Bioprospecting the Curculigoside-Cinnamic Acid-Rich Fraction from Molineria latifolia Rhizome as a Potential Antioxidant Therapeutic Agent
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extract/Fraction Yield, Total Phenolic Content and Total Flavonoid Content
2.2. Phenolic Compositions by HPLC-DAD Analysis
2.3. Antioxidant Activities
2.4. Non-Enzymatic Anti-Glycation Activities
2.5. Cell Viability Assessment
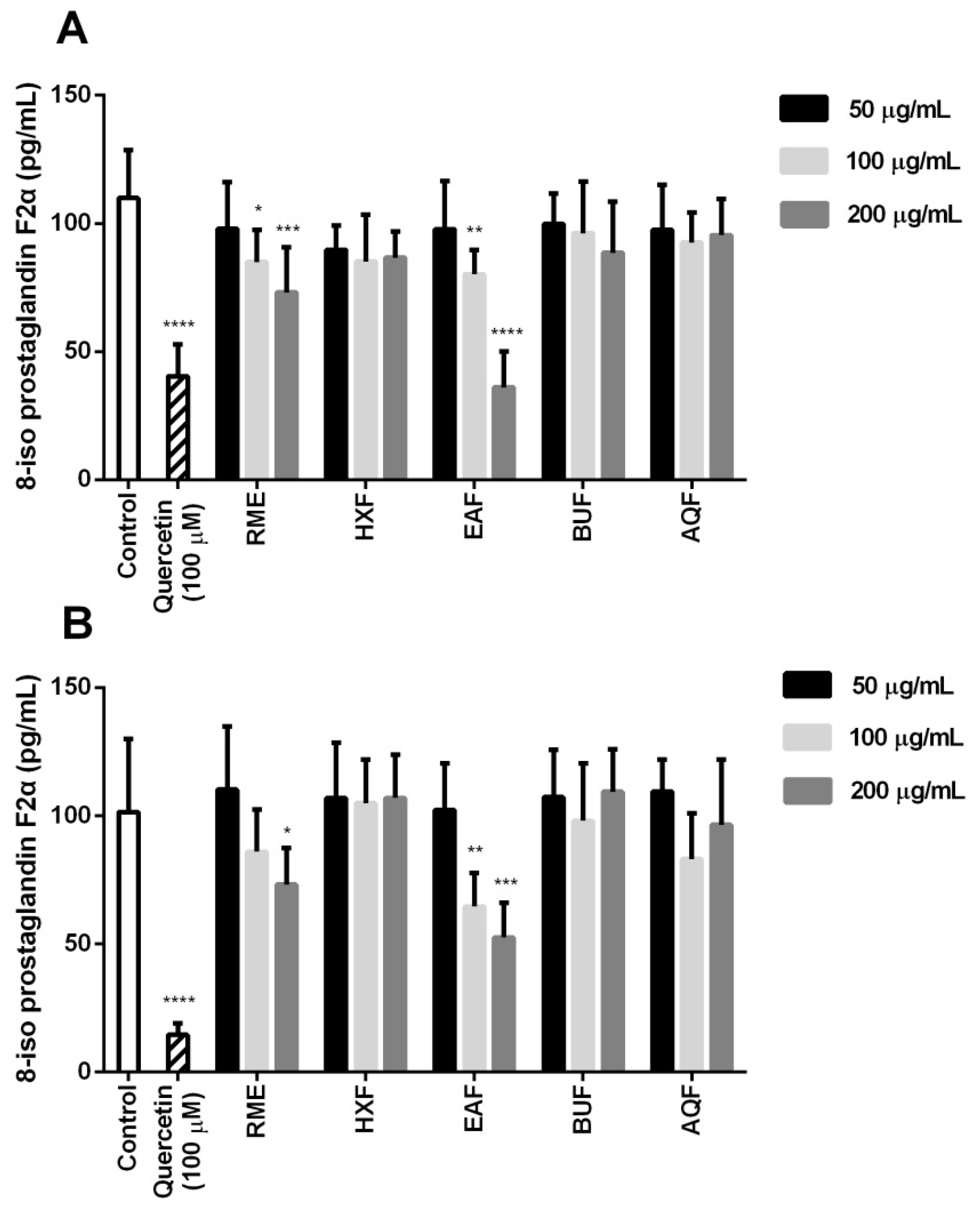
2.6. Cellular Antioxidant Activity and Oxidative Stress
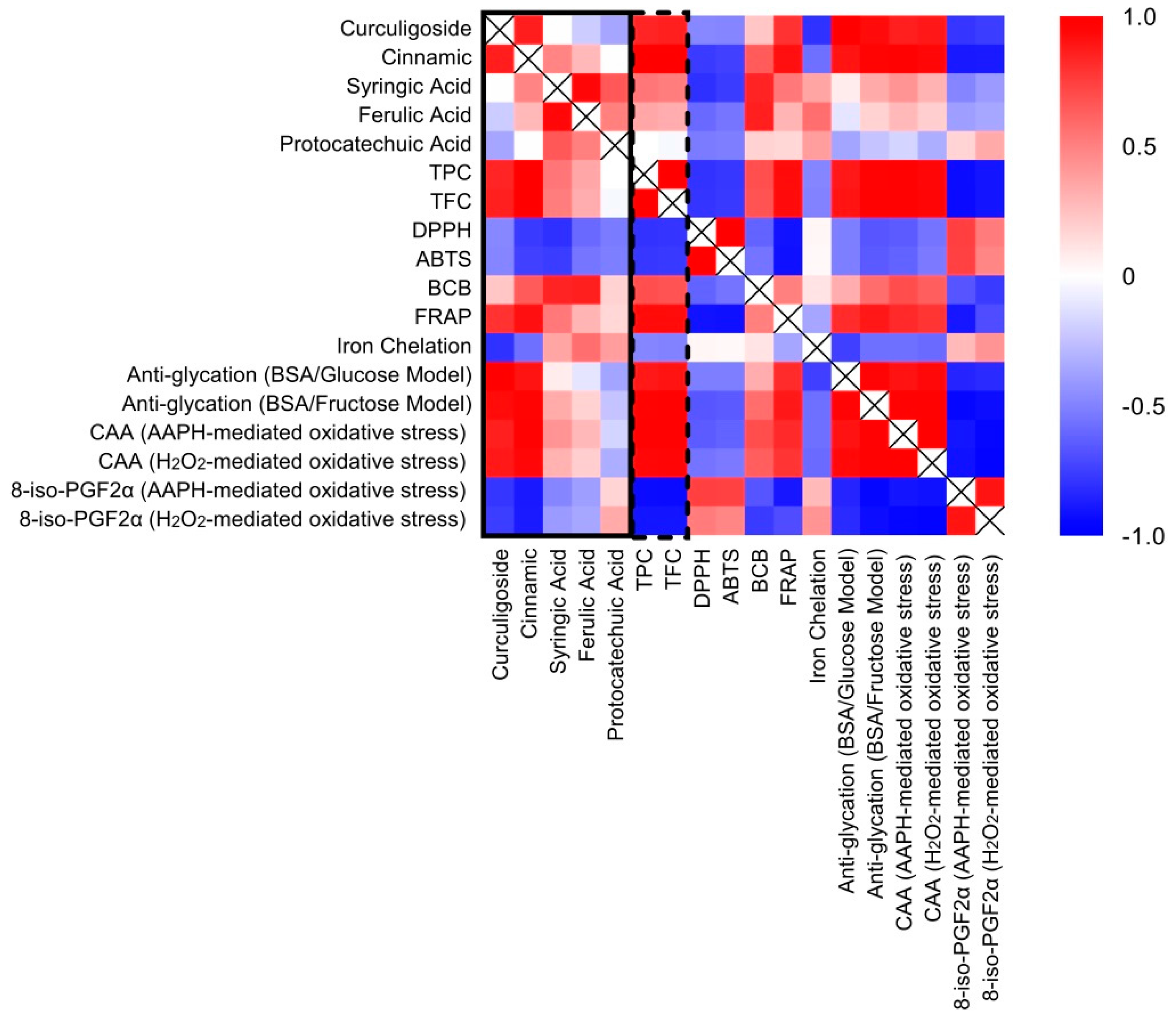
2.7. Correlation between Phenolic Compounds and Antioxidant Activities
3. Materials and Methods
3.1. Chemicals
3.2. Procurement and Preparation of Plant Materials
3.3. Extraction and Fractionation
3.4. Determination of Total Phenolic and Total Flavonoid Contents
3.5. HPLC-DAD Analysis for Phenolic Compounds
3.6. Antioxidant Activity Assays
3.6.1. DPPH Radical Scavenging Activity
3.6.2. ABTS Radical Cation Scavenging Activity
3.6.3. BCB Assay
3.6.4. FRAP Assay
3.6.5. Iron Chelating Activity
3.7. Non-Enzymatic Anti-Glycation Activities
3.8. Cell Culture
3.8.1. Cell Viability Assays
3.8.2. Cellular Antioxidant Activity
3.8.3. 8-iso-PGF2α Content
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| RME | rhizome methanolic extract |
| HXF | hexane fraction |
| EAF | ethyl acetate fraction |
| BUF | n-butanol fraction |
| AQF | aqueous fraction |
| GAE | gallic acid equivalent |
| RE | rutin equivalent |
| CE | catechin equivalent |
| TRE | trolox equivalent |
| NRU | neutral red uptake |
| CAA | cellular antioxidant activity |
| AUC | area under the curve |
References
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Dawane, J.S.; Pandit, V.A. Understanding redox homeostasis and its role in cancer. J. Clin. Diagn. Res. 2012, 6, 1796–1802. [Google Scholar] [CrossRef] [PubMed]
