Evaluation of the Antibacterial Effects and Mechanism of Action of Protocatechualdehyde against Ralstonia solanacearum
Abstract
:1. Introduction
2. Results
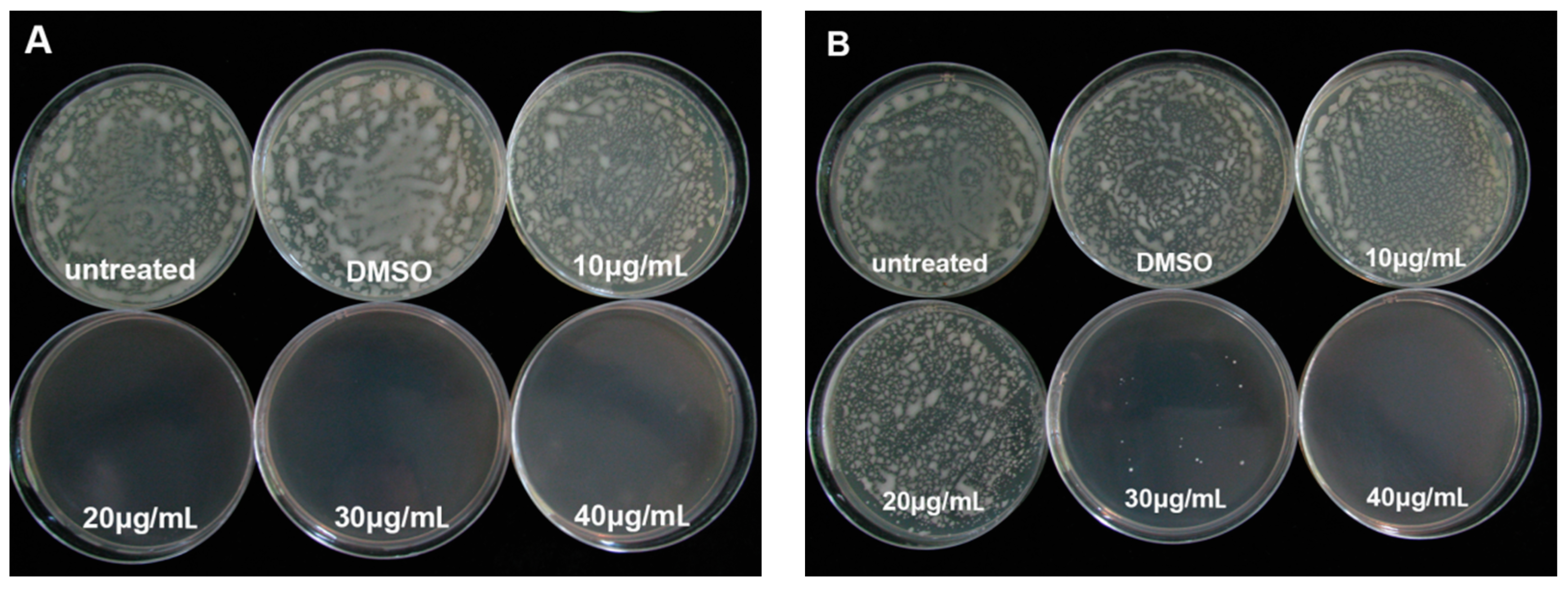
2.1. The Minimum Inhibitory Concentration and Minimum Bactericidal Concentration Values of PCA on R. solanacearum
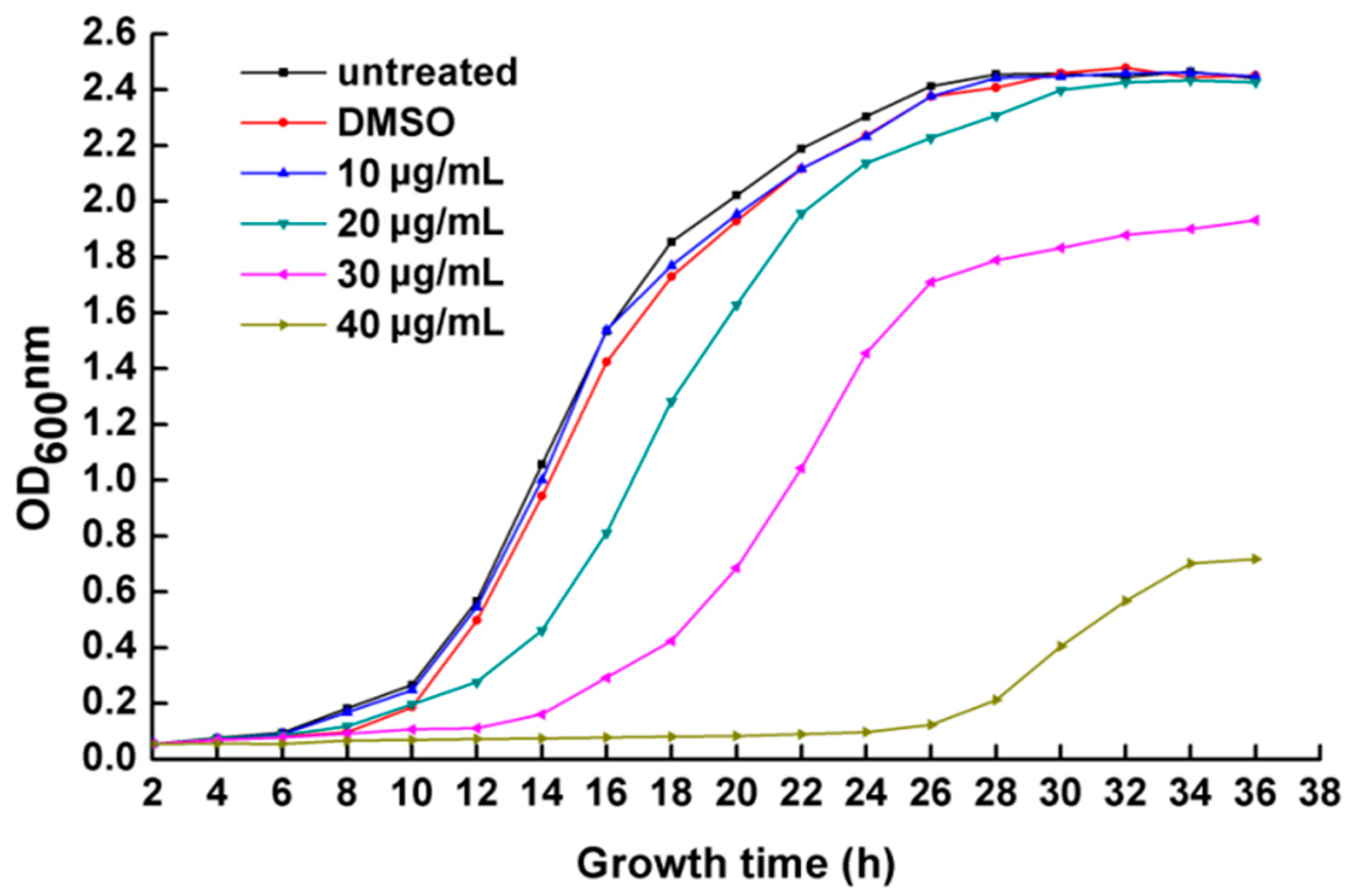
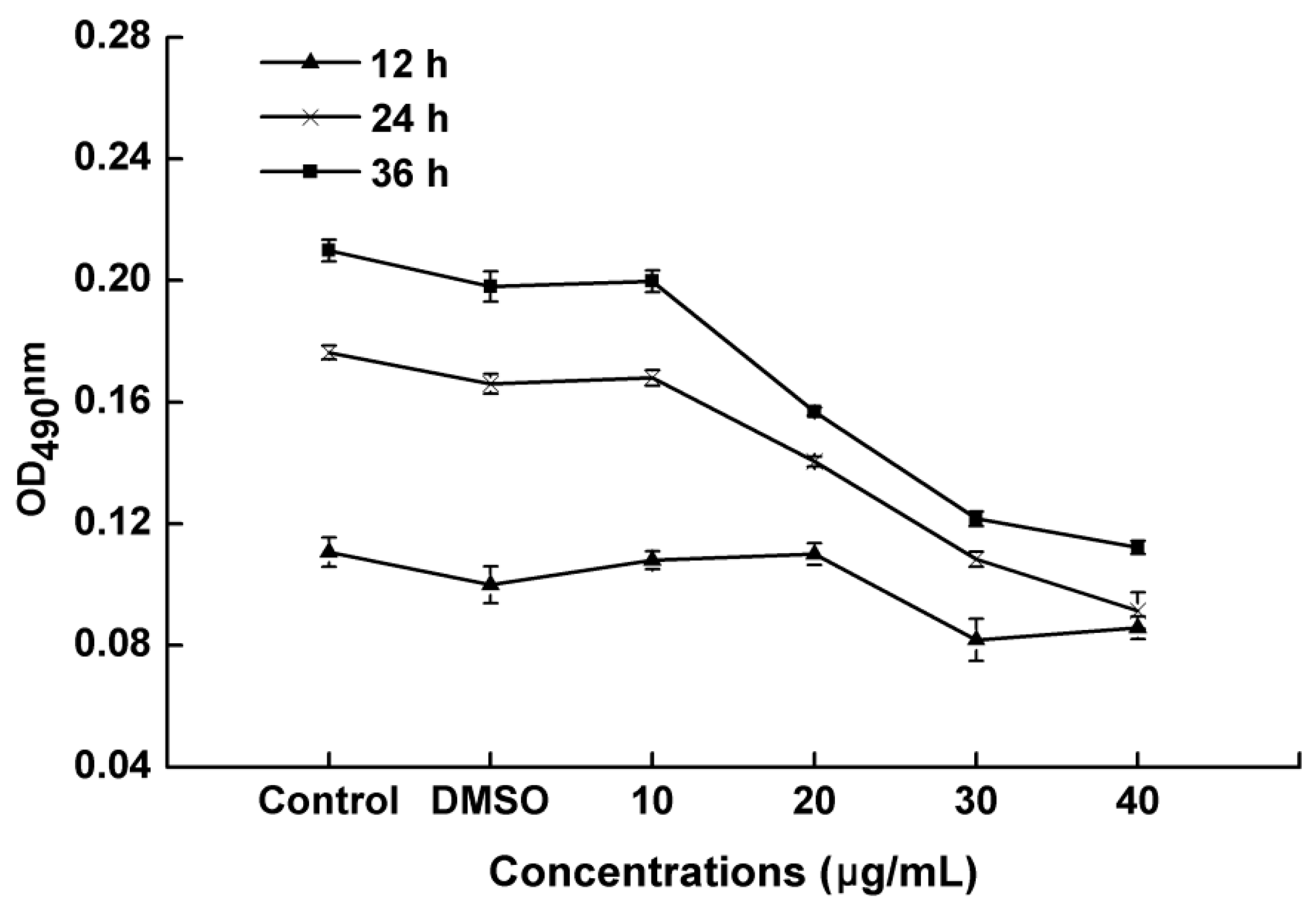
2.2. R. solanacearum Growth Curves with PCA Treatment
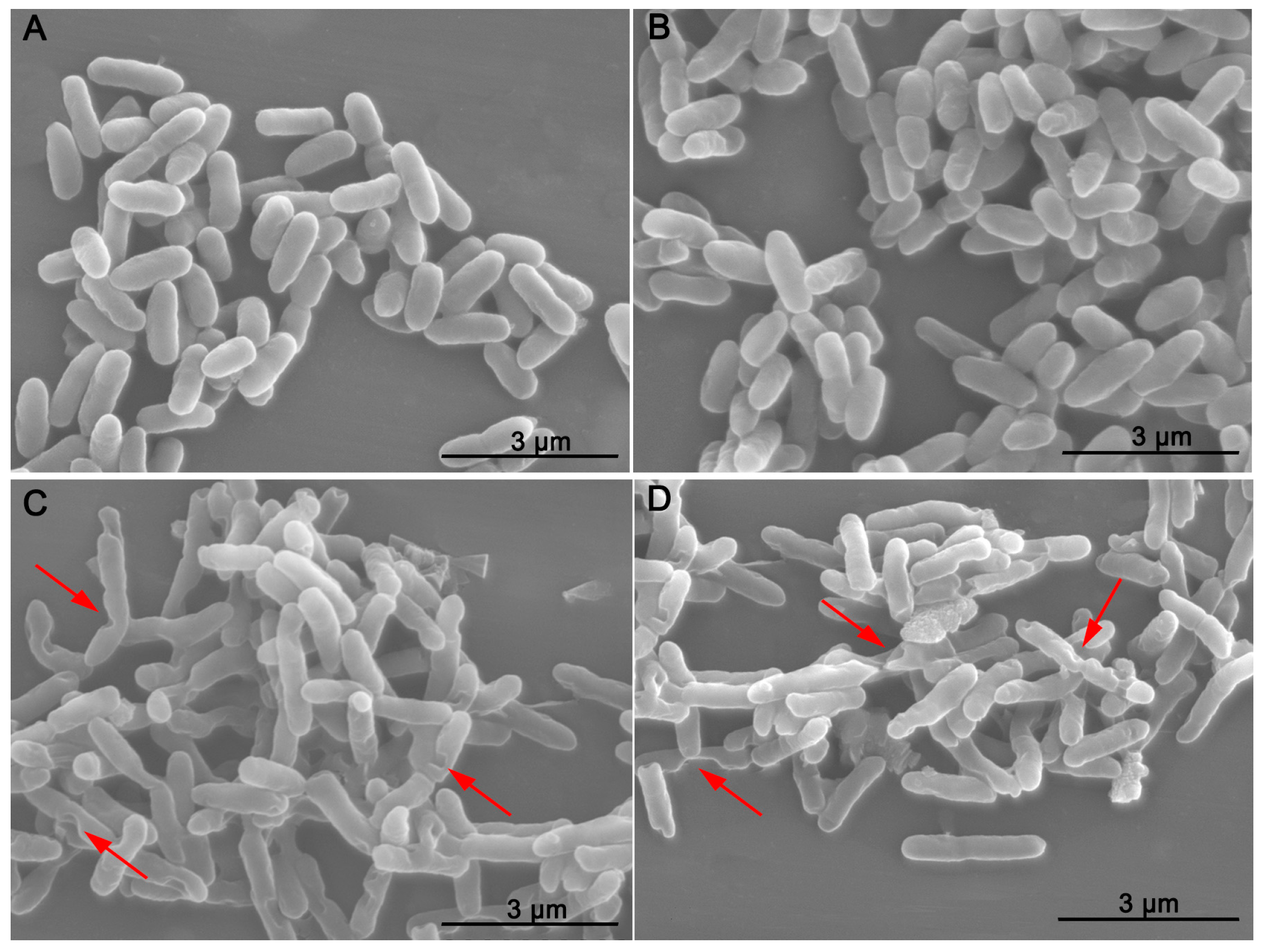
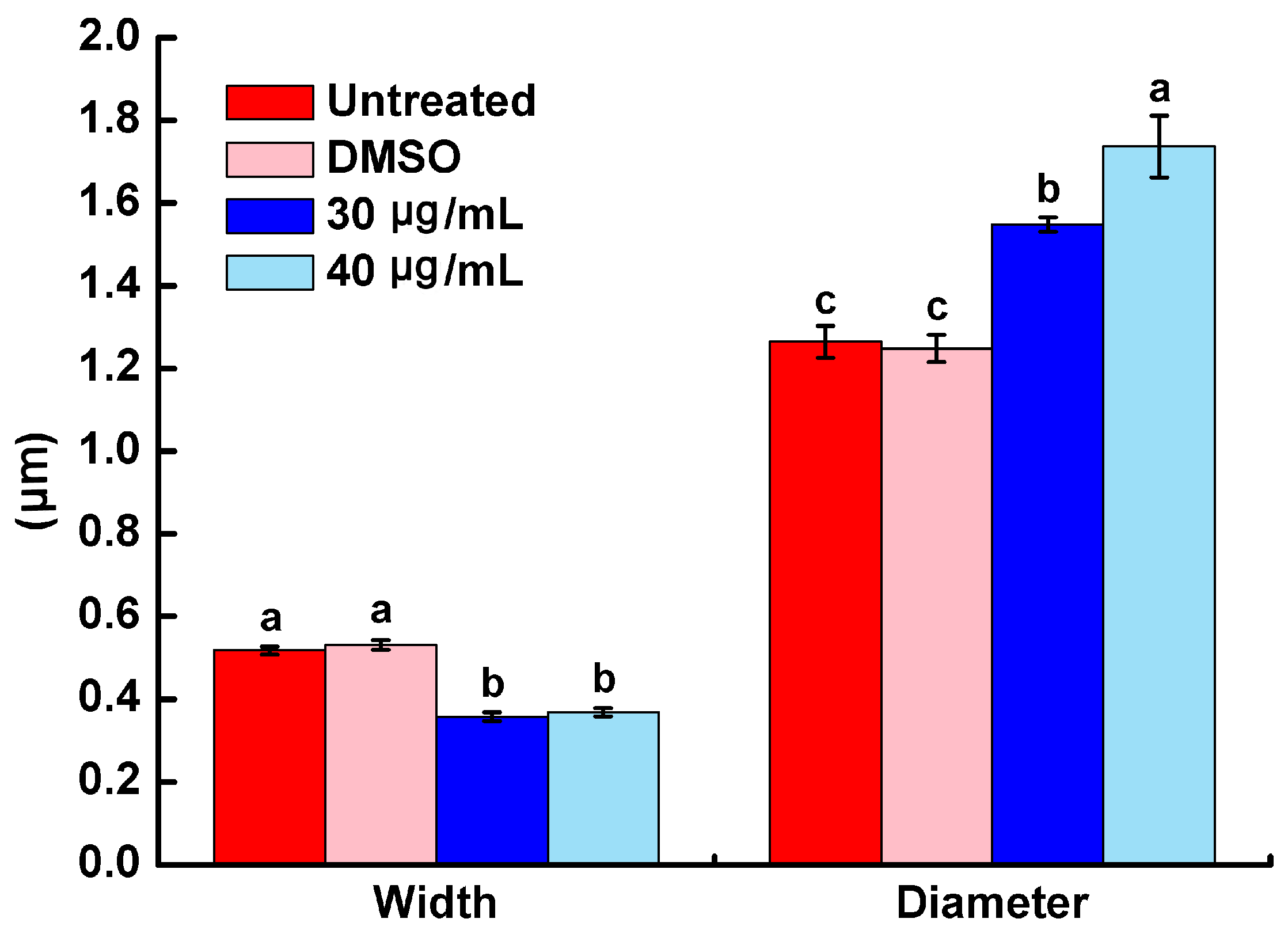
2.3. Morphology Characterization of R. solanacearum by SEM
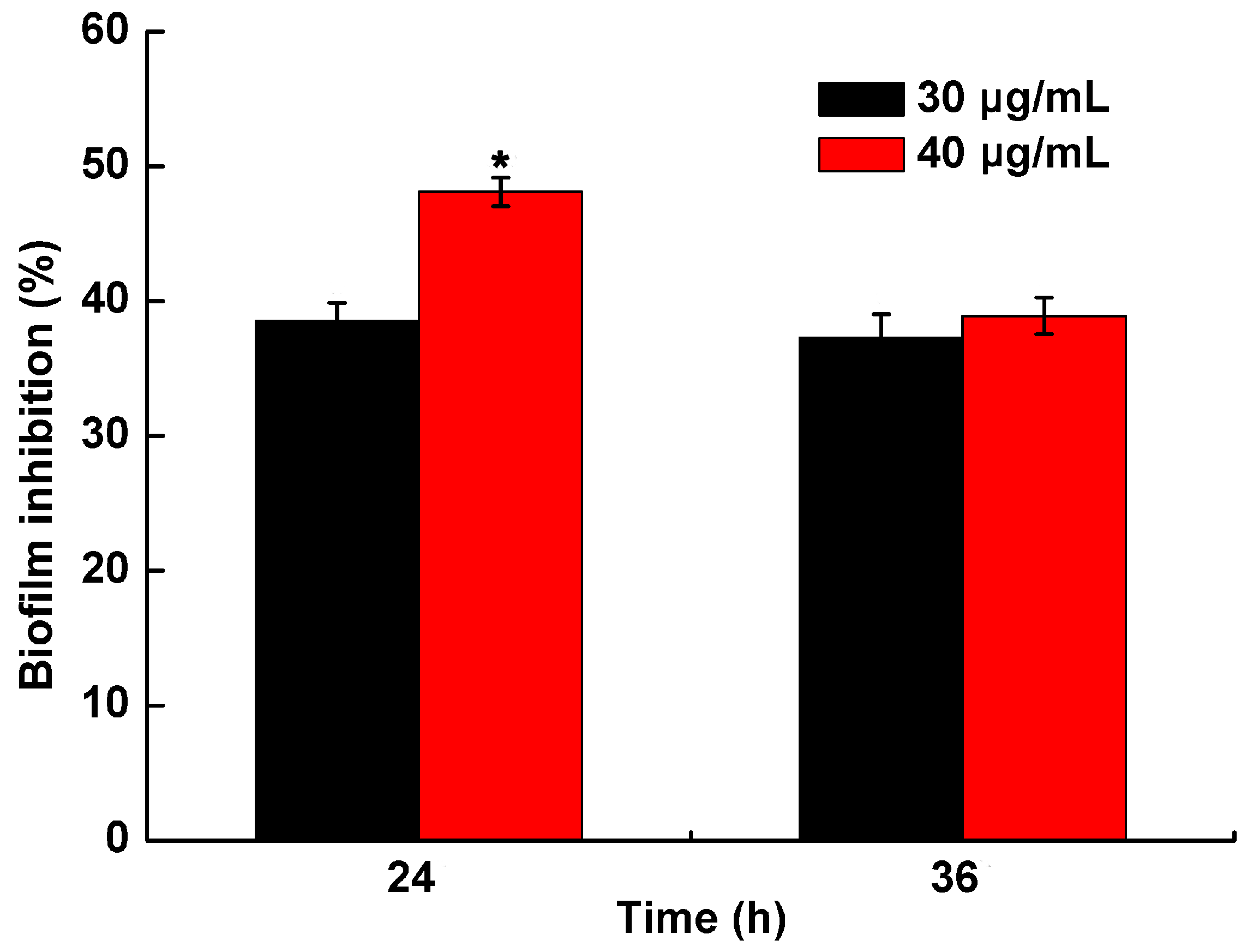
2.4. Effect of PCA on Biofilm Formation in R. solanacearum
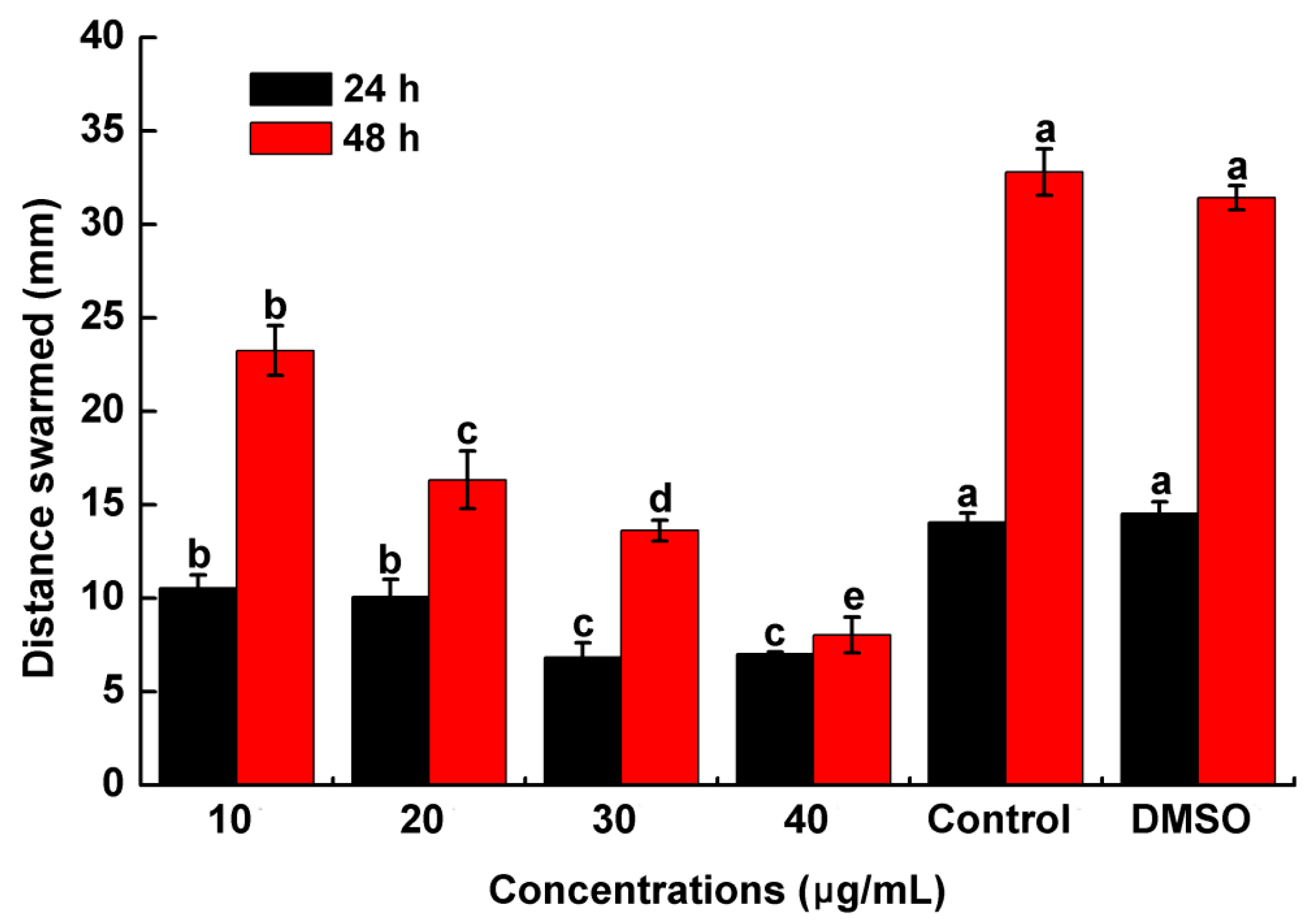
2.5. Assessment of R. solanacearum Swarming Motility in the Presence of PCA
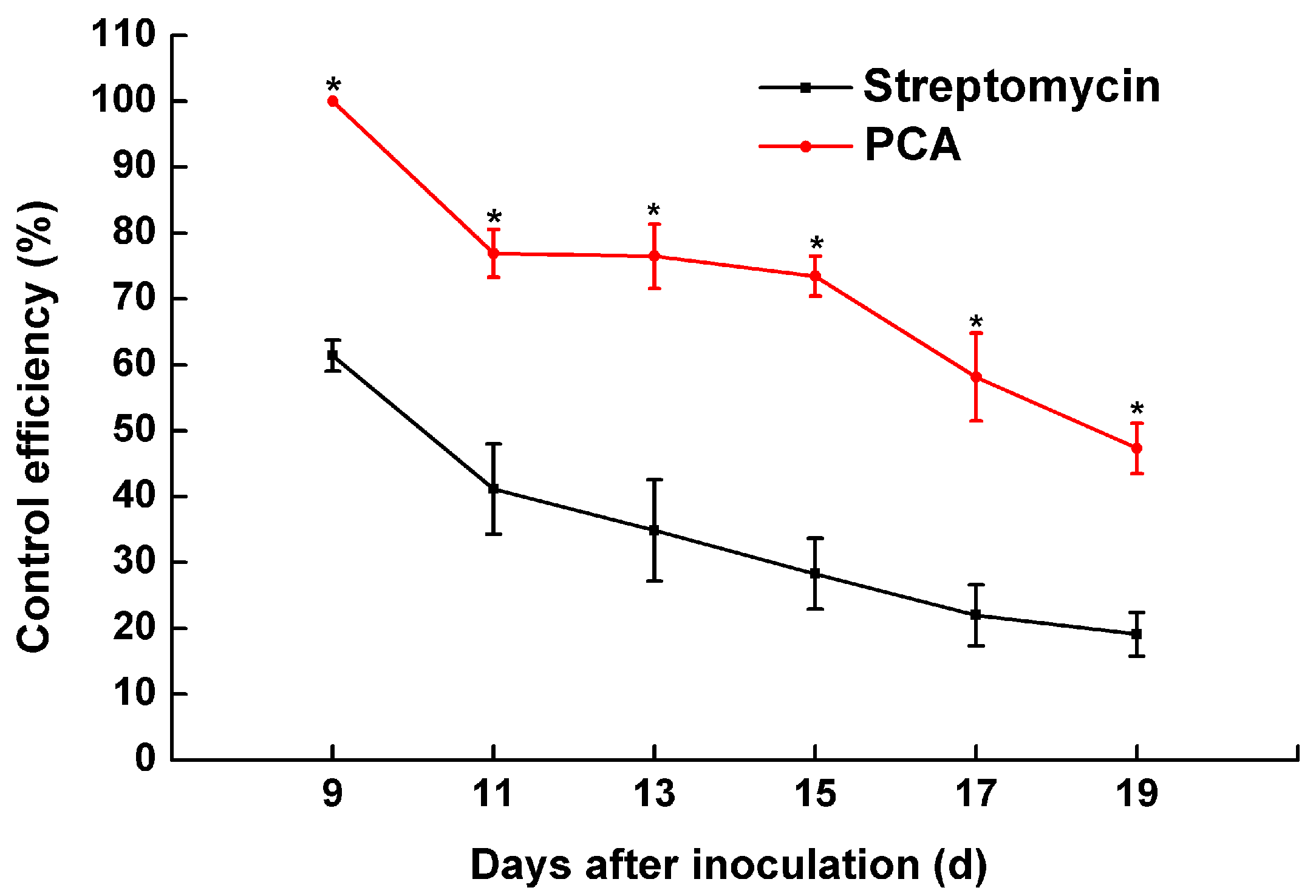
2.6. The Pathogenicity of R. solanacearum in a Greenhouse Treated with PCA
3. Discussion
4. Materials and Methods
4.1. Chemicals and Bacterial Strains
4.2. Determination of the MIC and the MBC
4.3. Antimicrobial Assay
4.4. Bacterial Morphology
4.5. Biofilm Formation Assay
4.6. Swarming Assay
4.7. Effect of PCA on Seedling Tobacco Incubated with R. solanacearum
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Genin, S.; Denny, T.P. Pathogenomics of the Ralstonia solanacearum species complex. Annu. Rev. Phytopathol. 2012, 50, 67–89. [Google Scholar] [CrossRef] [PubMed]
- Elphinstone, J.G.; Allen, C.; Prior, P.; Hayward, A.C. The current bacterial wilt situation: A global overview. In Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex; American Phytopathological Society: St Paul, MN, USA, 2005; pp. 9–28. [Google Scholar]
