Abstract
In the presence of transition metal ions and peroxides, polyphenols, well-known dietary antioxidants, can act as pro-oxidants. We investigated the effect of 13 polyphenols and their metabolites on oxidative degradation of deoxyribose by an •OH generating Fenton system (Fe2+-ethylenediaminetetraacetic acid (EDTA)-H2O2). The relationship between phenolics pro-oxidant/anti-oxidant effects and their molecular structure was analyzed using multivariate analysis with multiple linear regression and a backward stepwise technique. Four phenolics revealed a significant inhibitory effect on OH-induced deoxyribose degradation, ranging from 54.4% ± 28.6% (3,4-dihydroxycinnamic acid) to 38.5% ± 10.4% (catechin) (n = 6), correlating with the number of –OH substitutions (r = 0.58). Seven phenolics augmented the oxidative degradation of deoxyribose with the highest enhancement at 95.0% ± 21.3% (quercetin) and 60.6% ± 12.2% (phloridzin). The pro-oxidant effect correlated (p < 0.05) with the number of –OH groups (r = 0.59), and aliphatic substitutes (r = −0.22) and weakly correlated with the occurrence of a catechol structure within the compound molecule (r = 0.17). Selective dietary supplementation with phenolics exhibiting pro-oxidant activity may increase the possibility of systemic oxidative stress in patients treated with medications containing chelating properties or those with high plasma concentrations of H2O2 and non-transferrin bound iron.
1. Introduction
Plant derived polyphenolic compounds exhibit a wide range of antioxidant properties, which have been the subject of considerable research activity in recent years. These antioxidant properties can act as radical scavengers, terminators of free radical chain reactions and chelators of redox active metal ions [1]. Their antioxidant capacity is contingent on their molecular structure and has been shown to be more effective antioxidants in vitro than vitamins E and ascorbic acid on a molar basis [2]. Polyphenols may act directly on reactive oxygen species (ROS) or stimulate endogenous defense systems. They can inhibit the ability of myeloperoxidase to oxidize low-density lipoproteins, a potential anti-atherosclerotic effect [3]. Other potential antioxidant effects include the ability to inhibit cyclooxygenases (prostaglandin-endoperoxide synthase), lipoxygenases and NADPH oxidases [3]. Oxidation of polyphenols produces superoxide radicals (O2−•), hydrogen peroxide (H2O2) and an intricate composition of semiquinones and quinones all of which are potentially cytotoxic; however, quinone reductase, catechol-O-methyltransferase, and other conjugating enzymes limit the formation of such quinones in vivo [3]. Not surprisingly, there has been some effort to understand the pro-oxidant properties of some polyphenols in the presence of transition metals (Cu, Fe) and or in certain concentrations or a high pH accelerating hydroxyl radical (•OH) formation and oxidative strand breakage in DNA in vitro [2].
Green tea catechins, epicatechin and epigallocatechin enhanced Cu+-H2O2-mediated DNA damage, while myricetin, protocatechuic acid and epigallocatechin-3-gallate displayed an antioxidant effect [4]. On the other hand, gallic acid (GA) and epicatechin-3-gallate had both pro-oxidant and antioxidant activity depending on the concentration under the conditions of this in vitro model [4]. Aqueous and ethyl acetate extracts of rooibos leaves (used for redbush tea preparation) as well as dihydrochalcone aspalathin and tannin enhanced deoxyribose (a sugar component of DNA) oxidative degradation by the •OH generating system Fe3+-EDTA-H2O2 [5]. In addition, numerous phenolic acids revealed a dual effect on •OH (Fe2+-H2O2-EDTA system) mediated deoxyribose damage depending on the time of incubation: in the first several seconds, they inhibited and then later revealed an enhancing (pro-oxidant) effect [6]. Conversely, red wine polyphenols: resveratrol, quercetin, and caffeic acid inhibited deoxyribose degradation by two •OH generating systems: Fe3+-H2O2-ascorbate, and Fe3+-EDTA-H2O2-ascorbate [7]. In addition, polyphenol rich extracts of Moringa oleifera Lam (Moringaceae) inhibited DNA strand breaks induced by a Fe3+-H2O2-ascorbate system [8]. Moreover, the majority of the tested Mauritian endemic plant extracts revealed a protective effect on deoxyribose against the Fenton system supplemented with ascorbic acid [9]. These examples clearly show that polyphenols in general have a protective effect against Fenton systems supplemented with ascorbic acid, while, in Fenton systems without ascorbic acid, the situation is more complex, and these compounds can act as pro-oxidants or antioxidants and can have dual activity depending on their concentration in the reaction mixture [10,11,12]. Therefore, in this study, we investigated the effect of thirteen selected plant phenolics on deoxyribose oxidation induced by the Fenton system composed of Fe2+-H2O2-EDTA. Furthermore, the relationship between the phenolic pro-oxidant/antioxidant effect, their molecular structure and the ability to reduce Fe3+ ions into Fe2+ (ferric-reducing ability power—FRAP) was analyzed.