- Elahi, M.M.; Chuang, A.; Matata, B.M. Overview of oxidative stress and cardiovascular disease. In Systems Biology of Free Radicals and Antioxidants; Laher, I., Ed.; Springer Berlin Heidelberg: Berlin, Germany, 2014; pp. 719–764. [Google Scholar]
- Wang, X.; Hai, C. Redox modulation of adipocyte differentiation: Hypothesis of ‘Redox Chain’ and novel insights into intervention of adipogenesis and obesity. Free Radic. Biol. Med. 2015, 89, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, Y.; Lu, W.; Liu, K. Mitochondrial reactive oxygen species regulate adipocyte differentiation of mesenchymal stem cells in hematopoietic stress induced by arabinosylcytosine. PLoS ONE 2015, 10, e0120629. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jo, D.-G.; Park, D.; Chung, H.Y.; Mattson, M.P. Adaptive cellular stress pathways as therapeutic targets of dietary phytochemicals: Focus on the nervous system. Pharmacol. Rev. 2014, 66, 815–868. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.O.; Wightman, E.L. Herbal extracts and phytochemicals: Plant secondary metabolites and the enhancement of human brain function. Adv. Nutr. 2011, 2, 32–50. [Google Scholar] [CrossRef] [PubMed]
- Herranz-López, M.; Fernández-Arroyo, S.; Pérez-Sanchez, A.; Barrajón-Catalán, E.; Beltrán-Debón, R.; Menéndez, J.A.; Alonso-Villaverde, C.; Segura-Carretero, A.; Joven, J.; Micol, V. Synergism of plant-derived polyphenols in adipogenesis: Perspectives and implications. Phytomedicine 2012, 19, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.U.; Ismail, M.; Ooi, D.J.; Azmi, N.H.; Sarega, N.; Chan, K.W.; Bhanger, M.I. Are bioactive-rich fractions functionally richer? Crit. Rev. Biotechnol. 2015. [Google Scholar] [CrossRef] [PubMed]
- The Plant List. Version 1.1. 2013. Available online: http://www.theplantlist.org (accessed on 6 November 2015).