- Salanoubat, M.; Genin, S.; Artiguenave, F.; Gouzy, J.; Mangenot, S.; Arlat, M.; Chandler, M. Genome sequence of the plant pathogen Ralstonia solanacearum. Nature 2002, 415, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Feng, X.; Tang, M.; Hao, W.; Han, Y.; Zhang, G.; Wan, S. Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum). Microbiol. Res. 2014, 169, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Hayward, A.C. Biology and epidemiology of bacterial wilt caused by Pseudomonas solanacearum. Annu. Rev. Phytopathol. 1991, 29, 65–87. [Google Scholar] [CrossRef] [PubMed]
- Denny, T.P.; Brumbley, S.M.; Carney, B.F.; Clough, S.J.; Schell, M.A.; Hayward, A.C.; Hartman, G.L. Phenotype conversion of Pseudomonas solanacearum: Its molecular basis and potential function. In Bacterial Wilt: The Disease and Its Causative Agent, Pseudomonas solanacearum; CABI: Wallingford, UK, 1994; pp. 137–155. [Google Scholar]
- Meng, F. The Virulence Factors of the Bacterial Wilt Pathogen Ralstonia solanacearum. J. Plant Pathol. Microb. 2013, 4, 2. [Google Scholar] [CrossRef]
- Coll, N.S.; Valls, M. Current knowledge on the Ralstonia solanacearum type III secretion system. Microb. Biotechnol. 2013, 6, 614–620. [Google Scholar] [PubMed]
- Kao, C.C.; Barlow, E.; Sequeira, L. Extracellular polysaccharide is required for wild-type virulence of Pseudomonas solanacearum. J. Bacteriol. 1992, 174, 1068–1071. [Google Scholar] [PubMed]
