O-Carboxymethyl Chitosan Supported Heterogeneous Palladium and Ni Catalysts for Heck Reaction
Abstract
:1. Introduction
2. Results
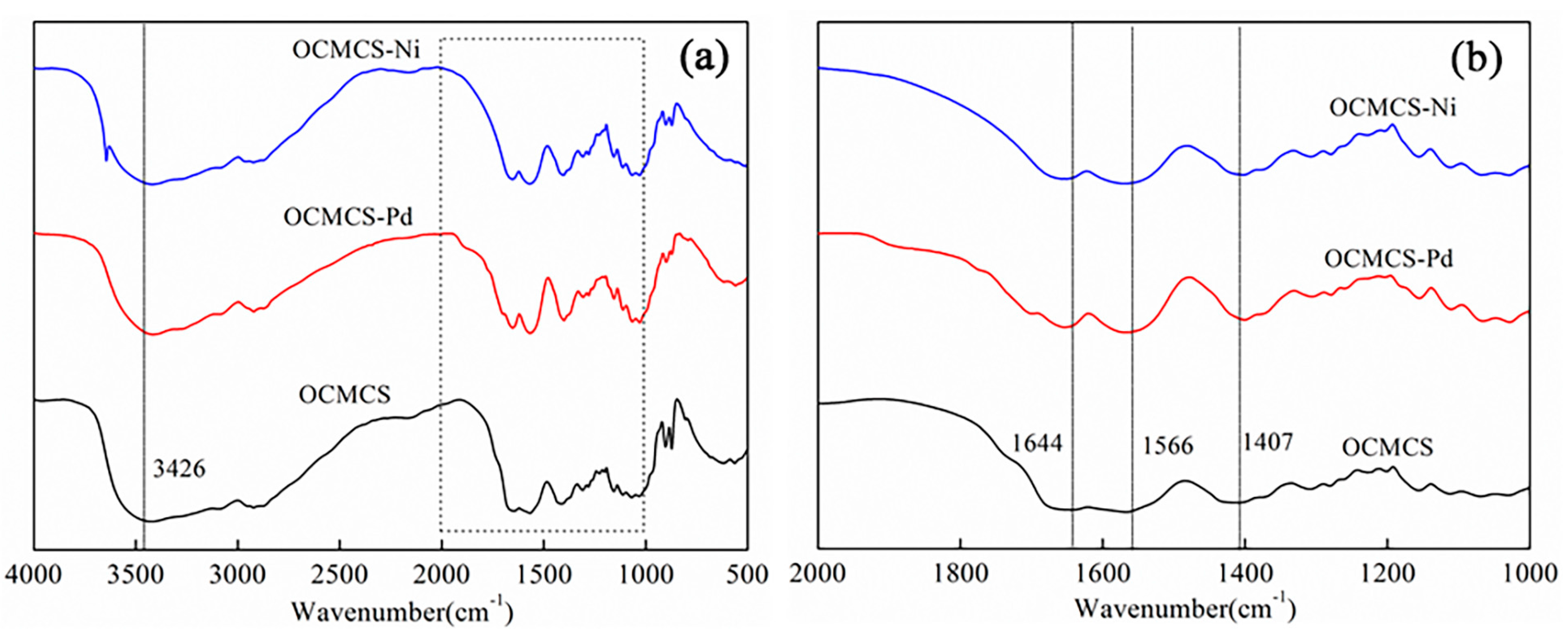
2.1. FT-IR Spectra
2.2. Thermal Analysis
2.3. XRD Analysis
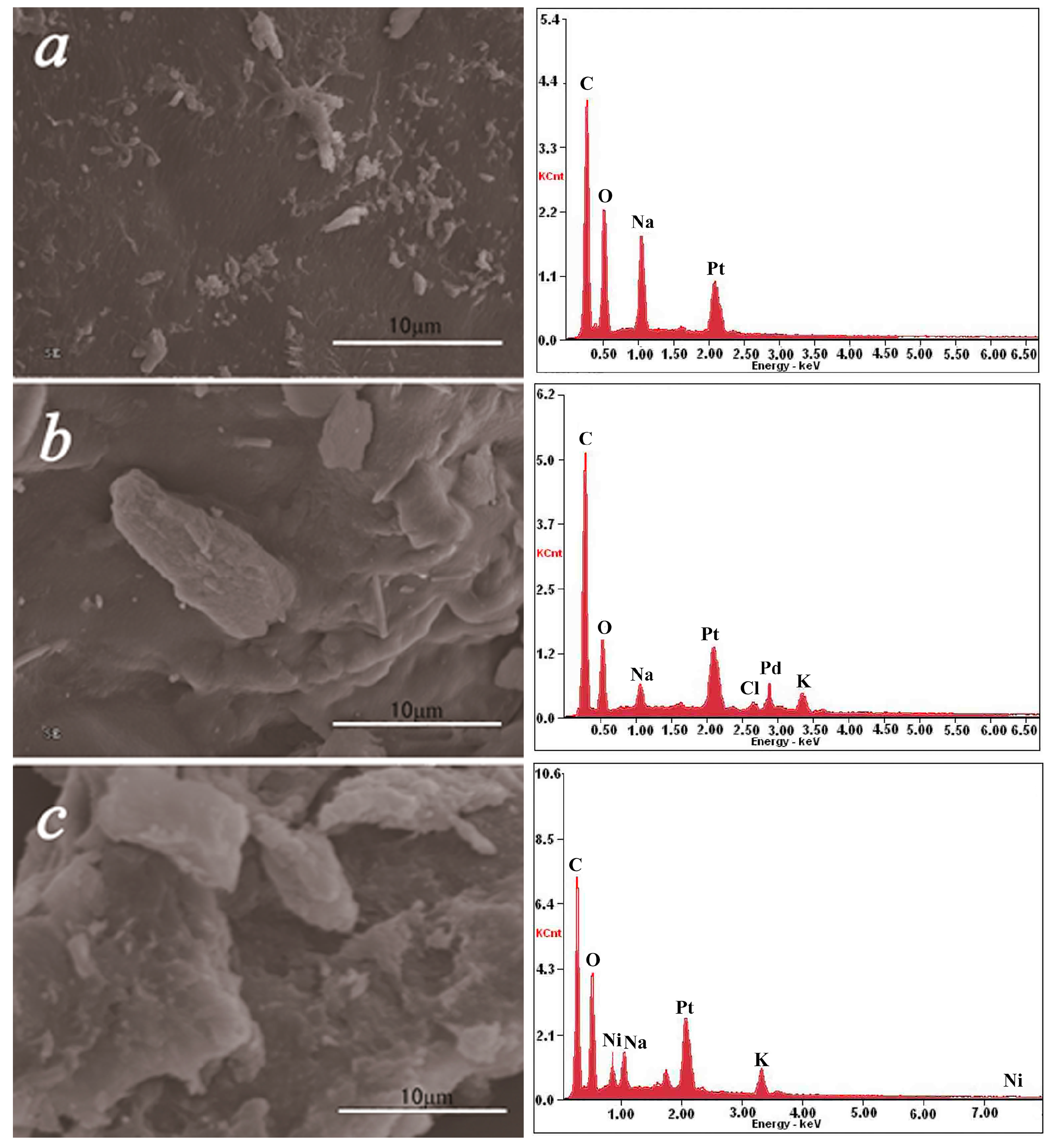
2.4. SEM–EDS Analysis
2.5. Catalytic Studies
2.5.1. Heck Cross-Coupling Reactions
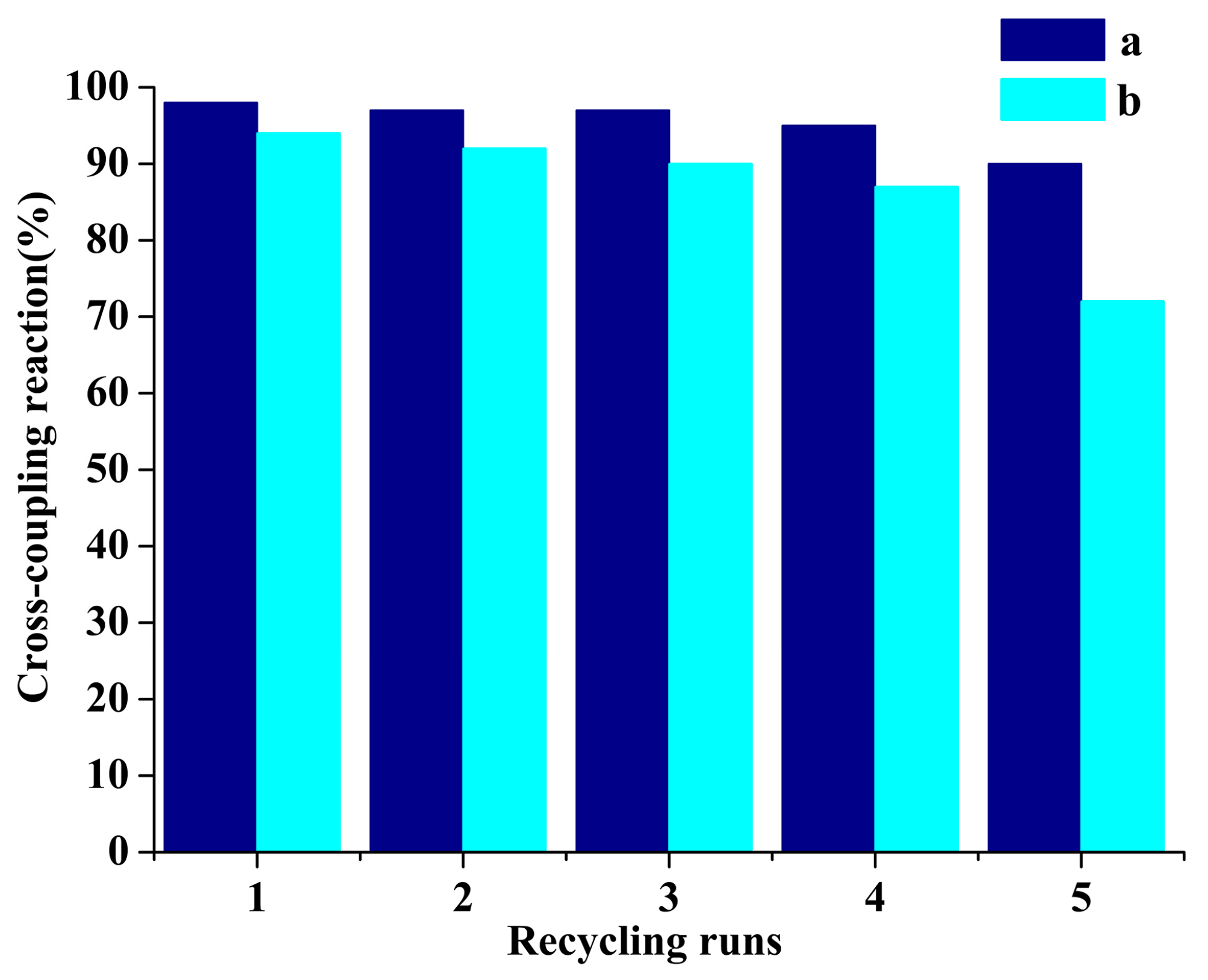
2.5.2. Reusability of Catalysts
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Physical Measurements
4.3. Synthesis of OCMCS-Pd and OCMCS-Ni
4.4. General Procedure for the Heck Reaction
Author Contributions
Conflicts of Interest
References
- Atla, S.B.; Kelkar, A.A.; Puranik, V.G.; Bensch, W.; Chaudhari, R.V. NC palladacycles in the Heck arylation of ethylene: Synthesis, structure and their reactivity. J. Organomet. Chem. 2009, 694, 683–690. [Google Scholar] [CrossRef]
- Tsai, C.J.; Chen, Y. Luminescent poly(p-phenylenevinylene) with 4-methylcoumarin side groups: Synthesis, optical properties and photo-crosslinking behaviors. React. Funct. Polym. 2006, 66, 1327–1335. [Google Scholar] [CrossRef]
- Mikroyannidis, J.A.; Damouras, P.A.; Maragos, V.G. Synthesis, photophysics and electroluminescence of new vinylene-copolymers with 2,4,6-triphenylpyridine kinked segments along the main chain. Eur. Polym. J. 2009, 45, 284–294. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Molnár, Á. Silica-supported Pd catalysts for Heck coupling reactions. Tetrahedron 2007, 63, 6949–6976. [Google Scholar] [CrossRef]
