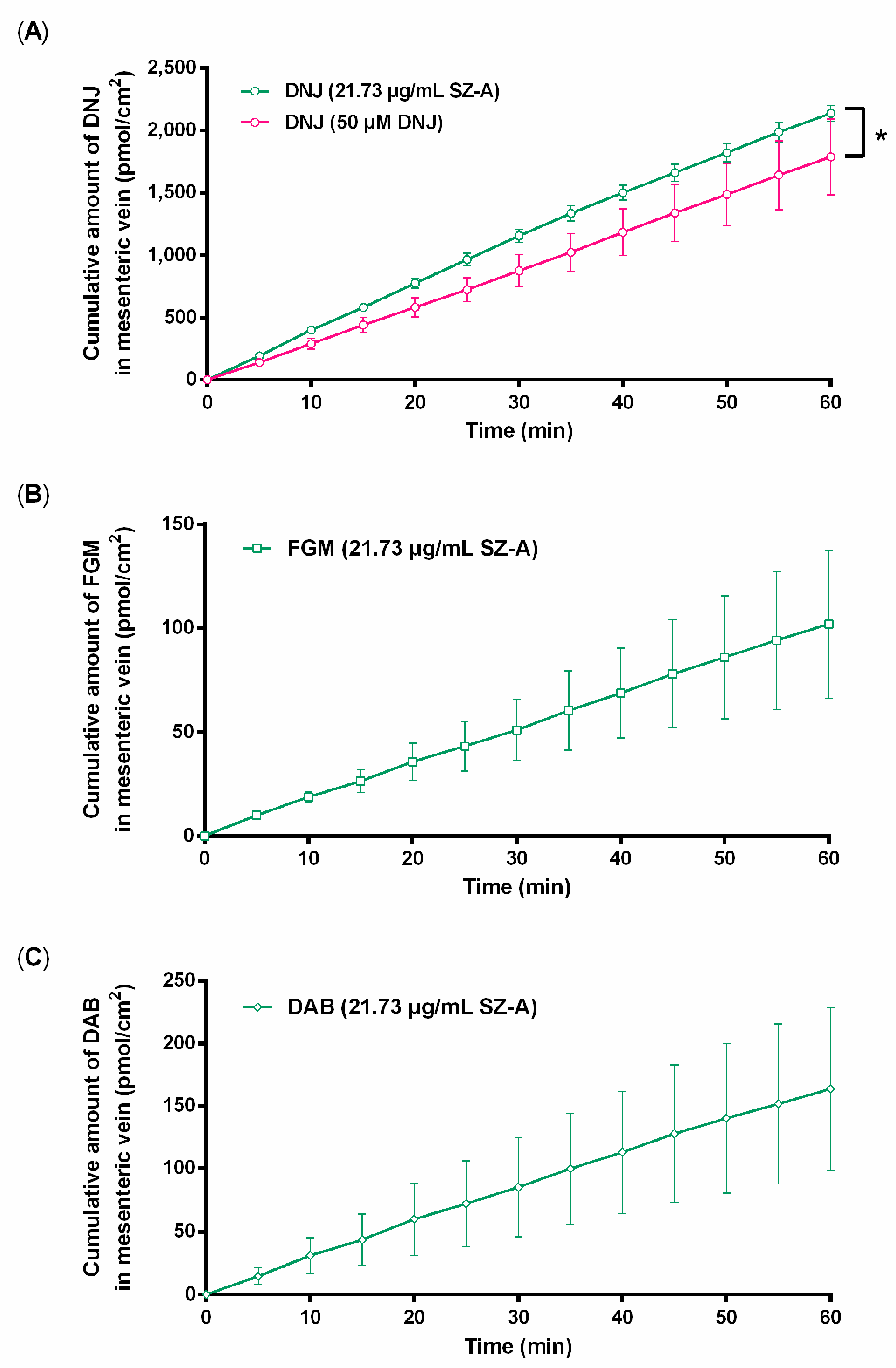
2.2. Impacts of Other Components in SZ–A on Absorption of DNJ
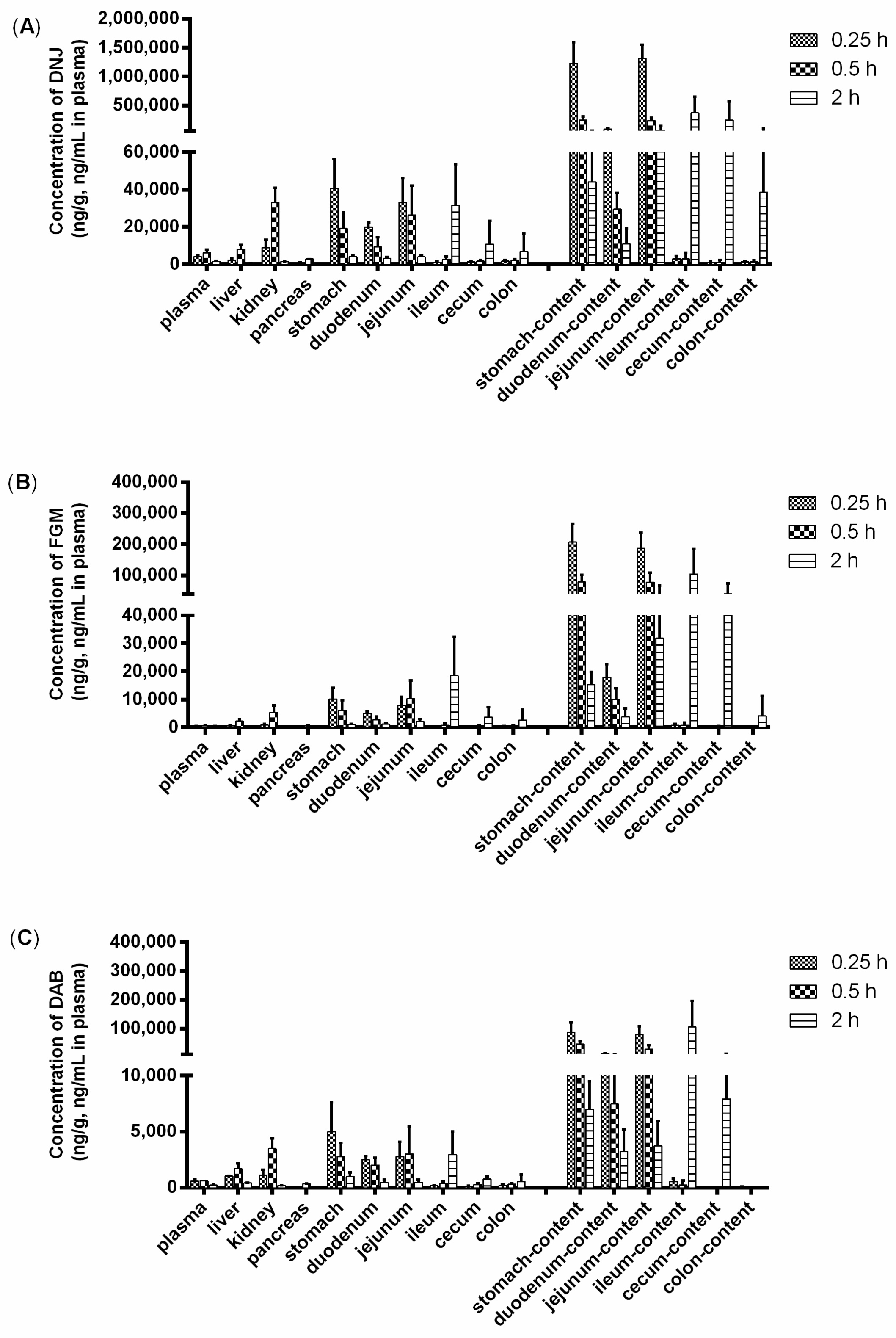
2.3. Tissue Distribution
2.4. Excretion of Three Alkaloids
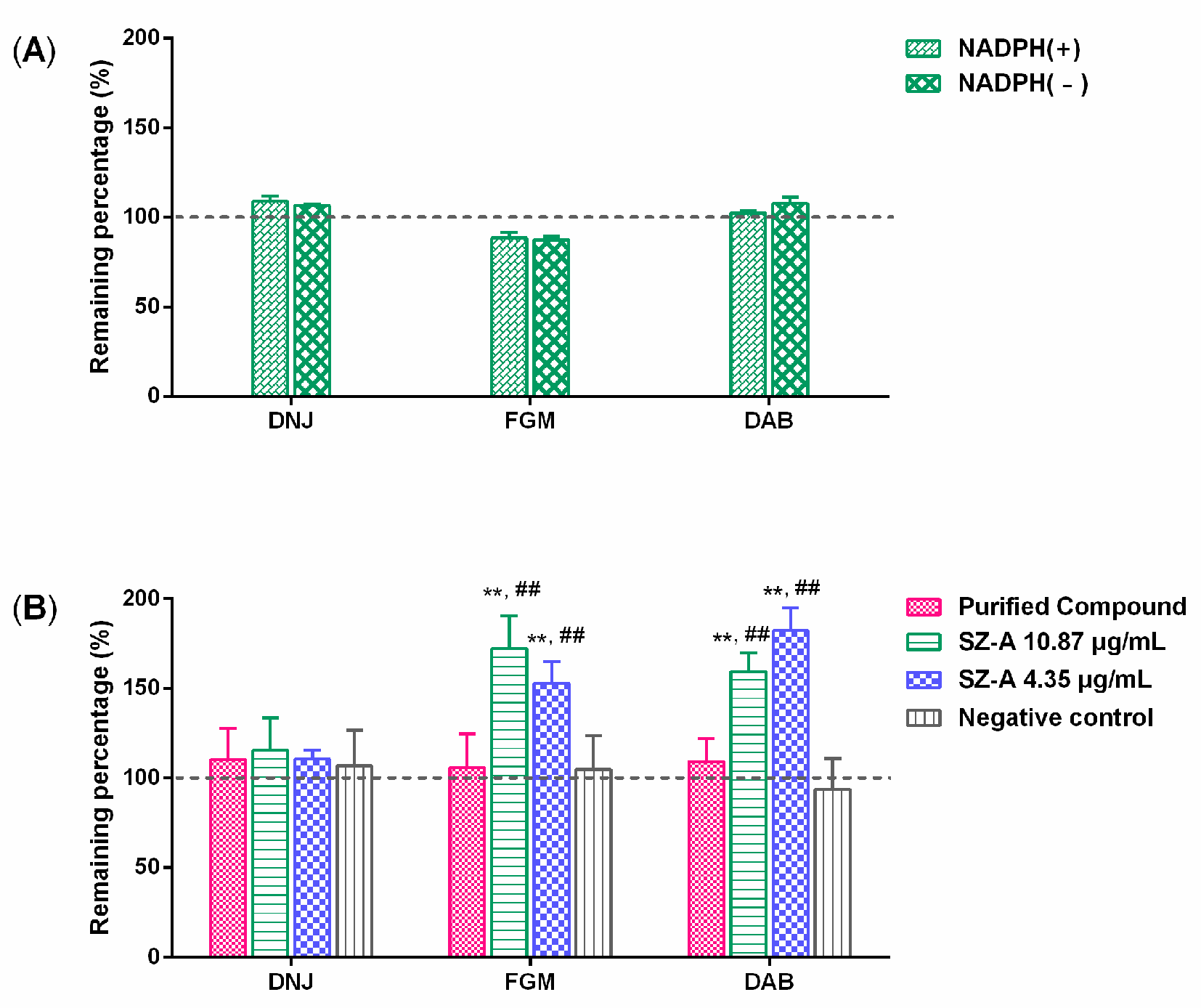
2.5. Biotransformation of Other Components in SZ–A
3. Discussion
4. Materials and Methods
4.1. Chemical and Reagents
4.2. Animals
4.3. Pharmacokinetic Study
4.4. Single-Pass Intestinal Perfusion In Situ
4.5. Tissue Distribution
4.6. Excretion
4.7. In Vitro Incubation of SZ–A with Rat Intestinal Homogenate and Caecal Microbiota Cultures
4.8. Sample Preparation and LC-MS/MS Analysis
4.9. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kitabchi, A.E.; Umpierrez, G.E.; Miles, J.M.; Fisher, J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care 2009, 32, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Federation, I.D. Idf Diabetes Atlas 7th Edition. Available online: http://www.diabetesatlas.org/ (accessed on 29 November 2015).