- Poueymiro, M.; Genin, S. Secreted proteins from Ralstonia solanacearum: A hundred tricks to kill a plant. Curr. Opin. Microbiol. 2009, 12, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Tans-Kersten, J.; Huang, H.; Allen, C. Ralstonia solanacearum needs motility for invasive virulence on tomato. J. Bacteriol. 2001, 183, 3597–3605. [Google Scholar] [CrossRef] [PubMed]
- Genin, S.; Boucher, C. Lessons learned from the genome analysis of Ralstonia solanacearum. Annu. Rev. Phytopathol. 2004, 42, 107–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, X.; Han, H. Evaluation of antibacterial effects of carbon nanomaterials against copper-resistant Ralstonia solanacearum. Colloids Surf. B Biointerfaces 2013, 103, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, S.; Shams-Bakhsh, M.; Hosseinzadeh, E. Effects of sub-bactericidal concentration of plant essential oils on pathogenicity factors of Ralstonia solanacearum. Arch. Phytopathol. Plant Prot. 2013, 46, 643–655. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, R.; Yin, H.; Luo, K. Isolation, identification and field control efficacy of endophytic strain against tobacco bacterial wilt. Chin. J. Appl. Ecol. 2007, 18, 554–558. [Google Scholar]
- Zhao, X.; Mei, W.; Gong, M.; Zuo, W.; Bai, H.; Dai, H. Antibacterial activity of the flavonoids from Dalbergia odorifera on Ralstonia solanacearum. Molecules 2011, 16, 9775–9782. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Momol, M.T.; Olson, S.M.; Pradhanang, P.M.; Jones, J.B. Evaluation of thymol as biofumigant for control of bacterial wilt of tomato under field conditions. Plant Dis. 2005, 89, 497–500. [Google Scholar] [CrossRef]
- Hong, J.C.; Momol, M.T.; Ji, P.; Olson, S.M.; Colee, J.; Jones, J.B. Management of bacterial wilt in tomatoes with thymol and acibenzolar-S-methyl. Crop Prot. 2011, 30, 1340–1345. [Google Scholar] [CrossRef]