- Yin, L.; Liebscher, J. Carbon-carbon coupling reactions catalyzed by heterogeneous palladium catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Beletskaya, I.P.; Cheprakov, A.V. The Heck reaction as a sharpening stone of palladium catalysis. Chem. Rev. 2000, 100, 3009–3066. [Google Scholar] [CrossRef] [PubMed]
- Casey, M.; Lawless, J.; Shirran, C. The Heck reaction: Mechanistic insights and novel ligands. Polyhedron 2000, 19, 517–520. [Google Scholar] [CrossRef]
- Polshettiwar, V.; Len, C.; Fihri, A. Silica-supported palladium: Sustainable catalysts for cross-coupling reactions. Coord. Chem. Rev. 2009, 253, 2599–2626. [Google Scholar] [CrossRef]
- Alonso, F.; Yus, M. Heterogeneous catalytic homocoupling of terminal alkynes. ACS Catal. 2012, 2, 1441–1451. [Google Scholar] [CrossRef]
- Wight, A.P.; Davis, M.E. Design and preparation of organic-inorganic hybrid catalysts. Chem. Rev. 2002, 102, 3589–3614. [Google Scholar] [CrossRef] [PubMed]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and applications of supramolecular-templated mesoporous materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Brunel, D.; Blanc, A.C.; Galarneau, A.; Fajula, F. New trends in the design of supported catalysts on mesoporoussilicas and their applications in fine chemicals. Catal. Today 2002, 73, 139–152. [Google Scholar] [CrossRef]
- El-Sherbiny, I.M. Synthesis, characterization and metal uptake capacity of a new carboxymethyl chitosan derivative. Eur. Polym. J. 2009, 45, 199–210. [Google Scholar] [CrossRef]