- Wang, Z.; Wang, J.; Chan, P. Treating type 2 diabetes mellitus with traditional Chinese and Indian medicinal herbs. Evid. Based Complement. Alternat. Med. 2013, 2013, 343594. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.J.; Spencer, C.M. Miglitol: A review of its therapeutic potential in type 2 diabetes mellitus. Drugs 2000, 59, 521–549. [Google Scholar] [CrossRef] [PubMed]
- Bolen, S.; Wilson, L.; Vassy, J.; Feldman, L.; Yeh, J.; Marinopoulos, S.; Wilson, R.; Cheng, D.; Wiley, C.; Selvin, E.; et al. Comparative Effectiveness and Safety of Oral Diabetes Medications for Adults with Type 2 Diabetes; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2007. [Google Scholar]
- Ye, F.; Shen, Z.; Xie, M. α-glucosidaseinhibition from a chinese medical herb (Ramulus Mori) in normal and diabetic rats and mice. Phytomedicine 2002, 9, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Shen, Z.F.; Qiao, F.X.; Zhao, D.Y.; Xie, M.Z. Experimental treatment of complications in alloxan diabetic rats with α-glucosidase inhibitor from the Chinese medicinal herb Ramulus Mori. Yao Xue Xue Bao 2002, 37, 108–112. [Google Scholar] [PubMed]
- Liu, Y.; Shen, Z.; Chen, Z.; Wang, R.; Xia, X.; Chen, Y.; Liu, Q.; Sun, S.; Xie, M. Use of the Effective Fraction of Alkaloids from Mulberry Twig in Preparing Hypoglycemic Agents. U.S. Patent 12/674532, 30 June 2015. [Google Scholar]
- Li, M.; Huang, X.; Ye, H.; Chen, Y.; Yu, J.; Yang, J.; Zhang, X. Randomized, double-blinded, double-dummy, active-controlled, and multiple-dose clinical study comparing the efficacy and safety of mulberry twig (Ramulus Mori, Sangzhi) alkaloid tablet and acarbose in individuals with type 2 diabetes mellitus. Evid. Based Complement. Alternat. Med. 2016, 2016, 10. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kubota, H.; Kimura, T.; Yamashita, S.; Tsuzuki, T.; Oikawa, S.; Miyazawa, T. Occurrence of orally administered mulberry 1-deoxynojirimycin in rat plasma. J. Agric. Food Chem. 2007, 55, 8928–8933. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wang, B.; Xia, X.; Li, X.; Wang, R.; Sheng, L.; Li, D.; Liu, Y.; Li, Y. Simultaneous quantification of three active alkaloids from a traditional Chinese medicine Ramulus Mori (Sangzhi) in rat plasma using liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015, 109, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Mackay, P.; Ynddal, L.; Andersen, J.V.; McCormack, J.G. Pharmacokinetics and anti-hyperglycaemic efficacy of a novel inhibitor of glycogen phosphorylase, 1,4-dideoxy-1,4-imino-d-arabinitol, in glucagon-challenged rats and dogs and in diabetic ob/ob mice. Diabetes Obes. Metab. 2003, 5, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Kwon, H.J.; Jung, J.Y.; Kwon, H.Y.; Baek, J.G.; Kim, Y.