2. Results
2.1. Validity of Experimental Conditions
Incubation of deoxyribose with incomplete (Fe2+-EDTA) and complete (Fe2+-EDTA-H2O2) Fenton systems and subsequent boiling with trichloroacetic acid (TCA) and thiobarbituric acid (TBA) solutions resulted in the formation of a chromogen, which gave rise to a sample absorbance at 532 nm (A532). As expected, an Fe2+-EDTA-H2O2-induced rise of A532 was many-times higher than that caused by an incomplete Fenton system. On the other hand, baseline A532 of deoxyribose alone was lower than in the case of deoxyribose with Fe2+-EDTA. This may result from the presence of trace amounts of peroxides in water [13]. Phenolics alone and in combination with deoxyribose did not induce formation of additional chromogen. In addition, incubation of all tested polyphenols with H2O2 did not result in the formation of oxidation products increasing A532. All of the afore-mentioned relationships were observed in all series of experiments on the effect of phenolics on the oxidative degradation of deoxyribose; furthermore, they ensured a high validity of obtained results. DMSO as a potent •OH scavenger [14] inhibited deoxyribose oxidation; accordingly, this activity was taken into account in the calculation of the antioxidant or pro-oxidant effect of polyphenols.
2.2. Inhibitory Effect of Polyphenols on the Oxidative Degradation of Deoxyribose by the Fenton System
The ability to inhibit the oxidative degradation of deoxyribose by a •OH generating system (Fe2+-EDTA-H2O2) was revealed by five of the 13 tested polyphenols in vitro (Table 1). Four out of the five inhibiting compounds (3,4-dihydroxycinnamic acid (3,4-DCA), 4-hydroxybenzoic acid (4-HBA), 3,4-dihydroxyhydrocinnamic acid ((3,4-DHCA) and catechin) significantly protected deoxyribose from oxidative degradation. This protection ranged from 54.4% ± 28.6% for 3,4-DCA to 38.5% ± 10.4% for catechin; however, no statistically significant differences between the compounds were observed. Furthermore, although the mean inhibition of deoxyribose oxidation by chlorogenic acid (CA) was 7.5%, this did not reach statistical significance (p > 0.05).

Table 1.
Inhibitory (antioxidant) effect of polyphenols on the oxidative degradation of deoxyribose by the system (Fe2+-EDTA-H2O2).
2.3. Enhancing Effect of Polyphenols on the Oxidative Degradation of Deoxyribose by the Fenton System
Seven polyphenols enhanced the oxidative degradation of deoxyribose under the Fe2+-EDTA-H2O2 system (Table 2). The highest pro-oxidative effect resulting in a mean enhancement of deoxyribose oxidation by 95.0% and 60.6% was observed for quercetin and phloridzin, respectively. Ferulic acid (FA) was without significant pro-oxidant activity (Table 2). Ascorbic acid, at a concentration of 10 μmol/L (as a standard of pro-oxidant activity) [15], enhanced the oxidative degradation of deoxyribose by 195.7% ± 19.5% (n = 6) under these conditions. Moreover, at the same concentration as the studied polyphenols, two other compounds known as antioxidants: TROLOX® and N-acetylcysteine [16] augmented the Fenton system-induced deoxyribose degradation by 8.1% ± 5.6% and 48.2% ± 31.2% (p < 0.05), respectively.

Table 2.
Enhancing (pro-oxidant) effect of polyphenols on the oxidative degradation of deoxyribose by the Fenton system (Fe2+- ethylenediaminetetraacetic acid (EDTA)-H2O2).
2.4. Factors Determining the Pro-Oxidant or Antioxidant Effect of Polyphenols on Deoxyribose Oxidation
Figure 1A shows the studied compounds (phenolics, TROLOX® and ascorbic acid) ranked (from left to right) according to their inhibiting (negative value) and enhancing effect (positive value) on the oxidative degradation of deoxyribose by the Fenton system. Below (Figure 1B), their FRAP at a concentration of 5 µmol/L [12] is shown. It clearly delineates that ascorbic acid, which revealed the highest FRAP, had the strongest pro-oxidant effect on deoxyribose oxidation. However, catechin and 3,4-DHCA having distinct FRAP significantly inhibited deoxyribose oxidation. Moreover, quercetin and 3,4-DPAA, the strongest Fe3+ reducing agents among the tested phenolics (Figure 1B), enhanced and inhibited deoxyribose oxidation, respectively. A lack of significant correlation between FRAP and the antioxidant/pro-oxidant effect in the group of phenolics (r = 0.22, p > 0.05) corresponded to the foregoing observations. However, when all compounds were analyzed, a linear correlation was revealed (r = 0.65, p < 0.001), probably due to the particularly high FRAP and pro-oxidant activity of ascorbic acid.
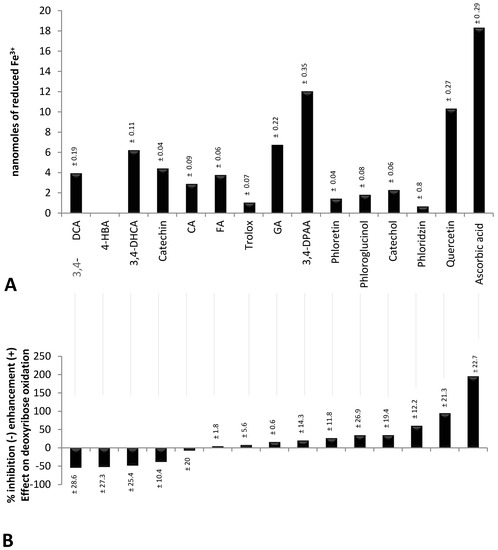
Figure 1.