- Wiart, C. Medicinal Plants of Southeast Asia; Pelanduk Publications: Kuala Lumpur, Malaysia, 2000. [Google Scholar]
- Ishak, N.A.; Ismail, M.; Hamid, M.; Ahmad, Z.; Abd Ghafar, S.A. Antidiabetic and Hypolipidemic Activities of Curculigo latifolia Fruit: Root Extract in High Fat Fed Diet and Low Dose STZ Induced Diabetic Rats. Evid. Based Complement. Altern. Med. 2013, 2013, 601838. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Maqueda, D.; Hernández-Ledesma, B.; Amigo, L.; Miralles, B.; Gómez-Ruiz, J.A. Extraction/Fractionation Techniques for Proteins and Peptides and Protein Digestion. In Proteomics in Foods; Toldrá, F., Nollet, L.M.L., Eds.; Springer: New York, NY, USA, 2013; pp. 21–50. [Google Scholar]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.E.; Lewers, K.S.; Medina, M.B.; Saftner, R.A. Comparative analysis of strawberry total phenolics via Fast Blue BB vs. Folin–Ciocalteu: Assay interference by ascorbic acid. J. Food Comp. Anal. 2012, 27, 102–107. [Google Scholar] [CrossRef]
- Mariod, A.A.; Ibrahim, R.M.; Ismail, M.; Ismail, N. Antioxidant activity and phenolic content of phenolic rich fractions obtained from black cumin (Nigella sativa) seedcake. Food Chem. 2009, 116, 306–312. [Google Scholar] [CrossRef]
- Lee, M.S.; Lee, I.I.; Kim, Y.; Kim, Y.J.; Heo, H.J.; Kim, D.-O. Inhibitory effect of the ethyl acetate fraction from astringent persimmon on H2O2-induced oxidative stress in HepG2 cells. Food Sci. Biotechnol. 2014, 23, 1247–1252. [Google Scholar] [CrossRef]
- Sutherland, I.A.; Fisher, D. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J. Chromatogr. A 2009, 1216, 740–753. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2016, 4, D1202–D1213. [Google Scholar] [CrossRef] [PubMed]
- Das, N.; Islam, M.E.; Jahan, N.; Islam, M.S.; Khan, A.; Islam, M.R.; Parvin, M.S. Antioxidant activities of ethanol extracts and fractions of Crescentia cujete leaves and stem bark and the involvement of phenolic compounds. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What makes good antioxidants in lipid-based systems? The next theories beyond the polar paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef] [PubMed]
- Ohyashiki, T.; Kadoya, A.; Kushida, K. The role of Fe3+ on Fe2+-dependent lipid peroxidation in phospholipid liposomes. Chem. Pharm. Bull. 2002, 50, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Ambigaipalan, P. Phenolics and polyphenolics in foods, beverages and spices: Antioxidant activity and health effects—A review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Chan, K.W.; Iqbal, S.; Khong, N.M.H.; Ooi, D.-J.; Ismail, M. Antioxidant activity of phenolics–saponins rich fraction prepared from defatted kenaf seed meal. LWT Food Sci. Technol. 2014, 56, 181–186. [Google Scholar] [CrossRef]
- Hatia, S.; Septembre-Malaterre, A.; Le Sage, F.; Badiou-Bénéteau, A.; Baret, P.; Payet, B.; Lefebvre d’hellencourt, C.; Gonthier, M.P. Evaluation of antioxidant properties of major dietary polyphenols and their protective effect on 3T3-L1 preadipocytes and red blood cells exposed to oxidative stress. Free Radic. Res. 2014, 48, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Sompong, W.; Meeprom, A.; Cheng, H.; Adisakwattana, S. A comparative study of ferulic acid on different monosaccharide-mediated protein glycation and oxidative damage in bovine serum albumin. Molecules 2013, 18, 13886–13903. [Google Scholar] [CrossRef] [PubMed]
- Vlassopoulos, A.; Lean, M.E.J.; Combet, E. Protein-phenolic interactions and inhibition of glycation—Combining a systematic review and experimental models for enhanced physiological relevance. Food Funct. 2014, 5, 2646–2655. [Google Scholar] [CrossRef] [PubMed]
- Adisakwattana, S.; Sompong, W.; Meeprom, A.; Ngamukote, S.; Yibchok-anun, S. Cinnamic Acid and Its Derivatives Inhibit Fructose-Mediated Protein Glycation. Int. J. Mol. Sci. 2012, 13, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Capanoglu, E.; Altay, F. A review on protein–phenolic interactions and associated changes. Food Res. Int. 2013, 51, 954–970. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Baile, C.A. Phytochemicals and regulation of the adipocyte life cycle. J. Nutr. Biochem. 2008, 19, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, R.; Raiola, A.; Tenore, G.C.; Frusciante, L.; Barone, A.; Monti, D.M.; Rigano, M.M. Antioxidant bioactive compounds in tomato fruits at different ripening stages and their effects on normal and cancer cells. J. Funct. Foods 2015, 18, 83–94. [Google Scholar] [CrossRef]
- Prior, R.L. Oxygen radical absorbance capacity (ORAC): New horizons in relating dietary antioxidants/bioactives and health benefits. J. Funct. Foods 2015, 18, 797–810. [Google Scholar] [CrossRef]
- Marimoutou, M.; Sage, F.L.; Smadja, J.; d’Hellencourt, C.L.; Gonthier, M.-P.; Silva, C.R.-D. Antioxidant polyphenol-rich extracts from the medicinal plants Antirhea borbonica, Doratoxylon apetalum and Gouania mauritiana protect 3T3-L1 preadipocytes against H2O2, TNFα and LPS inflammatory mediators by regulating the expression of superoxide dismutase and NF-κB genes. J. Inflamm. 2015, 12. [Google Scholar] [CrossRef]