- Kim, J.Y.; Park, S.C.; Hwang, I.; Cheong, H.; Nah, J.W.; Hahm, K.S.; Park, Y. Protease inhibitors from plants with antimicrobial activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.Q.; Li, Q.Q.; Qin, J.; Ye, Y.F.; Lin, W. Isolation of methyl gallate from Toxicodendron sylvestre and its effect on tomato bacterial wilt. Plant Dis. 2012, 96, 1143–1147. [Google Scholar] [CrossRef]
- Yuan, G.; Li, Q.; Wang, J.; Zeng, D.; Wu, D.; Yang, L.; Qin, J.; Cao, Z.; Li, H. Screening of plants for antimicrobial activity to phytopathogenic bacteria. Plant Prot. 2010, 4, 052. [Google Scholar]
- Fan, W.W.; Yuan, G.Q.; Li, Q.Q.; Lin, W. Antibacterial mechanisms of methyl gallate against Ralstonia solanacearum. Australas. Plant Pathol. 2014, 43, 1–7. [Google Scholar] [CrossRef]
- Paret, M.L.; Cabos, R.; Kratky, B.A.; Alvarez, A.M. Effect of plant essential oils on Ralstonia solanacearum race 4 and bacterial wilt of edible ginger. Plant Dis. 2010, 94, 521–527. [Google Scholar] [CrossRef]
- Pradhanang, P.M.; Momol, M.T.; Olson, S.M.; Jones, J.B. Effects of plant essential oils on Ralstonia solanacearum population density and bacterial wilt incidence in tomato. Plant Dis. 2003, 87, 423–427. [Google Scholar] [CrossRef]
- Paret, M.L.; Sharma, S.K.; Alvarez, A.M. Characterization of biofumigated Ralstonia solanacearum cells using micro-Raman spectroscopy and electron microscopy. Phytopathology 2012, 102, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.T.; Su, H.J.; Chen, C.T.; Ho, W.C.; Tsou, Y.R.; Chern, L.L. Inhibitory Effects of Chinese Medicinal Herbs on Plant-Pathogenic Bacteria and Identification of the Active Components from Gallnuts of Chinese Sumac. Plant Dis. 2012, 96, 1193–1197. [Google Scholar] [CrossRef]