- Pestov, A.; Bratskaya, S.; Pestov, A.; Bratskaya, S. Chitosan and its derivatives as highly efficient polymer ligands. Molecules 2016, 21, 330. [Google Scholar] [CrossRef] [PubMed]
- Khalil, K.D.; Almatar, H.M. Chitosan based heterogeneous catalyses: Chitosan-grafted-poly(4-vinylpyridne) as an efficient catalyst for michael additions and alkylpyridazinyl carbonitrile oxidation. Molecules 2013, 18, 5288–5305. [Google Scholar] [CrossRef] [PubMed]
- Hardy, J.J.E.; Hubert, S.; Macquarrie, D.J.; Wilson, A.J. Chitosan-based heterogeneous catalysts for Suzuki and Heck reactions. Green Chem. 2004, 6, 53–56. [Google Scholar] [CrossRef]
- Baran, T.; Menteş, A. Cu(II) and Pd(II) complexes of water soluble O-carboxymethyl chitosan Schiff bases: Synthesis, characterization. Int. J. Biol. Macromol. 2015, 79, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Han, F.S. Transition-metal-catalyzed Suzuki-Miyaura cross-coupling reactions: A remarkable advance from palladium to nickel catalysts. Chem. Soc. Rev. 2013, 42, 5270–5298. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, J.; Muto, K.; Itami, K. Recent progress in nickel-catalyzed biaryl coupling. Eur. J. Org. Chem. 2013, 18, 19–30. [Google Scholar] [CrossRef]
- Hassaninejad-Darzi, S.K. A novel, effective and low cost catalyst for formaldehyde electrooxidation based on nickel ions dispersed onto chitosan-modified carbon paste electrode for fuel cell. J. Electroceram. 2014, 33, 252–263. [Google Scholar] [CrossRef]