-S.; Kwon, O. Comparison of absorption of 1-deoxynojirimycin from mulberry water extract in rats. J. Agric. Food Chem. 2010, 58, 6666–6671. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Wang, Q.; Fan, L.; Kong, L.; Guo, S.; Chang, Q. Pharmacokinetic mechanism of enhancement by Radix Pueraria flavonoids on the hyperglycemic effects of Cortex Mori extract in rats. J. Ethnopharmacol. 2014, 151, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, G.; Ramanathan, S.; Nair, N.K.; Mansor, S.M.; Sattar, M.A.; Khan, M.A.; Navaratnam, V. Permeability of atenolol and propranolol in the presence of dimethyl sulfoxide in rat single-pass intestinal perfusion assay with liquid chromatography/UV detection. Biomed. Chromatogr. 2007, 21, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Bollen, M.; Vandebroeck, A.; Stalmans, W. 1-deoxynojirimycin and related compounds inhibit glycogenolysis in the liver without affecting the concentration of phosphorylase a. Biochem. Pharmacol. 1988, 37, 905–909. [Google Scholar] [CrossRef]
- Bollen, M.; Stalmans, W. The antiglycogenolytic action of 1-deoxynojirimycin results from a specific inhibition of the α-1,6-glucosidase activity of the debranching enzyme. Eur. J. Biochem. 1989, 181, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Reinkensmeier, G.; Butters, T.D.; Dwek, R.A.; Platt, F.M. Inhibition of glycogen breakdown by imino sugars in vitro and in vivo. Biochem. Pharmacol. 2004, 67, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Kato, A.; Asano, N.; Kizu, H.; Matsui, K. Fagomine isomers and glycosides from Xanthocercis zambesiaca. J. Nat. Prod. 1997, 60, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Padró, M.; Castillo, J.; Gómez, L.; Joglar, J.; Clapés, P.; Bolós, C. Cytotoxicity and enzymatic activity inhibition in cell lines treated with novel iminosugar derivatives. Glycoconj. J. 2010, 27, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Gómez, L.; Molinar-Toribio, E.; Calvo-Torras, M.Á.; Adelantado, C.; Juan, M.E.; Planas, J.M.; Cañas, X.; Lozano, C.; Pumarola, S.; Clapés, P.; et al. D-fagomine lowers postprandial blood glucose and modulates bacterial adhesion. Br. J. Nutr. 2012, 107, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Westergaard, N.; Quistorff, B.; Grunnet, N.; Kristiansen, M.; Lundgren, K. Kinetic and functional characterization of 1,4-dideoxy-1,4-imino-d-arabinitol: A potent inhibitor of glycogen phosphorylase with anti-hyperglyceamic effect in ob/ob mice. Arch. Biochem. Biophys. 2000, 380, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Varma, M.V.; Panchagnula, R. Prediction of in vivo intestinal absorption enhancement on P-glycoprotein inhibition, from rat in situ permeability. J. Pharmacol. Sci. 2005, 94, 1694–1704. [Google Scholar] [CrossRef] [PubMed]