(A) nanomoles of reduced Fe3+ by polyphenols, TROLOX® and ascorbic acid at a concentration of 5 µmol/L. 4-HBA observed no FRAP at this concentration; (B) percentage of inhibition (expressed as a negative value) and enhancement (expressed as a positive value) of deoxyribose oxidation by polyphenols in a concentration of 10 µmol/L. Deoxyribose oxidation was induced by a chemical system Fe2+-EDTA-H2O2; 3,4-DCA—3,4-dihydroxycinnamic acid, 4-HBA—4-hydroxybenzoic acid, 3,4-DHCA—3,4-dihydroxyhydrocinnamic acid, CA—chlorogenic acid, FA—ferulic acid, GA—gallic acid, 3,4-DPAA—3,4-dihydroxyphenylacetic acid, EDTA—ethylenediaminetetraacetic acid.
A multivariate analysis with multiple linear regressions was performed to determine which component of the molecular structure (aliphatic substituent, catechol ring, the number of –OH and –COOH) of polyphenols had a significant effect on the inhibition or enhancement of deoxyribose oxidation (Table 3). In the compounds inhibiting deoxyribose oxidation (Table 1), the correlation between the % inhibition and the number of –OH groups was significant, r = 0.58 (p = 0.004). The number of –OH groups in the compound molecule accounted for 34.6% of the variance in the inhibitory effect on deoxyribose oxidation (Table 3); however, this has some limitation due to a low number of analyzed compounds. Furthermore, three of the five compounds inhibited deoxyribose oxidation with similar intensity. With the compounds enhancing the oxidation of deoxyribose (Table 2), the number of –OH groups revealed a significant positive correlation r = 0.59 (p = 0.001) responsible for 32.7% of the variance in pro-oxidant activity (Table 3). This activity also correlated with the occurrence of a catechol structure within the compound molecule (r = 0.17, p = 0.05) and the presence of an aliphatic substitute at the catechol ring (r = −0.22, p = 0.04) as estimated with univariate analyses, respectively.

Table 3.
Factors influencing the anti-oxidant and pro-oxidant properties of studied plant phenolics—a summary of multivariate regression.
3. Discussion
In this study, we evaluated the effect of various polyphenols on the oxidative degradation of deoxyribose through a Fe2+-EDTA-H2O2 system. This system generates •OH via the Fenton reaction (Fe2+ + H2O2 → Fe3+ + OH− + •OH) while EDTA chelates Fe ions (Fe2+ or Fe3+), thereby preventing Fe ions from directly binding to deoxyribose [17,18]. Thus, •OH generated from the reaction of H2O2 with an Fe2+-EDTA complex enter the “free” milieu and can react with deoxyribose and any added scavenger (e.g., polyphenol molecule). The number of Fe2+ ions was the limiting factor for •OH production in this Fe2+-EDTA-H2O2 system. Therefore, the addition of any compound that can reduce Fe3+ into Fe2+ will further stimulate •OH release and subsequent deoxyribose oxidation leading to the rise of sample absorbance at 532 nm. This was noted when ascorbic acid was added to the reaction mixture. Although ascorbic acid itself can scavenge •OH radicals [19], it is a strong Fe3+ reducer [12] and thus enhanced deoxyribose degradation about twice as much under the conditions of our experiment. On the other hand, ascorbic acid did not enhance and, in fact, slightly inhibited the aromatic hydroxylation of salicylic acid via the Fe2+-EDTA-H2O2 system [20]. However, in these experiments, the concentration of Fe2+ ions and the ratio of Fe2+ to H2O2 were 30 and 10.5 times higher than in our study. Hence, ascorbate-induced regeneration of Fe2+ ions (via reduction of Fe3+) could have a weak enhancing effect on the generation of •OH radicals. Thus, the scavenging of •OH radicals by ascorbic acid prevailed and resulted in a weak inhibition of salicylic acid hydroxylation.