- Labuschagne, C.F.; van den Broek, N.J.F.; Postma, P.; Berger, R.; Brenkman, A.B. A protocol for quantifying lipid peroxidation in cellular systems by F2-isoprostane analysis. PLoS ONE 2013, 8, e80935. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Chen, D.; Chen, S. Antioxidant activity and mechanism of protocatechuic acid in vitro. Funct. Food Health Dis. 2011, 7, 232–244. [Google Scholar]
- Zeraik, M.L.; Petrônio, M.S.; Coelho, D.; Regasini, L.O.; Silva, D.H.S.; Fonseca, L.M.; Machado, S.A.S.; Bolzani, V.S.; Ximenes, V.F. Improvement of Pro-Oxidant Capacity of Protocatechuic Acid by Esterification. PLoS ONE 2014, 9, e110277. [Google Scholar] [CrossRef] [PubMed]
- Natella, F.; Nardini, M.; Di Felice, M.; Scaccini, C. Benzoic and Cinnamic Acid Derivatives as Antioxidants: Structure-Activity Relation. J. Agric. Food Chem. 1999, 47, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Wang, Y.; Xu, J.; Nie, Y.; Guo, Y.; Tong, Y.; Qin, L.; Zhang, Q. Curculigoside isolated from Curculigo orchioides prevents hydrogen peroxide-induced dysfunction and oxidative damage in calvarial osteoblasts. Acta Biochim. Biophys. Sin. 2012, 44, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yu, W.; Liu, H.; Zhang, N.; Li, X.; Zhao, M.; Liu, S. Neuroprotective effects of curculigoside against NMDA-induced neuronal excitoxicity in vitro. Food Chem. Toxicol. 2012, 50, 4010–4015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.K.; Hong, Y.J.; Wei, M.; Wu, Y.; Huang, Z.Q.; Chen, R.Z.; Chen, H.Z. Curculigoside attenuates human umbilical vein endothelial cell injury induced by H2O2. J. Ethnopharmacol. 2010, 132, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, S.; Wang, Y.; Zhang, Q.; Zhao, W.; Wang, Z.; Yin, M. Effects of Curculigoside on Memory Impairment and Bone Loss via Anti-Oxidative Character in APP/PS1 Mutated Transgenic Mice. PLoS ONE 2015, 10, e0133289. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Rajan, P.; Vedernikova, I.; Calomme, M.; Pieters, L.; Vlietinck, A.J.; Augustyns, K.; Haemers, A.; Berghe, D.V. In vitro antioxidant profile of phenolic acid derivatives. Free Radic. Res. 2002, 36, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Avanesyan, A.A.; Pashkov, A.N.; Simonyan, N.A.; Simonyan, A.V.; Myachina, O.V. Antiradical activity of cinnamic acid derivatives. Pharm. Chem. J. 2009, 43, 249–250. [Google Scholar] [CrossRef]
- Kang, X.; Guo, W.; Zhao, C.; Song, J. Adsorption-parallel catalytic waves of cinnamic acid in hydrogen peroxide-tetra-n-butylammonium bromide-acetate system. Sci. China Ser. B 2000, 43, 275–282. [Google Scholar] [CrossRef]
- Patra, K.; Bose, S.; Sarkar, S.; Rakshit, J.; Jana, S.; Mukherjee, A.; Roy, A.; Mandal, D.P.; Bhattacharjee, S. Amelioration of cyclophosphamide induced myelosuppression and oxidative stress by cinnamic acid. Chem. Biol. Interact. 2012, 195, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Lukmanul Hakkim, F.; Miura, M.; Matsuda, N.; Alharassi, A.S.; Guillemin, G.; Yamauchi, M.; Arivazhagan, G.; Song, H. An in vitro evidence for caffeic acid, rosmarinic acid and trans cinnamic acid as a skin protectant against γ-radiation. Int. J. Low Radiat. 2014, 9, 305–316. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Ooi, D.-J.; Iqbal, S.; Ismail, M. Proximate composition, nutritional attributes and mineral composition of Peperomia pellucida L. (Ketumpangan Air) grown in Malaysia. Molecules 2012, 17, 11139–11145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Wan, L.; Wu, C.; Fang, Y.; Han, G.; Li, H.; Zhang, Z.; Wang, H. Simultaneous determination of 14 phenolic compounds in grape canes by HPLC-DAD-UV using wavelength switching detection. Molecules 2013, 18, 14241–14257. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.; Khong, N.M.H.; Iqbal, S.; Umar, I.M.; Ismail, M. Antioxidant property enhancement of sweet potato flour under simulated gastrointestinal pH. Int. J. Mol. Sci. 2012, 13, 8987–8997. [Google Scholar] [CrossRef] [PubMed]
- Yusri, N.M.; Chan, K.W.; Iqbal, S.; Ismail, M. Phenolic content and antioxidant activity of Hibiscus cannabinus L. seed extracts after sequential solvent extraction. Molecules 2012, 17, 12612–12621. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, F.M.; Subratty, A.H.; Gurib-Fakim, A.; Choudhary, M.I. Antioxidant, antiglycation and cytotoxicity evaluation of selected medicinal plants of the Mascarene Islands. BMC Complement. Altern. Med. 2012, 12. [Google Scholar] [CrossRef] [PubMed]
- Foo, J.B.; Yazan, L.S.; Tor, Y.S.; Armania, N.; Ismail, N.; Imam, M.U.; Yeap, S.K.; Cheah, Y.K.; Abdullah, B.; Ismail, M. Induction of cell cycle arrest and apoptosis in caspase-3 deficient MCF-7 cells by Dillenia suffruticosa root extract via multiple signalling pathways. BMC Complement. Altern. Med. 2014, 14. [Google Scholar] [CrossRef] [PubMed]
- Kamba, A.S.; Ismail, M.; Tengku Ibrahim, T.A.; Zakaria, Z.A.B.; Gusau, L.H. In Vitro Ultrastructural Changes of MCF-7 for Metastasise Bone Cancer and Induction of Apoptosis via Mitochondrial Cytochrome C Released by CaCO3/Dox Nanocrystals. BioMed Res. Int. 2014, 2014, 391869. [Google Scholar] [PubMed]
- Ramful, D.; Tarnus, E.; Rondeau, P.; Da Silva, C.R.; Bahorun, T.; Bourdon, E. Citrus fruit extracts reduce advanced glycation end products (AGEs)- and H2O2-induced oxidative stress in human adipocytes. J. Agric. Food Chem. 2010, 58, 11119–11129. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the extracts and fractions are available from the authors.