- Deberdt, P.; Perrin, B.; Coranson-Beaudu, R.; Duyck, P.F.; Wicker, E. Effect of Allium fistulosum extract on Ralstonia solanacearum populations and tomato bacterial wilt. Plant Dis. 2012, 96, 687–692. [Google Scholar] [CrossRef]
- Cho, J.Y.; Choi, G.J.; Son, S.W.; Jang, K.S.; Lim, H.K.; Lee, S.O.; Kim, J.C. Isolation and antifungal activity of lignans from Myristica fragrans against various plant pathogenic fung. Pest Manag. Sci. 2007, 63, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Jiang, X.; Jeong, J.B.; Lee, S.H. Anticancer activity of protocatechualdehyde in human breast cancer cells. J. Med. Food 2014, 17, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.H.; Wang, B.Y.; Wang, C.Q.; Xie, X.L.; Yu, G.R.; Yao, Z.Y.; Yang, B.J. Effect of danshensu, protocatechualdehyde and danshen injection on calcium ion concentration in cytoplasm of human erythrocytes. China J. Chin. Mater. Med. 2004, 29, 984–988. [Google Scholar]
- Prachayasittikul, S.; Buraparuangsang, P.; Worachartcheewan, A.; Isarankura-Na-Ayudhya, C.; Ruchirawat, S.; Prachayasittikul, V. Antimicrobial and antioxidative activities of bioactive constituents from Hydnophytum formicarum Jack. Molecules 2008, 13, 904–921. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.O.; Schwinn, F.J. Control of soil-borne pathogens with living bacteria and fungi: Status and outlook. Pestic. Sci. 1993, 37, 355–363. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Sansores-Peraza, P.; Rosado-Vallado, M.; Brito-Loeza, W.; Mena-Rejon, G.J.; Quijano, L. Cassine, an antimicrobial alkaloid from Sennaracemosa. Fitoterapia 2000, 71, 690–692. [Google Scholar] [CrossRef]