- Portnyagin, A.S.; Bratskaya, S.Y.; Pestov, A.V.; Voit, A.V. Binding Ni(II) ions to chitosan and its N-heterocyclic derivatives: Density functional theory investigation. Comput. Theor. Chem. 2015, 1069, 4–10. [Google Scholar] [CrossRef]
- Antony, R.; Manickam, S.T.D.; Saravanan, K.; Karuppasamy, K.; Balakumar, S. Synthesis, spectroscopic and catalytic studies of Cu(II), Co(II) and Ni(II) complexes immobilized on Schiff base modified chitosan. J. Mol. Struct. 2013, 1050, 53–60. [Google Scholar] [CrossRef]
- Baran, T.; Menteş, A.; Arslan, H. Synthesis and characterization of water soluble O-carboxymethyl chitosan Schiff bases and Cu(II) complexes. Int. J. Biol. Macromol. 2015, 72, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Wang, Z.-W.; Wang, G.-S.; Lin, X.-D.; Ren, J.-G. Catalytic performance of chitosan-Schiff base supported Pd/Co bimetallic catalyst for acrylamide with phenyl halide. Polym. Adv. Technol. 2010, 21, 244–249. [Google Scholar]
- Yi, S.S.; Lee, D.H.; Sin, E.; Lee, Y.-S. Chitosan-supported palladium (0) catalyst for microwave-prompted Suzuki cross-coupling reaction in water. Tetrahedron Lett. 2007, 48, 6771–6775. [Google Scholar] [CrossRef]
- Baran, T.; Açıksöz, E.; Menteş, A. Carboxymethyl chitosan Schiff base supported heterogeneous palladium(II) catalysts for Suzuki cross-coupling reaction. J. Mol. Catal. A-Chem. 2015, 407, 47–52. [Google Scholar] [CrossRef]