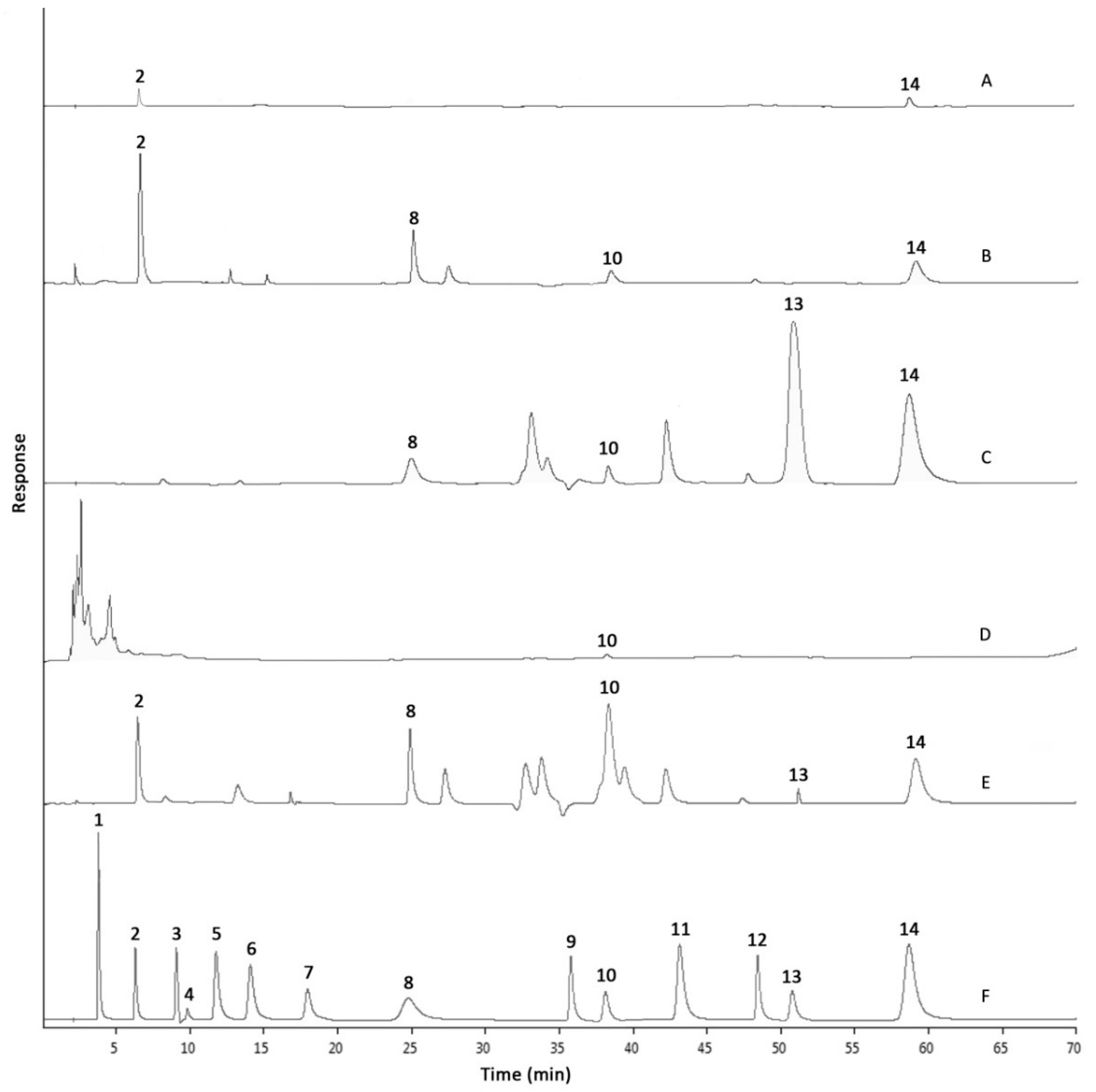
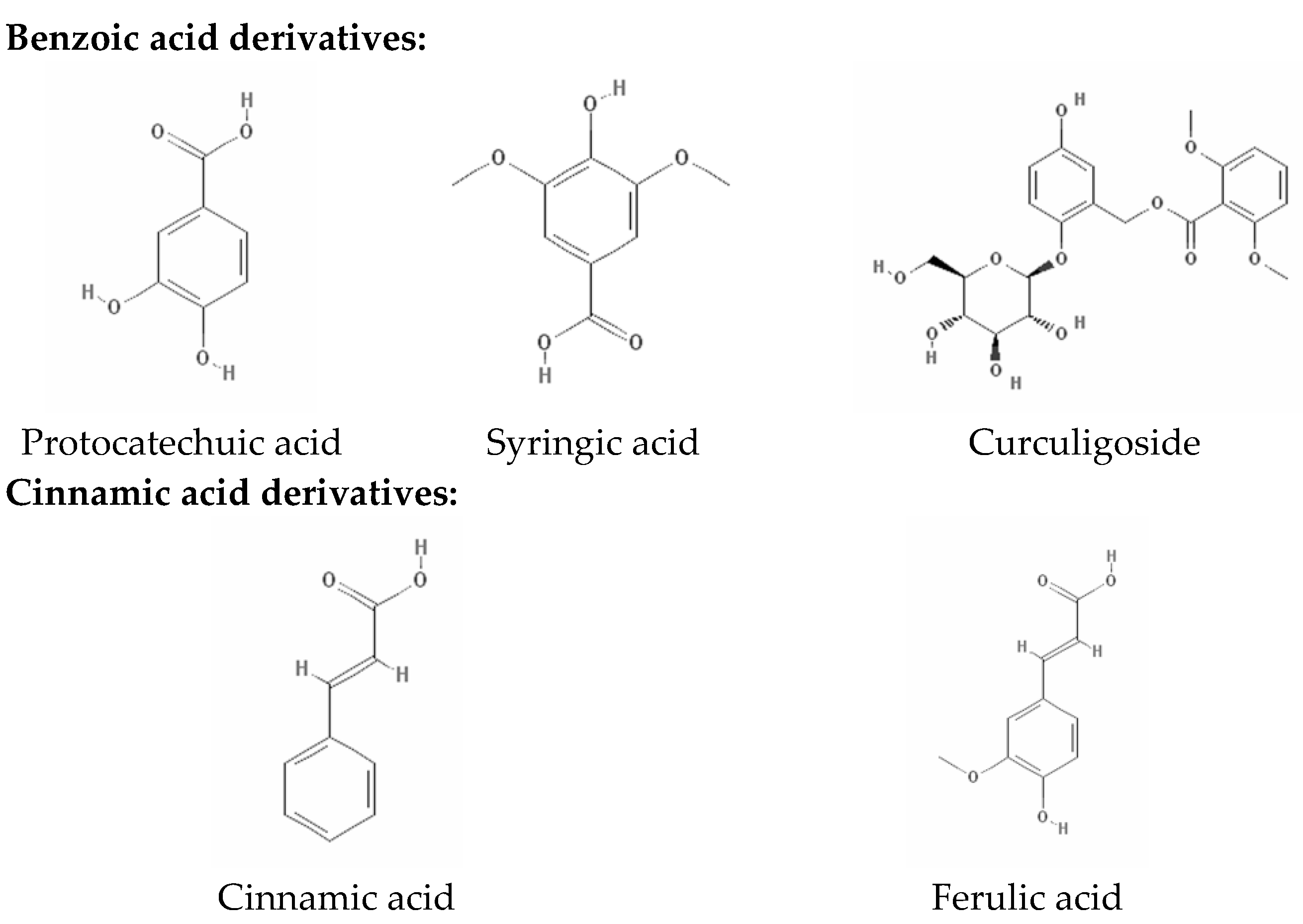
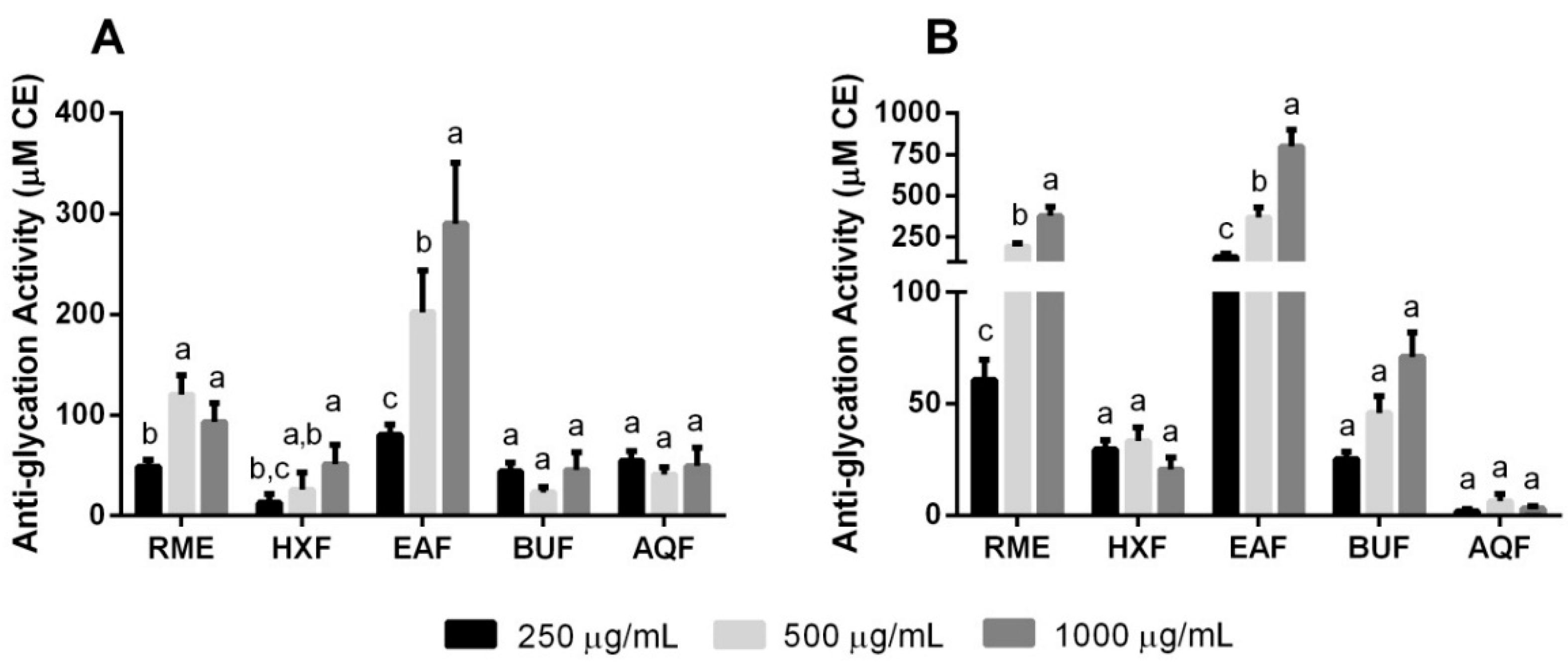
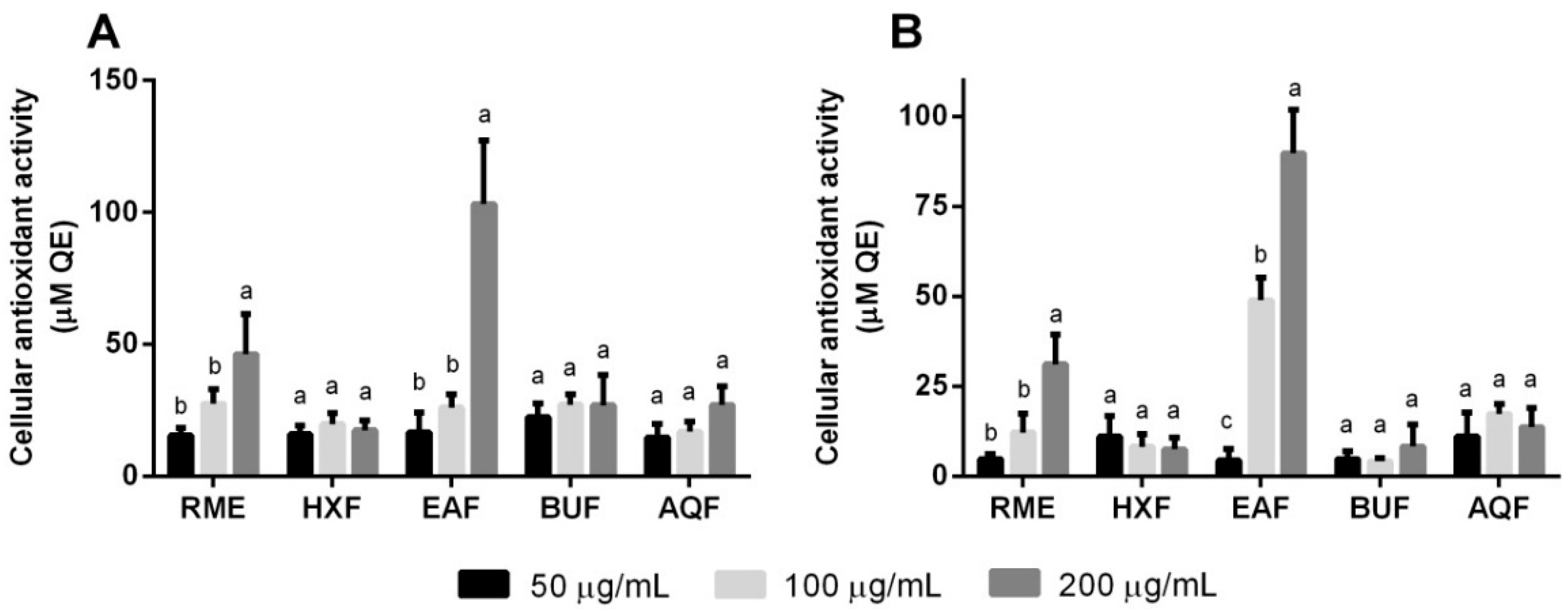
| Extract or Fraction | Yield (g/100 g Dry Weight) | Total Phenolic Content (mg GAE/g Extract or Fraction) | Total Flavonoid Content (mg RE/g Extract or Fraction) |
|---|---|---|---|
| RME | 7.95 ± 0.02 d | 48.35 ± 10.54 b | 21.97 ± 3.33 b |
| HXF | 0.71 ± 0.02 e | 0.30 ± 0.27 e | 0.18 ± 0.10 d |
| EAF | 10.38 ± 0.13 c | 187.30 ± 9.28 a | 76.77 ± 2.68 a |
| BUF | 36.37 ± 0.29 b | 24.96 ± 4.34 c | 10.84 ± 2.05 c |
| AQF | 42.54 ± 0.59 a | 4.62 ± 0.85 d | 1.62 ± 0.63 d |
| Phenolic Compound | Retention Time (min) | Individual Phenolic Content (mg/g Extract or Fraction) | ||||
|---|---|---|---|---|---|---|
| RME | HXF | EAF | BUF | AQF | ||
| Gallic acid | 3.835 ± 0.014 | Nd | Nd | Nd | Nd | Nd |
| Protocatechuic acid | 6.391 ± 0.019 | 1.75 ± 0.27 b | Nd | Nd | 4.58 ± 0.09 a | 0.11 ± 0.02 c |
| p-Hydroxybenzoic acid | 9.135 ± 0.012 | Nd | Nd | Nd | Nd | Nd |
| Gentisic acid | 9.962 ± 0.011 | Nd | Nd | Nd | Nd | Nd |
| Chlorogenic acid | 11.917 ± 0.013 | Nd | Nd | Nd | Nd | Nd |