- Yang, L.; Ding, W.; Xu, Y.; Wu, D.; Li, S.; Chen, J.; Guo, B. New Insights into the Antibacterial Activity of Hydroxycoumarins against Ralstonia solanacearum. Molecules 2016, 21, 468. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Lee, S.H. Protocatechualdehyde possesses anti-cancer activity through downregulating cyclin D1 and HDAC2 in human colorectal cancer cells. Biochem. Biophys. Res. Commun. 2013, 430, 381–386. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Shim, S.H.; Kim, J.S.; Shin, K.H.; Kang, S.S. Aldose reductase inhibitors from the fruiting bodies of Ganoderma applanatum. Biol. Pharm. Bull. 2005, 28, 1103–1105. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.Y.; Yin, M.C. Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2009, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Hudson, E.A.; Dinh, P.A.; Kokubun, T.; Simmonds, M.S.; Gescher, A. Characterization of potentially chemopreventive phenols in extracts of brown rice that inhibit the growth of human breast and colon cancer cells. Cancer Epidemiol. Biomark. Prev. 2000, 9, 1163–1170. [Google Scholar]
- Guan, S.; Bao, Y.M.; Jiang, B.; An, L.J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.L.; Wang, J.M.; Chu, C.Y.; Cheng, M.T.; Tseng, T.H. In vivo protective effect of protocatechuic acid on tert-butyl hydroperoxide-induced rat hepatotoxicity. Food Chem. Toxicol. 2002, 40, 635–641. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A review on protocatechuic acid and its pharmacological potential. ISRN Pharmacol. 