- Tremaroli, V.; Backhed, F. Functional interactions between the gut microbiota and host metabolism. Nature 2012, 489, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Kolars, J.C.; Awni, W.M.; Merion, R.M.; Watkins, P.B. First-pass metabolism of cyclosporin by the gut. Lancet 1991, 338, 1488–1490. [Google Scholar] [CrossRef]
- Paine, M.F.; Shen, D.D.; Kunze, K.L.; Perkins, J.D.; Marsh, C.L.; McVicar, J.P.; Barr, D.M.; Gillies, B.S.; Thummel, K.E. First-pass metabolism of midazolam by the human intestine. Clin. Pharmacol. Ther. 1996, 60, 14–24. [Google Scholar] [CrossRef]
- Sousa, T.; Paterson, R.; Moore, V.; Carlsson, A.; Abrahamsson, B.; Basit, A.W. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int. J. Pharm. 2008, 363, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Hu, J.; Li, X.; Li, Y. Role of P-glycoprotein in intestinal absorption of fb2, a promising abl/src dual tyrosine kinase inhibitor. Drug Metab. Pharmacok. 2012, 27, 486–494. [Google Scholar] [CrossRef]
Sample Availability: Samples of SZ–A, DNJ, FGM and DAB are available from the authors. |



| Analyte | Group | Cmax | Tmax | t1/2 | AUC0–t | AUC0–∞ | Vd | CLz | MRT0–t | MRT0–∞ | F |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (ng/mL) | (h) | (h) | (h * ng/mL) | (h * ng/mL) | (mL/kg) | (mL/h/kg) | (h) | (h) | (%) | ||
| DNJ | SZ–A 40 mg/kg (p.o.) | 6370.0 ± 1116.9 | 0.67 ± 0.29 | 1.30 ± 0.25 | 10,114.3 ± 1412.0 | 10,153.0 ± 1444.6 | 2830.1 ± 741.1 | 1500.8 ± 195.5 | 1.71 ± 0.67 | 1.78 ± 0.69 | 72.41 |
| SZ–A 200 mg/kg (p.o.) | 10,482.2 ± 2351.8 | 0.67 ± 0.20 | 2.11 ± 0.47 | 26,963.6 ± 3750.6 | 27,032.7 ± 3735.8 | 8617.8 ± 2317.5 | 2823.7 ± 401.6 | 2.63 ± 0.48 | 2.71 ± 0.49 | 38.61 | |
| SZ–A 1000 mg/kg (p.o.) | 25,090.5 ± 5126.1 | 0.43 ± 0.09 | 3.52 ± 0.85 | 116,270.3 ± 10547.8 | 116,695.9 ± 10709.1 | 16,468.3 ± 4217.0 | 3242.7 ± 298.1 | 5.12 ± 1.48 | 5.25 ± 1.53 | 33.29 | |
| SZ–A 4 mg/kg (i.v.) | 6083. 1 ± 1549.9 | 0.033 | 0.29 ± 0.08 | 1396.9 ± 174.5 | 1399.2 ± 174.6 | 453.4 ± 130.4 | 1086.5 ± 127.7 | 0.80 ± 0.50 | 0.82 ± 0.51 | ||
| DNJ 15 mg/kg (p.o.) | 4863.3 ± 1116.4 | 0.67 ± 0.29 | 0.88 ± 0.14 * | 6731.5 ± 1146.2 ** | 6758.6 ± 1124. 1 ** | 2826.9 ± 347. 8 | 2261.6 ± 382.4 * | 1.77 ± 0.25 | 1.85 ± 0.21 | 59.36 | |
| DNJ 1.5 mg/kg (i.v.) | 4522.4 ± 576.1 # | 0.03 | 0.25 ± 0.05 | 1133.9 ± 108.1 ## | 1137.2 ± 108.4 ## | 486.1 ± 85.40 | 1332.6 ± 128.4 # | 0.48 ± 0.08 | 0.50 ± 0.08 | ||
| FGM | SZ–A 40 mg/kg (p.o.) | 1489.7 ± 756.0 | 0.67 ± 0.29 | 0.93 ± 0.29 | 2156.9 ± 778.3 | 2170.2 ± 770.4 | 1894.0 ± 1357.8 | 1317.9 ± 584.9 | 1.41 ± 0.27 | 1.48 ± 0.34 | 77.50 |