| Vanillic acid | 14.211 ± 0.012 | Nd | Nd | Nd | Nd | Nd |
| Caffeic acid | 18.158 ± 0.014 | Nd | Nd | Nd | Nd | Nd |
| Syringic acid | 25.075 ± 0.018 | 4.69 ± 0.09 a | Nd | 1.75 ± 0.02 c | 3.26 ± 0.03 b | Nd |
| p-Coumaric acid | 36.165 ± 0.013 | Nd | Nd | Nd | Nd | Nd |
| Ferulic acid | 38.534 ± 0.019 | 2.47 ± 0.04 a | 0.09 ± 0.03 d | 0.29 ± 0.06 c | 1.06 ± 0.04 b | Nd |
| Sinapic acid | 43.609 ± 0.013 | Nd | Nd | Nd | Nd | Nd |
| Salicylic acid | 48.985 ± 0.015 | Nd | Nd | Nd | Nd | Nd |
| Curculigoside | 51.353 ± 0.021 | 6.47 ± 0.04 b | Nd | 90.49 ± 0.04 a | Nd | Nd |
| Cinnamic acid | 59.323 ± 0.020 | 7.05 ± 0.01 b | Nd | 12.43 ± 0.10 a | 4.39 ± 0.06 c | 1.70 ± 0.08 d |
| Sum of Phenolic Content | 22.43 b | 0.09 d | 104.96 a | 13.29 b | 1.81 c | |
| Extract or Fraction | DPPH Radical Scavenging Activity | ABTS Radical Cation Scavenging Activity | β-Carotene Bleaching Activity | Ferric Reducing Antioxidant Power | Iron Chelating Activity |
|---|---|---|---|---|---|
| IC50 (μg/mL) | IC50 (μg/mL) | (μg TRE/mg Extract or Fraction) | (μg TRE/mg Extract or Fraction) | IC50 (mg/mL) | |