2014. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.M.; Kim, W.S.; Lim, J.; Nam, S.Y.; Youn, M.; Nam, S.W.; Kim, Y.h.; Kim, S.H.; Park, W.J.; Park, S.S. Antipathogenic properties of green tea polyphenol epigallocatechin gallate at concentrations below the MIC against enterohemorrhagic Escherichia coli O157:H7. J. Food Prot. 2009, 72, 325–331. [Google Scholar] [PubMed]
- Farag, M.A.; Al-Mahdy, D.A.; Salah El Dine, R.; Fahmy, S.; Yassin, A.; Porzel, A.; Brandt, W. Structure Activity Relationships of Antimicrobial Gallic Acid Derivatives from Pomegranate and Acacia Fruit Extracts against Potato Bacterial Wilt Pathogen. Chem. Biodivers. 2015, 12, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.J.; Lee, H.B.; Kim, I.S.; Kim, K.Y.; Park, R.D.; Jung, W.J. Antifungal activity of gallic acid purified from Terminalia nigrovenulosa bark against Fusarium solani. Microb. Pathogen. 2013, 56, 8–15. [Google Scholar]
- French, G.L. Bactericidal agents in the treatment of MRSA infections—The potential role of daptomycin. J. Antimicrob. Chemother. 2006, 58, 1107–1117. [Google Scholar] [CrossRef] [PubMed]
- Ikigai, H.; Nakae, T.; Hara, Y.; Shimamura, T. Bactericidal catechins damage the lipid bilayer. Biochim. Biophys. Acta (BBA) Biomembr. 1993, 1147, 132–136. [Google Scholar] [CrossRef]
- Cushnie, T.P.; Hamilton, V.E.; Chapman, D.G.; Taylor, P.W.; Lamb, A.J. Aggregation of Staphylococcus aureus following treatment with the antibacterial flavonol galangin. J. Appl. Microbiol. 2007, 103, 1562–1567. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Ding, W.; Zhang, Y.; Liu, X.; Yang, L. Oleanolic acid induces the type III secretion system of Ralstonia solanacearum. Front. Microbiol. 2015, 6, 1466. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Englert, D.L.; Jayaraman, A.; Manson, M.D. Microfluidic techniques for the analysis of bacterial chemotaxis. Chemotaxis 2009, 571, 1–23. [Google Scholar]
- Kelman, A.; Hruschka, J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. Microbiology 1973, 76, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Sample of the compound protocatechualdehyde is available from the authors.

© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yu, Y.; Chen, J.; Guo, B.; Yang, L.; Ding, W. Evaluation of the Antibacterial Effects and Mechanism of Action of Protocatechualdehyde against Ralstonia solanacearum. Molecules 2016, 21, 754. https://doi.org/10.3390/molecules21060754
Li S, Yu Y, Chen J, Guo B, Yang L, Ding W. Evaluation of the Antibacterial Effects and Mechanism of Action of Protocatechualdehyde against Ralstonia solanacearum. Molecules. 2016; 21(6):754. https://doi.org/10.3390/molecules21060754
Chicago/Turabian StyleLi, Shili, Yanmei Yu, Juanni Chen, Bing Guo, Liang Yang, and Wei Ding. 2016. "Evaluation of the Antibacterial Effects and Mechanism of Action of Protocatechualdehyde against Ralstonia solanacearum" Molecules 21, no. 6: 754. https://doi.org/10.3390/molecules21060754
APA StyleLi, S., Yu, Y., Chen, J., Guo, B., Yang, L., & Ding, W. (2016). Evaluation of the Antibacterial Effects and Mechanism of Action of Protocatechualdehyde against Ralstonia solanacearum. Molecules, 21(6), 754. https://doi.org/10.3390/molecules21060754