3.1. Plausible Mechanisms of the Anti- or Pro-Oxidant Activity of Polyphenols
While polyphenols can directly react with •OH [2], they also possess distinct FRAP activity [12]. Moreover, iron ions can form complexes with phenolics, which can decrease their reactivity with H2O2 [2,21]. However, excess EDTA, a strong chelator in the reaction mixture has been shown to prevent the binding of Fe2+ and Fe3+ to polyphenols [21,22]. Thus, under the conditions of our experiments, the net effect of a tested compound on deoxyribose degradation will be the sum of these two counteracting processes: scavenging of •OH and enhancement of •OH production via regeneration of Fe2+ ions. Seven of the thirteen tested polyphenols enhanced deoxyribose oxidation by an Fe2+-EDTA-H2O2 system, while four inhibited this process and two compounds had no significant effect. This differs from previous studies showing significant protection of deoxyribose by polyphenols against •OH-induced degradation [7,8,9]. For instance, quercetin protected deoxyribose degradation from a •OH generating system [7], while, in our experiments; it revealed a significant pro-oxidant effect. In the aforementioned studies, the antioxidant activities of polyphenols or polyphenols containing plant extracts were tested in Fe3+-H2O2-ascorbate or Fe3+-EDTA-H2O2-ascorbate systems [7,8,9]. Since the FRAP of ascorbic acid is stronger than that of phenolics, especially those tested in our study [12], the addition of a polyphenol to a reaction mixture containing Fe3+, H2O2 and ascorbate did not significantly increase the accessibility of Fe2+ ions for a reaction with H2O2 and the subsequent formation of •OH. Thus, the •OH scavenging activity of polyphenols prevailed and was responsible for the protective antioxidant effect under such conditions. In our experiments, ascorbic acid was not used for Fe2+ regeneration; however, such capability was evident to some extent by the tested polyphenols. Hence, seven phenolics revealed an enhancing pro-oxidant effect. Accordingly, our results are in line with studies demonstrating the pro-oxidant (enhancing •OH production) activity of polyphenols (e.g., myricetin, quercetin and catechin) in a Fe3+-EDTA-H2O2 system [23,24]. This effect was also observed in the presence of other metal chelators (adenosine diphosphate (ADP), bleomycin) [16,23,25] and disappeared when the •OH generating system was supplemented with ascorbic acid [24]. It is believed that polyphenols as well as other antioxidants (e.g., α-tocopherol, N-acetylcysteine) [16] can exert a pro-oxidant effect in systems containing Fe3+, H2O2 and a potent metal chelator that prevent binding of iron to the polyphenol [22]. However, we demonstrated that some phenolics as well as N-acetylcysteine and TROLOX® can also act as pro-oxidants in a •OH-generating system composed of Fe2+, EDTA and H2O2. Conversely, four phenolics (3,4-DCA, 4-HBA, 3,4-DHCA and catechin) showed a significant inhibitory effect on the oxidative degradation of deoxyribose through an Fe2+-EDTA-H2O2 system. This is revealing in that, under conditions that unmask and favor pro-oxidant activities, these phenolics behaved as antioxidants most probably due to direct scavenging of •OH.
3.2. Determinants of the Polyphenols Effect on Deoxyribose Oxidation by the Fenton System
Although the reduction of Fe3+ into Fe2+ is supposedly the main mechanism of phenolic pro-oxidant action, under the aforementioned conditions [22], there was no significant correlation between the effect of polyphenols on Fe2+-EDTA-H2O2-induced deoxyribose oxidation and their FRAP. This suggests the occurrence of other mechanisms responsible for the pro-oxidant activity of polyphenols. Polyphenols can undergo autoxidation especially in the presence of iron ions [26] with subsequent generation of H2O2 and O2−•. O2−• can reduce Fe3+ into Fe2+ and thus promote •OH generation. The inhibitory effect of superoxide dismutase on polyphenol (e.g., quercetin) induced acceleration of •OH formation by the Fe3+-EDTA-H2O2 system [23] supports this hypothesis. In addition, the reaction between Fe2+ and H2O2 can also produce ferryl ion (FeO2+) with an oxidizing potential that degrades deoxyribose to thiobarbituric acid reactive products [27]. On the other hand, the polyphenols’ FRAP was measured at a pH of 3.6 [12], while the effect on deoxyribose oxidation measured at a pH of 7.4. Moreover, the first variable was obtained for the concentration of phenolics at 5 µmol/L while the second was at 10 µmol/L. However, the FRAP of some polyphenols were linear within a wide range (up to 50 µmol/L for quercetin, GA or catechin), and the abovementioned reasons may account for the negative results seen in the correlation analysis. The protective effect of polyphenols correlated with the number of –OH substitutions in the backbone structure. It is possible that •OH can grab a hydrogen atom from one of the hydroxyl groups at the phenolic ring to form water and a less reactive and more stable radical. This corresponds well with a previous report demonstrating an intensification of the •OH scavenging activity of flavonoids with an increased number of –OH substituted in an aromatic B-ring [28]. Furthermore, hydroxyl groups (catechol group, –OH substitutions at position 3, 5, 7 and 4′) are critical for the effective scavenging of peroxynitrite by flavonoids [29], and the inhibition of total ROS generation in kidney homogenates by these compounds intensifies as the number of total –OH groups in their structure increases [30]. Previously, we demonstrated that the presence of a catechol group positively correlated with the ability of phenolics to reduce Fe3+ to Fe2+ [12]. In this process, both hydroxyl groups of catechol donate two electrons simultaneously losing two hydrogen atoms that results in the formation of a benzoquinone [12]. It is possible that other –OH substitutions could be electron donors for Fe3+ reduction [18]. Analysis of the antioxidant activity of eugenol (a phenolic compound and the main component of clove oil) derivatives obtained by the acylation and alkylation of the phenolic hydroxyl group underscores the important role of –OH as a hydrogen atom donor. Four of the 16 derivatives had efficient antioxidant properties; however, such properties were lost with the remaining 12 compounds [31]. Nonetheless, these results propose the presence of other antioxidant mechanisms in addition to those involving –OH substitutions in the phenolic ring [31], which may be responsible for the previously discussed lack of correlation between the effect of phenolics under the Fenton system-induced oxidation of deoxyribose and their FRAP. Since the reduction of Fe3+ into Fe2+ maintained a high generation of •OH under the Fe2+-EDTA-H2O2 system, this may well explain the positive correlation between the enhanced oxidative degradation of deoxyribose, the presence of a catechol group and the total number of –OH substitutions in phenolics with pro-oxidant activities. Hydroxyl groups can directly scavenge •OH radicals [18,28] and also (especially those present in the catechol structure) reduce Fe3+ ions [12]. Both of these activities are recognized as measures of antioxidant potential [32,33]. However, under the Fe2+-EDTA-H2O2 system, the first activity decreased the number of •OH, thus acting as a protectant against the oxidative degradation of deoxyribose, and the second activity enhanced •OH production operating in fact as a pro-oxidant. These activities may explain the seemingly contradictory dual role of these compounds: positive correlation between the total number of –OH substitutions and the pro-oxidant and antioxidant properties of phenolics, respectively. Aliphatic substitutes (e.g., –CH3, –CH2–CH3) in the catechol ring can increase the ability of its –OH groups to be a superior donor of electrons, thus intensifying the potential to reduce Fe3+ ions [34]. The negative correlation between the presence of an aliphatic substitute and pro-oxidant phenolic activity is in contrast with the preceding data, thus its elucidation requires further studies.