| RME | 90.76 ± 2.48 c | 431.00 ± 36.90 c | 3.97 ± 0.22 a | 204.44 ± 16.66 b | 6.13 ± 1.35 a |
| HXF | 495.47 ± 24.04 b | 1588.50 ± 261.61 b | 0.57 ± 0.04 e | 126.90 ± 13.54 c | 5.72 ± 1.88 a |
| EAF | 36.96 ± 1.10 c | 132.24 ± 19.20 c,d | 2.34 ± 0.06 b | 308.94 ± 16.98 a | 1.90 ± 0.04 c |
| BUF | 93.36 ± 0.38 c | 232.60 ± 14.68 c,d | 1.61 ± 0.09 c | 198.36 ± 18.46 b | 5.01 ± 2.29 a,b |
| AQF | 707.41 ± 143.29 a | 2537.98 ± 379.22 a | 1.34 ± 0.17 d | 57.68 ± 6.64 d | 3.55 ± 0.02 b,c |
| Trolox | 23.87 ± 0.40 c | 20.54 ± 0.07 d | |||
| Na2EDTA | 0.03 ± 0.00 d |
| MTT | NRU | |||
|---|---|---|---|---|
| IC20 (μg/mL) | IC50 (μg/mL) | IC20 (μg/mL) | IC50 (μg/mL) | |
| RME | 155.93 ± 9.83 d | 561.28 ± 69.53 c | 206.85 ± 15.57 c | 663.43 ± 88.62 b |
| HXF | 448.49 ± 21.26 a | >2000 a | 522.59 ± 27.74 a | >2000 a |
| EAF | 111.73 ± 9.57 e | 509.59 ± 49.75 c | 148.11 ± 16.23 d | 536.99 ± 45.94 c |
| BUF | 192.65 ± 12.33 c | 649.97 ± 53.82 b | 229.63 ± 20.12 c | 729.65 ± 61.27 b |
| AQF | 413.73 ± 28.75 b | >2000 a | 382.59 ± 27.66 b | >2000 a |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, D.J.; Chan, K.W.; Sarega, N.; Alitheen, N.B.; Ithnin, H.; Ismail, M. Bioprospecting