- Demetgül, C. Synthesis of the ketimine of chitosan and 4,6-diacetylresorcinol, and study of the catalase-like activity of its copper chelate. Carbohydr. Polym. 2012, 89, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.


| Entry | ArX | Product | Yield b (%) |
|---|---|---|---|
| 1 |  |  | 98 |
| 2 |  |  | 94 |
| 3 |  |  | 99 |
| 4 |  |  | 89 |
| 5 |  |  | 94 |
| 6 |  |  | 93 |
| 7 |  |  | 96 |
| 8 |  |  | 51 |
| 9 |  |  | 6 |
| Entry | ArX | Product | Yield b/% |
|---|---|---|---|
| 1 |  |  | 94 |
| 2 |  |  | 86 |
| 3 |  |  | 88 |
| 4 |  |  | 67 |
| 5 |  |  | 79 |
| 6 |  |  | 72 |
| 7 |  | 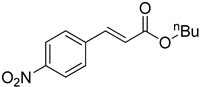 | 87 |
| 8 | 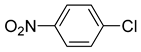 |  | - |
| 9 |  |  | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, D.; Zhang, M. O-Carboxymethyl Chitosan Supported Heterogeneous Palladium and Ni Catalysts for Heck Reaction. Molecules 2017, 22, 150. https://doi.org/10.3390/molecules22010150
Lv D, Zhang M. O-Carboxymethyl Chitosan Supported Heterogeneous Palladium and Ni Catalysts for Heck Reaction. Molecules. 2017; 22(1):150. https://doi.org/10.3390/molecules22010150
Chicago/Turabian StyleLv, Dongjun, and Mingjie Zhang. 2017. "O-Carboxymethyl Chitosan Supported Heterogeneous Palladium and Ni Catalysts for Heck Reaction" Molecules 22, no. 1: 150. https://doi.org/10.3390/molecules22010150
APA StyleLv, D., & Zhang, M. (2017). O-Carboxymethyl Chitosan Supported Heterogeneous Palladium and Ni Catalysts for Heck Reaction. Molecules, 22(1), 150. https://doi.org/10.3390/molecules22010150