3.3. Applicability to In Vivo Conditions
H2O2 is detectable in circulating blood and its concentration in human plasma can even reach 80 µmol/L in some pathological conditions [35]. In plasma, the vast majority of iron is bound to transferrin, thus, in healthy subjects, the non-transferrin bound iron that can be involved in ROS generation does not exceed 1 µmol/L. However, this pool of iron can be higher than 15 µmol/L in patients with end stage renal insufficiency or cancer patients undergoing chemotherapy [36]. In healthy subjects, the plasma concentration of phenolics (e.g., 3,4-DCA, 3,4-DHCA, and 3,4-DPAA) ranged between 0.1 to 6 µmol/L [37]. Irrespective of its clinical efficacy, chelation therapy with disodium EDTA has long been used to treat atherosclerotic coronary and peripheral artery disease. It was estimated that roughly 100,000 patients in the United States alone underwent such therapy in 2007 [38]. The plasma EDTA levels in patients that underwent chelation therapy ranged from 0.2 to 1 mmol/L [39]. In all, these studies demonstrate that a reaction between Fe2+-EDTA-H2O2 and selected phenolics can occur in circulating blood in humans and alter •OH generation. Although the concentration of reagents (especially H2O2) used in our in vitro experiments differ from those found in vivo, a study showing essential acute oxidative injury to circulating lipids, proteins and leukocyte DNA after the addition of ascorbic acid to a standard chelation therapy cocktail containing EDTA in male and female subjects [40] substantiates the applicability of this hypothesis. On the other hand, in healthy subjects, the interaction of ingested polyphenols with iron ions and H2O2 is more probable. In this case, polyphenols can form complexes with Fe2+ and change its reactivity with H2O2. Thus, the net effect of polyphenols on •OH generation will depend on at least three factors: direct •OH scavenging, reduction of Fe3+ ions to Fe2+ promoting •OH generation and the formation of polyphenol-iron complexes with an increased or decreased potential to partially reduce H2O2 to •OH.
4. Materials and Methods
4.1. Reagents
Ascorbic acid, dimethyl sulfoxide (DMSO), FeSO4, TROLOX® (a water-soluble analog of vitamin E), TBA, TCA, disodium EDTA, N-acetylcysteine, H2O2 30% solution (w/w) and 2-deoxy-d-ribose were purchased from Sigma-Aldrich Chemical (St. Louis, MO, USA). Sterile phosphate buffered saline (PBS, pH 7.4, without Ca2+ and Mg2+; osmolarity 300 mOsmol/L) was obtained from Biomed (Lublin, Poland). The following plant polyphenols and their metabolites of the highest purity available acquired from Sigma-Aldrich Chemie GmbH (Steinheim, Germany) or from Fluka, Sigma-Aldrich (Buchs, Steinheim, Germany) were tested: catechin, catechol, CA, 3,4-DCA, 3,4-dihydroxyhydrocinnamic acid (3,4-DHCA), 3,4-dihydroxyphenylacetic acid (3,4-DPAA), FA, GA, 4-hydroxybenzoic acid (4-HBA), phloretin, phloridzin, phloroglucinol and quercetin. Sterile deionized pyrogen-free H2O (resistance > 18 MΩ·cm, HPLC H2O Purification System, USF Elga, Buckinghamshire, UK) was used throughout the study. Polyphenols were dissolved in DMSO to a final concentration of 100 mmol/L and stored in 20 μL aliquots at −80 °C prior to the assay for no more than 2 weeks. Preparation of the working solution of polyphenol: 10 µL of thawed 100 mmol/L polyphenol stock solution in DMSO was mixed with 990 µL of PBS. Then, 500 µL of this solution was mixed with 500 µL of PBS. The resulting working solution had a polyphenol concentration of 0.5 mmol/L and was used in experiments with deoxyribose oxidation. Control solution of DMSO was prepared in the same way: 10 µL of pure DMSO was mixed with 990 µL of PBS, afterwards 500 µL of this solution was mixed with 500 µL of PBS and subsequently used as a control solvent of polyphenols. Aqueous solution (10 mmol/L) of ascorbic acid, TROLOX® and N-acetylcysteine were prepared just before the assay.