| SZ–A 200 mg/kg (p.o.) | 2246.6 ± 1064.4 | 0.57 ± 0.09 | 1.44 ± 0.16 | 5355.7 ± 1489.8 | 5404.0 ± 1471.7 | 5332.0 ± 1855.9 | 2557.5 ± 786.6 | 2.66 ± 0.52 | 2.78 ± 0.54 | 38.49 | |
| SZ–A 1000 mg/kg (p.o.) | 4388.6 ± 2361.4 | 1.30 ± 0.67 | 2.73 ± 1.16 | 20,801.4 ± 7026.0 | 21,753.6 ± 6005.6 | 14,243.4 ± 9987.6 | 3385.8 ± 1262.0 | 5.44 ± 1.77 | 6.15 ± 2.11 | 29.90 | |
| SZ–A 4 mg/kg (i.v.) | 1240.4 ± 304.0 | 0.033 | 0.23 ± 0.03 | 278.3 ± 36.81 | 284.2 ± 36.69 | 308.1 ± 60.98 | 922.8 ± 109.5 | 0.26 ± 0.06 | 0.30 ± 0.06 | ||
| DAB | SZ–A 40 mg/kg (p.o.) | 708.0 ± 171.3 | 0.67 ± 0.29 | 1.22 ± 0.16 | 968.9 ± 147.2 | 978.2 ± 140.5 | 3548.6 ± 990.1 | 1991.3 ± 298.6 | 1.32 ± 0.18 | 1.42 ± 0.25 | 78.23 |
| SZ–A 200 mg/kg (p.o.) | 1997.3 ± 655.7 | 0.57 ± 0.09 | 1.38 ± 0.23 | 3506.2 ± 716.0 | 3555.0 ± 729.0 | 5472.4 ± 571.7 | 2815.7 ± 563.4 | 1.95 ± 0.35 | 2.09 ± 0.29 | 56.62 | |
| SZ–A 1000 mg/kg (p.o.) | 3771. 4 ± 1457.8 | 0.47 ± 0.14 | 2.38 ± 0.97 | 12,412.0 ± 2823.4 | 12,946.9 ± 2691.1 | 13,892.2 ± 7160.0 | 3926.0 ± 882.8 | 4.09 ± 1.19 | 4.66 ± 1.52 | 40.08 | |
| SZ–A 4 mg/kg (i.v.) | 624.9 ± 182.7 | 0.033 | 0.18 ± 0.03 | 123.9 ± 20.82 | 128.6 ± 20.07 | 400.7 ± 40.84 | 1535.6 ± 239.2 | 0.18 ± 0.04 | 0.21 ± 0.05 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Mi, J.; Liu, Z.; Wang, B.; Xia, X.; Wang, R.; Liu, Y.; Li, Y. Pharmacokinetics, Tissue Distribution, and Elimination of Three Active Alkaloids in Rats after Oral Administration of the Effective Fraction of Alkaloids from Ramulus Mori, an Innovative Hypoglycemic Agent. Molecules 2017, 22, 1616. https://doi.org/10.3390/molecules22101616
Yang S, Mi J, Liu Z, Wang B, Xia X, Wang R, Liu Y, Li Y. Pharmacokinetics, Tissue Distribution, and Elimination of Three Active Alkaloids in Rats after Oral Administration of the Effective Fraction of Alkaloids from Ramulus Mori, an Innovative Hypoglycemic Agent. Molecules. 2017; 22(10):1616. https://doi.org/10.3390/molecules22101616
Chicago/Turabian StyleYang, Shuang, Jiaqi Mi, Zhihao Liu, Baolian Wang, Xuejun Xia, Renyun Wang, Yuling Liu, and Yan Li. 2017. "Pharmacokinetics, Tissue Distribution, and Elimination of Three Active Alkaloids in Rats after Oral Administration of the Effective Fraction of Alkaloids from Ramulus Mori, an Innovative Hypoglycemic Agent" Molecules 22, no. 10: 1616. https://doi.org/10.3390/molecules22101616
APA StyleYang, S., Mi, J., Liu, Z., Wang, B., Xia, X., Wang, R., Liu, Y., & Li, Y. (2017). Pharmacokinetics, Tissue Distribution, and Elimination of Three Active Alkaloids in Rats after Oral Administration of the Effective Fraction of Alkaloids from Ramulus Mori, an Innovative Hypoglycemic Agent. Molecules, 22(10), 1616. https://doi.org/10.3390/molecules22101616