the Curculigoside-Cinnamic Acid-Rich Fraction from Molineria latifolia Rhizome as a Potential Antioxidant Therapeutic Agent. Molecules 2016, 21, 682. https://doi.org/10.3390/molecules21060682
Ooi DJ, Chan KW, Sarega N, Alitheen NB, Ithnin H, Ismail M. Bioprospecting the Curculigoside-Cinnamic Acid-Rich Fraction from Molineria latifolia Rhizome as a Potential Antioxidant Therapeutic Agent. Molecules. 2016; 21(6):682. https://doi.org/10.3390/molecules21060682
Chicago/Turabian StyleOoi, Der Jiun, Kim Wei Chan, Nadarajan Sarega, Noorjahan Banu Alitheen, Hairuszah Ithnin, and Maznah Ismail. 2016. "Bioprospecting the Curculigoside-Cinnamic Acid-Rich Fraction from Molineria latifolia Rhizome as a Potential Antioxidant Therapeutic Agent" Molecules 21, no. 6: 682. https://doi.org/10.3390/molecules21060682
APA StyleOoi, D. J., Chan, K. W., Sarega, N., Alitheen, N. B., Ithnin, H., & Ismail, M. (2016). Bioprospecting the Curculigoside-Cinnamic Acid-Rich Fraction from Molineria latifolia Rhizome as a Potential Antioxidant Therapeutic Agent. Molecules, 21(6), 682. https://doi.org/10.3390/molecules21060682