4.2. Determining the Effect of Plant Phenolics on the Process of Oxidative Degradation of Deoxyribose in the Fenton System
Antioxidant or pro-oxidant properties of polyphenols and their metabolites were examined in the presence of an •OH generating system that subsequently induced oxidative degradation of deoxyribose. •OH was generated at 37 °C in air-exposed incubation PBS buffer (pH = 7.4) by the following system: 10 µmol/L Fe2+, 20 µmol/L EDTA and 280 µmol/L H2O2 (final concentrations in the 500 µL volume of reaction mixture containing 10 mmol/L deoxyribose) [10]. The mechanism for the generation of ROS damaging deoxyribose in the experimental model was based on the Fenton reaction, where intensely reactive •OH are produced through the reaction of H2O2 with Fe2+ ions [11]. Phenolics and the other compounds were tested at a final concentration of 10 µmol/L. The experimental and control systems were prepared simultaneously by adding to a 460 µL deoxyribose solution in PBS with the 10 µL volumes of working solutions of polyphenol, FeSO4, EDTA, and H2O2. Some negative control samples (blank) contained H2O instead of H2O2. Some additional controls received a control solution of DMSO instead of a working polyphenol solution (Table 4). Samples were incubated for 10 min at 37 °C. This incubation time was chosen based on our preliminary experiments, which resulted in a mere oxidation of approximately 30% of deoxyribose molecules in the assayed sample [10]. Therefore, under these conditions, it was possible to study the inhibitory (antioxidant) or enhancing (pro-oxidant) effect of polyphenols on deoxyribose oxidation. After incubation, all samples were mixed with 0.5 mL 60 g/L TCA and 0.25 mL TBA solution (1 g TBA in 100 mL of 0.05 N NaOH), boiled for 20 min, and then their absorbance at 532 nm (A532) was measured against a blank containing deoxyribose alone [10]. The polyphenol antioxidant or pro-oxidant effect was expressed as % inhibition or % enhancement of oxidative degradation of deoxyribose by a complete Fenton system (Fe2+-EDTA-H2O2). Results were obtained from six series of separate experiments with each tested compound and were corrected for the antioxidant activity of DMSO present in the working polyphenol solutions.

Table 4.
Design of experiments on the effect of polyphenols on the oxidative degradation of deoxyribose in the Fenton system (Fe2+-EDTA-H2O2).
4.3. Statistical Analysis
Results (% inhibition or % enhancement of deoxyribose oxidation) were expressed as mean (standard deviation) and median. The effect of polyphenols on the oxidative degradation of deoxyribose (comparison between deoxyribose in the Fenton system and deoxyribose with polyphenol in the Fenton system) was analyzed with Mann–Whitney U test. To determine the statistical difference within the group of antioxidant (inhibiting deoxyribose oxidation) and pro-oxidant (enhancing deoxyribose oxidation) phenolics, a Kurskal–Wallis non-parametric one-way ANOVA was performed with an appropriate Bonferroni correction post hoc test.
In order to determine the molecular structures contributing to the effect of polyphenols on deoxyribose oxidation, a multivariate analysis was carried out with a multiple linear regression, using the backward stepwise technique. The percent of inhibition or enhancement of deoxyribose oxidation in the presence of polyphenols at a concentration of 10 µmol/L were the dependent variables, and the independent variables included the number of –OH and carboxyl (–COOH) substitutions in the backbone structure, the existence of a catechol structure within the compound molecule, and the presence of an aliphatic substitute at a catechol ring. The relationship between the effects of polyphenols on deoxyribose oxidation, and their FRAP was assessed with Pearson’s correlation. FRAP values of polyphenols were taken from our previously published study [12]. In all cases, a p-value of <0.05 was considered significant. Prior to commencing the study, ethical clearance and study protocol was accepted by the Ethics Committee of the Medical University of Lodz.
5. Conclusions
Seven of the 13 tested phenolics enhanced •OH generation while the remaining six inhibited or had no significant effect on •OH activity under the Fe2+-EDTA-H2O2 system, which favors the unmasking of pro-oxidant effects in the investigated compounds. Since this system can occur in the blood of patients that have undergone chelation therapy, the possibility of some ingested dietary polyphenols enhancing •OH production and inducing oxidative damage to various circulating biomolecules may be envisaged.
Fruits and vegetables contain a variety of polyphenols including those enhancing and inhibiting •OH production under the Fe2+-EDTA-H2O2 system. It is possible that, in vivo, their pro- and antioxidant action counteract; therefore, the risk of an induction of oxidative stress via a high intake of dietary polyphenols seem negligible. On the other hand, selective supplementation of the diet with phenolics exhibiting pro-oxidant activity under the Fe2+-EDTA-H2O2 system in vitro may increase the possibility of systemic oxidative stress in patients treated with EDTA or other medications with chelating properties (e.g., bleomycin) or those with high plasma concentrations of H2O2 and non-transferrin bound iron. However, confirmation of this hypothesis requires further clinical studies.
Acknowledgements
This study was supported by a research grant from the European Regional Development Fund through the Polish Innovative Economy Operational Program, contract N. UDAPOIG 01.03.01-10-109/08-00 and a grant from the Medical University of Lodz No: 503/1-079-01/503-11-001.
Author Contributions
Jeffrey de Graft-Johnson and Dariusz Nowak conceived and designed the experiments. Jeffrey de Graft-Johnson performed the experiments and Jeffrey de Graft-Johnson and Dariusz Nowak analyzed the data. Dariusz Nowak and Jeffrey de Graft-Johnson contributed reagents, materials and analysis tools. Jeffrey de Graft-Johnson wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Appendix A—Chemical Compounds Studied in This Article
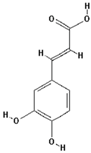
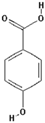
3,4-Dihydroxycinnamic acid (PubChem CID: 689043); 4-Hydroxybenzoic acid (PubChem CID: 135); 3,4-Dihydroxyhydrocinnamic acid (PubChem CID: 91697097);
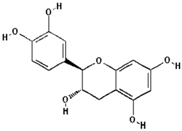
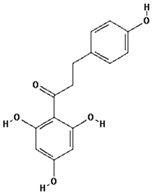
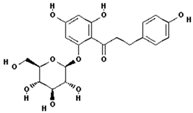
Catechin (PubChem CID: 9064); Chlorogenic acid (PubChem CID: 1794427); Ferulic acid (PubChem CID: 445858); Gallic acid (PubChem CID: 370); 3,4-Dihydroxyphenylacetic acid (PubChem CID: 547); Phloretin (PubChem CID: 4788); Phloroglucinol (PubChem CID: 359); Catechol (PubChem CID: 289); Phloridzin (PubChem CID: 6072); Quercetin (PubChem CID: 5280343).
References
- Shen, Y.; Zhang, H.; Cheng, L.; Wang, L.; Qian, H.; Qi, X. In vitro and in vivo antioxidant activity of polyphenols extracted from black highland barley. Food Chem. 2016, 194, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; García, C.R.; Pinzón, J.R.; Chaur, M.N.; Brumaghim, J.L. Antioxidant and prooxidant effects of polyphenol compounds on copper-mediated DNA damage. J. Inorg. Biochem. 2011, 105, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Joubert, E.; Winterton, P.; Britz, T.J.; Gelderblom, W.C. Antioxidant and pro-oxidant activities of aqueous extracts and crude polyphenolic fractions of rooibos (Aspalathus linearis). J. Agric. Food Chem. 2005, 53, 10260–10267. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Y.; Chang, W. Kinetic deoxyribose degradation assay and its application in assessing the antioxidant activities of phenolic compounds in a Fenton-type reaction system. Anal. Chim. Acta 2003, 478, 129–137. [Google Scholar] [CrossRef]
- Kurin, E.; Mučaji, P.; Nagy, M. In vitro antioxidant activities of three red wine polyphenols and their mixtures: An interaction study. Molecules 2012, 17, 14336–14348. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Singh, B.R.; Singh, R.L.; Prakash, D.; Dhakarey, R.; Upadhyay, G.; Singh, H.B. Oxidative DNA damage protective activity, antioxidant and anti-quorum sensing potentials of Moringa oleifera. Food Chem. Toxicol. 2009, 47, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Neergheen, V.S.; Soobrattee, M.A.; Bahorun, T.; Aruoma, O.I. Characterization of the phenolic constituents in Mauritian endemic plants as determinants of their antioxidant activities in vitro. J. Plant Physiol. 2006, 168, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Mozdzan, M.; Szemraj, J.; Rysz, J.; Nowak, D. Antioxidant properties of carnosine re-evaluated with oxidizing systems involving Fe and copper ions. Basic Clin. Pharmacol. Toxicol. 2005, 96, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Son, Y.O.; Pratheeshkumar, P.; Shi, X. Oxidative stress and metal carcinogenesis. Free Radic. Biol. Med. 2012, 53, 742–757. [Google Scholar] [CrossRef] [PubMed]
- De Graft-Johnson, J.; Kolodziejczyk, K.; Krol, M.; Nowak, P.; Krol, B.; Nowak, D. Ferric-reducing ability power of selected plant polyphenols and their metabolites: Implications for clinical studies on the antioxidant effects of fruits and vegetable consumption. Basic Clin. Pharmacol. Toxicol. 2007, 100, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, Y.; Wu, F.; Ni, Y.; Kokot, S. A colorimetric method of analysis for trace amounts of hydrogen peroxide with the use of the nano-properties of molybdenum disulfide. Analyst 2015, 140, 1119–1126. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, W.I.; El-Sabban, F. Dimethyl sulfoxide (DMSO) and glycerol, hydroxyl radical scavengers, impair platelet aggregation within and eliminate the accompanying vasodilation of injured mouse pial arterioles. Stroke 1982, 13, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Buettner, G.R.; Jurkiewicz, B.A. Catalytic metals, ascorbate and free radicals: Combinations to avoid. Radiat. Res. 1996, 145, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, S.; Oikawa, S.; Murata, M. Evaluation for safety of antioxidant chemopreventive agents. Antioxid. Redox Signal. 2005, 7, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.; Aruoma, O.I. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal. Biochem. 1987, 165, 215–219. [Google Scholar] [CrossRef]
- Gutteridge, J.M. Ferrous-salt-promoted damage to deoxyribose and benzoate. The increased effectiveness of hydroxyl-radical scavengers in the presence of EDTA. Biochem. J. 1987, 243, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.; Frei, B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999, 13, 1007–1024. [Google Scholar] [PubMed]
- Wang, S.Y.; Jio, H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef] [PubMed]
- Minakata, K.; Fukushima, K.; Nakamura, M.; Iwahashi, H. Effect of some naturally occurring iron ion chelators on the formation of radicals in the reaction mixtures of rat liver microsomes with ADP, Fe and NADPH. J. Clin. Biochem. Nutr. 2011, 49, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.R.; Brumaghim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Laughton, M.J.; Halliwell, B.; Evans, P.J.; Hoult, J.R.S. Antioxidant and pro-oxidant actions of the plant phenolics quercetin, gossypol and myricetin. Effects on lipid peroxidation, hydroxyl radical generation and bleomycin dependent damage to DNA. Biochem. Pharmacol. 1989, 38, 2859–2865. [Google Scholar] [CrossRef]
- Puppo, A. Effect of flavonoids on hydroxyl radical formation by Fenton-type reactions; influence of the iron chelator. Phytochemistry 1992, 31, 85–88. [Google Scholar] [CrossRef]
- Moran, J.F.; Klucas, R.V.; Grayer, R.J.; Abian, J.; Becana, M. Complexes of iron with phenolic compounds from soybean nodules and other legume tissues: Prooxidant and antioxidant properties. Free Radic. Biol. Med. 1997, 22, 861–870. [Google Scholar] [CrossRef]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Sutton, H.C.; Vile, G.F.; Winterbourn, C.C. Radical driven Fenton reactions--evidence from paraquat radical studies for production of tetravalent iron in the presence and absence of ethylenediaminetetraacetic acid. Arch. Biochem. Biophys. 1987, 256, 462–471. [Google Scholar] [CrossRef]
- Hussain, R.S.; Cillard, J.; Cillard, P. Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 1987, 26, 2489–2491. [Google Scholar] [CrossRef]
- Heijnen, C.G.; Haenen, G.R.; van Acker, F.A.; van der Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers: The role of the hydroxyl groups. Toxicol In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, M.J.; Kim, J.Y.; Chung, H.Y.; Choi, J.S. Inhibitory activity of flavonoids from Prunus davidiana and other flavonoids on total ROS and hydroxyl radical generation. Arch. Pharm. Res. 2003, 26, 809–815. [Google Scholar] [CrossRef] [PubMed]
- D’Avila Farias, M.; Oliveira, P.S.; Dutra, F.S.; Fernandes, T.J.; de Pereira, C.M.; de Periera, C.M.; de Oliveira, S.Q.; Stefanello, F.M.; Lencina, C.L.; Barschak, A.G. Eugenol Derivatives as potential anti-oxidants: Is phenolic hydroxyl necessary to obtain an effect? J. Pharm. Pharmacol. 2014, 66, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Moniczewski, A.; Librowski, T.; Lochyński, S.; Strub, D. Evaluation of the irritating influence of carane derivatives and their antioxidant properties in a deoxyribose degradation test. Pharmacol. Rep. 2011, 63, 120–129. [Google Scholar] [CrossRef]
- Benzie, I.F.; Choi, S.W. Antioxidants in food: Content, measurement, significance, action, cautions, caveats, and research needs. Adv. Food Nutr. Res. 2014, 71, 1–53. [Google Scholar] [PubMed]
- Nguyen, M.T.; Kryachko, E.S.; Vanquickenborne, L.G. General and Theoretical Aspects of Phenols. In The Chemistry of Phenols; Rappoport, Z., Ed.; John Wiley & Sons: Chichester, UK, 2003; pp. 3–179. [Google Scholar]
- Tsukimori, K.; Yoshitomi, T.; Morokuma, S.; Fukushima, K.; Wake, N. Serum uric acid levels correlate with plasma hydrogen peroxide and protein carbonyl levels in preeclampsia. Am. J. Hypertens. 2008, 21, 1343–1346. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Ramavataram, D.V. Non transferrin bound iron: Nature, manifestations and analytical approaches for estimation. Indian J. Clin. Biochem. 2012, 27, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Prymont-Przyminska, A.; Zwolinska, A.; Sarniak, A.; Wlodarczyk, A.; Krol, M.; Nowak, M.; de Graft-Johnson, J.; Padula, G.; Bialasiewicz, P.; Markowski, J.; et al. Consumption of strawberries on a daily basis increases the non-urate 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activity of fasting plasma in healthy subjects. J. Clin. Biochem. Nutr. 2014, 55, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Anstrom, K.J.; Clapp-Channing, N.E.; Knight, J.D.; Boineau, R.; Goertz, C.; Rozema, T.C.; Liu, D.M.; Nahin, R.L.; Rosenberg, Y.; et al. TACT Investigators. Quality-of-life outcomes with a disodium EDTA chelation regimen for coronary disease: Results from the trial to assess chelation therapy randomized trial. Circ. Cardiovasc. Qual. Outcomes 2014, 7, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Harich, K.C.; Cranton, E.M. A laboratory method to measure plasma EDTA levels. In A Textbook on EDTA Chelation Therapy; Cranton, E.M., Ed.; Hampton Roads Publishing Inc.: Newburyport, MA, USA, 2001; pp. 468–476. [Google Scholar]
- Hininger, I.; Waters, R.; Osman, M.; Garrel, C.; Fernholz, K.; Roussel, A.M.; Anderson, R.A. Acute prooxidant effects of vitamin C in EDTA chelation therapy and long-term antioxidant benefits of therapy. Free Radic. Biol. Med. 2005, 38, 1565–1570. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not Available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).